Abstract
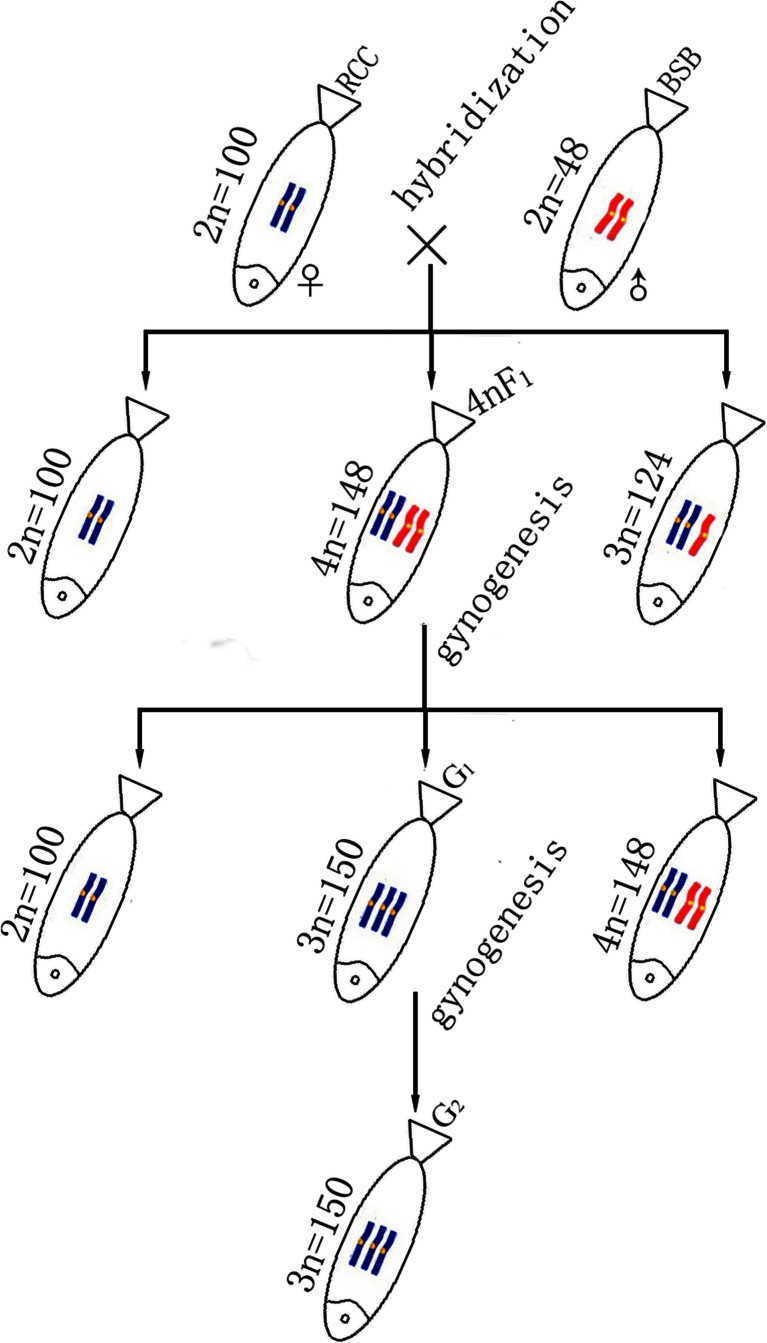
Following activation by UV-irradiated BSB sperm, the fertilized eggs of tetraploid hybrids (abbreviated as 4nF1) (4n = 148, AABB) of Carassius auratus red var. (abbreviated as RCC) (2n = 100, AA) (♀) × Megalobrama amblycephala (abbreviated as BSB) (2n = 48, BB) (♂) developed into normal live gynogenetic offspring without chromosome doubling treatment. Some of these were autotriploids with three sets of red crucian carp chromosomes (abbreviated as G1) (3n = 150, AAA). G1 were all-females, and can produce unreduced (3n) eggs at age 1 year. After activation by UV-irradiated BSB sperm, the fertilized eggs of G1 developed into a second generation of autotriploid gynogenetic offspring (abbreviated as G2) (3n = 150) without chromosome doubling treatment. G1 were obviously different from both 4nF1 and RCC in their morphological traits and showed a significantly higher growth rate than RCC. In aquaculture, the autotriploid fish could provide an important source of gametes for the production of all-female triploid fish and for the establishment of autotriploid gynogenetic lines.
Keywords: Distant hybridization, Gynogenesis, Autotriploid, Fertile, Unreduced gamete
Introduction
The introduction of triploid individuals into culture systems potentially minimizes the risks associated with accidental releases because triploids are usually sterile. In aquaculture, polyploids have been obtained by exposing fertilized eggs to physical or chemical agents (Teskered et al. 1993; Maclean et al. 1999; Linhart et al. 2001). Induction of triploid fish involves the retention of the second polar body of fertilized eggs, and tetraploid fish can be produced by suppression of the first mitotic division in fertilized eggs (Liu et al. 2007). Distant hybridization is an efficient way to produce crossbred fish that show heterosis with respect to growth rate and disease tolerance in culture (Hulata 1995; Hulata et al. 2008), and may generate tetraploid lines that can produce fast-growing sterile triploids by inter-ploidy hybridization (Liu et al. 2001, 2007, 2010; Liu 2010).
Usually, allopolyploids contain both parental genomes that undergo bivalent pairing at meiosis because only homologous chromosomes pair (Soltis and Soltis 2000; Wu et al. 2001). In face, abnormal chromosome behavior during meiosis has been observed among the polyploid hybrid progeny of many plants, which may lead to the production of unexpected gametes (Li and Heneen 1999).
In our previous study, we successfully obtained sterile triploid hybrids (3n = 124, AAB), fertile tetraploid hybrids (4n = 148, AABB), and fertile natural gynogenetic diploid (2n = 100, AA), but no diploid hybrids (2n = 74, AB) were found to survive in the first generation of Carassius auratus red var. (RCC, 2n = 100, AA) (♀) × Megalobrama amblycephala (BSB, 2n = 48, BB) (♂) (Liu et al. 2007; Liu 2010). The diploid hybrids probably did not survive because of the large difference in chromosome number between RCC (2n = 100) and BSB (2n = 48), presumably preventing the embryos from developing into viable fish. However, some diploid hybrid embryos developed into tetraploid hybrids through inhibition of the first cleavage which resulted in chromosome doubling (Liu et al. 2010).
Allotetraploids of RCC (♀) × BSB (♂) contain two sets of RCC-derived chromosomes and two sets of BSB-derived chromosomes, and would be supposed to produce allodiploid gamete (2n = 74, AB). However, abnormal chromosome behavior during meiosis in allotetraploids may lead to the formation of autotriploid gametes (3n = 150, AAA). There have been many reports on the fish gynogenesis using haploid eggs (Kavumpurath and Pandian 1994; Gomelsky et al. 1998; Hulata 2001), which have to be subjected to harmful treatment for doubling the chromosomes. The prominent advantage of the gynogenesis using autotriploid eggs is that it does not require the treatment for doubling the chromosomes. Thus, the autotriploids may be successfully obtained in gynogenetic progeny of allotetraploids. Interestingly, artificial autotriploids are usually sterile (Cherfas et al. 1994) but these autotriploids reached sexual maturity at age 1 year. This paper is the first to report of the formation of fertile autotriploid gynogenetic fish by distant hybridization and gynogenesis. These observations are of importance for both aquaculture and the genetic breeding of fish.
Methods
Animals and Crosses
BSB and RCC were obtained from the Protection Station of Polyploid Fish, Hunan Normal University. During the reproductive seasons (April) of 2004, 2005, and 2006, 4nF1 hybrids of RCC (♀) × BSB (♂) were produced. During the reproductive seasons of 2010, 2011, and 2012, gynogenetic offspring (G1) were obtained from 4nF1 eggs by artificial gynogenesis. During the reproductive season of 2011 and 2012, gynogenetic offspring (G2) were obtained from G1 eggs by artificial gynogenesis. The method of gynogenesis helps to clarify the ploidy of the eggs because polyploid eggs are able to develop into living fish but haploid eggs do not. The formation of G1 and G2 suggest that 4nF1 hybrids and G1 can produce the triploid egg.
UV Irradiation for Sperm Inactivation
The milt was stripped from male BSB, diluted with Hank’s solution (1:4), and then poured into cold culture dishes to allow formation of a thin (0.1–0.2 mm) layer. UVC irradiation was performed using two quartz UV lamps (ZSZ20D) emitting a wavelength of 253.7 nm. The total UV dosage administered to the sperm over a period of 25–45 min was in the range 3000–3600 mJ/cm2. The milt suspension was continuously shaken at 30 rpm and 4 °C during the exposure period. The irradiation was monitored by observing the vitality of the sperm under a microscope. After UV irradiation, sperm were stored in glass tubes at 4 °C. All processes were conducted in the dark. This method enhanced the success rate of gynogenesis and decreased the number of hybrids in the offspring of gynogenesis.
Preparation of Chromosome Spreads
Chromosome preparation is carried out on the kidney tissues of RCC, BSB, 4nF1, G1, and G2, according to the procedures reported by Liu et al. (2001, 2007). For each type of fish, 200 metaphase spreads (20 metaphase spreads from each of 10 fish) of chromosomes were counted and analyzed for further determination of ploidy and chromosome number. Examining the chromosomal spreads can directly identify the chromosomal number of RCC, BSB, 4nF1, G1 and G2.
Gonadal Structure
Ten RCC, 10 4nF1, and 40 G1 at age 9 months were randomly selected for histological observation of gonad structure. Gonads were fixed in Bouin’s solution, embedded in paraffin, sectioned with a Leica RM2016 microtome, and stained with hematoxylin and eosin. Tissue sections were observed and photographed with an Olympus CX41 microscope. Gonadal development was staged according to criteria established for cyprinid fish (Liu 1993). Observation of gonadal development was used to determine whether the gynogenetic progenies were fertile.
Morphological Traits
At 1 year of age, 20 RCC, 20 4nF1, and 20 G1 were randomly selected for morphological examination with reference to the standards set by Zou et al. (2008). Measurable traits recorded were the mean values of the whole length, the body length (excluding tail) and width, the head length and width, and the tail length and width. These values were used to calculate ratios of whole length to body length (WL/BL), body length to body width (BL/BW), body length to head length (BL/HL), head length to head width (HL/HW), tail length to tail width (TL/TW), and body width to head width (BW/HW) are calculated. Countable traits recorded for each fish were the number of: lateral line scales; scales above and below the lateral line; dorsal fin rays; abdominal fin rays; and anal fin rays. ANOVA and pairwise comparisons among the different types of fish were analyzed using SPSS Statistics 17.0 and used to identify similarities and significant differences (P < 0.01) between the gynogenetic progenies and their parents. Comparing the measurable and countable traits between the gynogenesis progenies and their parents is useful to identify the similarities and differences between the gynogenesis progenies and their parents.
Fluorescence In Situ Hybridization
Species-specific centromere probe of fluorescence in situ hybridization (FISH) was made from RCC and amplified by PCR using the primers 5′-TTCGAAAAGAGAGAATAATCTA-3′ and 5′-AACTCGTCTAAA CCCGAACTA-3′. The FISH probes were produced by Dig-11-dUTP labeling (using a nick translation kit; Roche, Germany) of purified PCR products. FISH was performed according to the method described by He et al. (2012). For each type of fish (RCC, 4nF1, G1 and G2), 200 metaphase spreads of chromosomes from ten individuals are analyzed under an inverted microscope (CW4000, Leica, Germany) with a confocal imaging system (LCS SP2, Leica). Captured images were colored and superimposed in Adobe Photoshop CS6. FISH was used to identify the origin of the chromosomes in the gynogenetic offspring at the molecular level.
Evaluation on the Aquacultural Performance of G1 and G2
Aquaculture data were collected in 2012 from three sites in Hunan: Wangcheng, Changsha, and Changde.
RCC, G1, and G2 fry were transported to their destinations 3 days after hatching and kept in separate, aerated aquaculture net cages at 21–25 °C for 1 week. They were fed two boiled egg yolks per 40–60 thousand fry. On the first and the last day, the net cage was thoroughly stirred, and then three 100-ml water samples were randomly taken from each net cage, combined and diluted to 2000 ml. Three 10-ml water samples were then taken from the 2000-ml sample and the numbers of fry in them were counted to estimate the mean density. The total number of fry was calculated from the mean density and the total volume of water in each cage. The survival rate was calculated based on the total number of fry on the first and last days.
On day 11 post-hatching, RCC, G1, and G2 were transferred to different ponds at a density of 120,000 fry/667 m2 at each site. They were fed with plankton and 3–5 kg of soybean milk daily. Twenty-one days later, subsamples of these fish were randomly selected and stocked at a density of 1800 fish/667 m2 for the adult grow-out stage. These were fed with aquatic compound feed containing 32 % protein. Three ponds were prepared for each fish taxon at each site. All fish were bred under similar conditions. The number of dead fish in each pond was recorded to calculate the survival rate during the adult grow-out stage from day 32 to day 217 post-hatching. The body weights of 30 randomly selected individuals of each taxon at each site were measured monthly from June to December. As there were no significant differences among the three sites in the mean survival rates and body weights of fish of the same type, the data were pooled for comparison of growth performance among RCC, G1, and G2. This method was used to determine whether the autotriploids (G1 and G2) grew more rapidly than RCC.
Results
Formation of Experimental Fish
Following activation by UV-irradiated BSB sperm, the fertilized eggs of 4nF1 developed into viable gynogenetic offspring of normal appearance without chromosome doubling treatment. Autotriploids gynogenetic offspring (G1) accounted for 37.5 % of the total. Likewise, the fertilized eggs of G1 developed into second-generation gynogenetic offspring (G2) without chromosome doubling treatment after activation by UV-irradiated BSB sperm (Fig. 1). Autotriploids gynogenetic offspring accounted for 100 % of the total. The fertilization rates, hatching rates, and survival rates of the gynogenetic progeny of 4nF1 and G1 are shown in Table 1.
Fig. 1.
Crossing procedure used in the formation of the experimental fish. The parental origins of the chromosomes are indicated by blue and red colors
Table 1.
The fertilization rates, hatching rates, and surviving rate in gynogenetic progeny of 4nF1 and G1
| Egg source | Sperm source | UV-irradiated (min) | Fertilization rate (%) | Hatching rate (%) | Surviving rate (%) |
|---|---|---|---|---|---|
| 4nF1 | BSB | 0 | 67.14 ± 5.21 | 56.45 ± 4.12 | 46.34 ± 3.14 |
| 4nF1 | BSB | 28 | 35.22 ± 3.52 | 28.56 ± 1.23 | 20.56 ± 1.24 |
| 4nF1 | BSB | 40 | 18.87 ± 3.21 | 11.65 ± 2.67 | 9.65 ± 2.65 |
| 4nF1 | BSB | 45 | 8.72 ± 2.32 | 5.29 ± 1.25 | 4.12 ± 1.48 |
| G1 | BSB | 28 | 19.36 ± 3.21 | 11.63 ± 2.73 | 9.53 ± 2.19 |
| G1 | BSB | 40 | 42.57 ± 2.92 | 38.65 ± 4.91 | 33.65 ± 2.23 |
| G1 | BSB | 45 | 35.18 ± 6.21 | 28.89 ± 5.31 | 24.89 ± 3.54 |
Fertility rate = (number of fertilized eggs/number of eggs) × 100 %; Hatching rate = (number of hatching fry/number of eggs) × 100 %; Survival rate = (number of normal fry/number of eggs) × 100 %
Examination of Chromosome Number
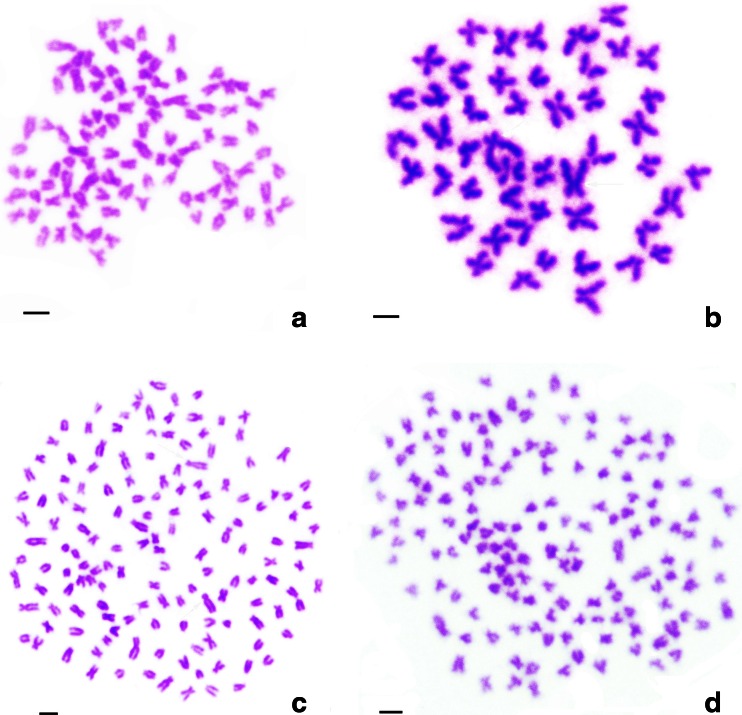
Table 1 presents the distributions of chromosome numbers in RCC, BSB, 4nF1, G1, and G2. For RCC, 93 % of chromosomal metaphases had 100 chromosomes (Fig. 2a; Table 2). For BSB, 86 % of chromosomal metaphases possessed 48 chromosomes (Fig. 2b; Table 2). For 4nF1, 83 % of chromosomal metaphases had 148 chromosomes (Fig. 2c; Table 2). For G1, 81 % of chromosomal metaphases had 150 chromosomes (Fig. 2d; Table 2). For G2, 84.5 % of chromosomal metaphases have 150 chromosomes (Table 2).
Fig. 2.
Chromosome spreads at metaphase in RCC, BSB, 4nF1, and G1. a The 100 chromosomes of RCC; b The 48 chromosomes of BSB; c The 148 chromosomes of 4nF1; d The 150 chromosomes of G1; Bar in a–d, 3 μm
Table 2.
Examination of chromosome number in RCC, BSB, 4nF1, G1, and G2
| Fish type | No. of metaphase | Distribution of chromosome number | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <48 | 48 | <100 | 100 | <148 | 148 | <150 | 150 | ||
| RCC | 200 | 14 | 186 | ||||||
| BSB | 200 | 28 | 172 | ||||||
| 4nF1 | 200 | 34 | 166 | ||||||
| G1 | 200 | 38 | 162 | ||||||
| G2 | 200 | 31 | 169 | ||||||
Fluorescence In Situ Hybridization
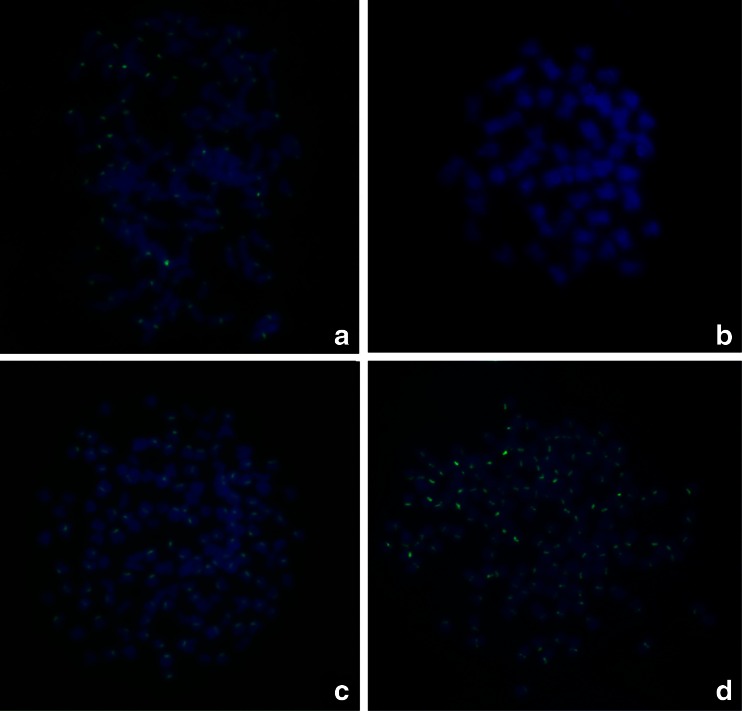
The species-specific centromere probe (GenBank accession No.: JQ086761) hybridized with 100 chromosomes of RCC (Fig. 3a; Table 3), whereas no chromosomes of BSB showed hybridization (Fig. 3b; Table 3). Therefore, RCC and BSB-derived chromosomes could be discriminated by FISH using the centromere probes. As expected, the centromere probe also hybridized with 100 chromosomes of 4nF1 (Fig. 3c; Table 3), indicating that they possessed two sets of RCC-derived chromosomes. The centromere probe hybridized with 150 RCC-derived chromosomes in G1 and G2 (Fig. 3d; Table 3), indicating that they possessed three sets of RCC-derived chromosomes.
Fig. 3.
Examination of the FISH signals in RCC, BSB, 4nF1 and G1. a The centromere probe hybridized to 100 chromosomes in RCC; b No chromosomes of BSB hybridized; c The centromere probe hybridized to 100 RCC-derived chromosomes in 4nF1; d The centromere probe hybridized to 150 chromosomes of G1
Table 3.
Examination of hybridizing signals by FISH in RCC, BSB, 4nF1, G1, and G2
| Fish type | No. of fish | No. of metaphase | No. of loci |
|---|---|---|---|
| RCC | 10 | 200 | 100 |
| BSB | 10 | 200 | 0 |
| 4nF1 | 10 | 200 | 100 |
| G1 | 10 | 200 | 150 |
| G2 | 10 | 200 | 150 |
Morphological Traits
There were clear morphological differences among RCC (Fig. 4a), 4nF1 (Fig. 4b) and G1 (Fig. 4c). The body color of RCC was red, while that of 4nF1 and G1 are green-brown. An interesting difference was the presence of barbels in 4nF1 and their absence in G1. Tables 4 and 5 show the values for the measurable and countable traits in RCC, 4nF1 and G1. The ratios of the measurable traits all differed significantly between RCC and G1 (P < 0.01), except for HL/HW and BW/HW. The ratios BL/BW and BW/HW were significantly different between G1 and 4nF1. The ratios of all measurable traits differed significantly between 4nF1 and RCC (P < 0.01), with the exception of BL/BW and HL/HW.
Fig. 4.
Morphological appearances of RCC, 4nF1, and G1. a RCC; b 4nF1; c G1; Bar in a–c, 4 cm
Table 4.
Comparison of ratios of the measurable traits between RCC, 4nF1 and G1
| Fish type | WL/BL | BL/BW | BL/HL | HL/HW | TL/TW | BW/HW |
|---|---|---|---|---|---|---|
| RCC | 1.22 ± 0.02a | 2.18 ± 0.02a | 3.72 ± 0.03a | 1.07 ± 0.03a | 0.82 ± 0.03a | 1.84 ± 0.03a |
| 4nF1 | 1.18 ± 0.02b | 2.18 ± 0.02a | 3.83 ± 0.03b | 1.08 ± 0.04a | 0.75 ± 0.04b | 1.92 ± 0.02b |
| G1 | 1.19 ± 0.03b | 2.21 ± 0.02b | 3.82 ± 0.02b | 1.08 ± 0.01a | 0.75 ± 0.02b | 1.85 ± 0.02a |
The same superscript letters in the same column indicate no significant difference (P > 0.01); different superscript letters in the same column indicate significant difference (P < 0.01); mean value ± standard deviation
Table 5.
Comparison of the countable traits between RCC, 4nF1 and G1
| Fish type | No. lateral line scales | No. of scales above the lateral line | No. of scales below the lateral line | No. of dorsal fins rays | No. of abdominal fins rays | No. of anal fins rays |
|---|---|---|---|---|---|---|
| RCC | 29.20 ± 0.70a (28–30) | 5.60 ± 0.50a (5–6) | 5.70 ± 0.47a (5–6) | III + 18.65 ± 0.49a (18–19) | 8.55 ± 0.51a (8–9) | III + 5.65 ± 0.49a (5–6) |
| 4nF1 | 31.65 ± 0.49b (31–32) | 6.55 ± 0.51b (6–7) | 6.45 ± 0.51b (6–7) | III + 18.70 ± 0.98a (17–20) | 8.60 ± 0.50a (8–9) | III + 6.40 ± 0.68b (5–7) |
| G1 | 31.34 ± 0.43b (31–32) | 5.75 ± 0.23a (5–6) | 5.73 ± 0.27a (5–6) | III + 18.2 ± 0.87a (17–19) | 9.12 ± 0.26b (9–10) | III + 6.57 ± 0.36b (6–7) |
The same superscript letters in the same column indicate no significant difference (P > 0.01); different superscript letters in the same column indicate significant difference (P < 0.01); Ш represents hard fin rays and Arabic numerals represent soft fin ray; mean value ± standard deviation; the numbers in brackets indicate range for the value
All countable traits differed significantly between G1 and RCC (P < 0.01), with the exception of the number of scales above the lateral line, lower scales below the lateral line and dorsal fins rays. However, the numbers of scales above the lateral line, lower scales below the lateral line and abdominal fins rays differed significantly between G1 and 4nF1 (P < 0.01). With the exception of the numbers of dorsal fins and abdominal fins, all other countable traits differed significantly between 4nF1 and RCC (P < 0.01).
Analysis of Gonadal Development
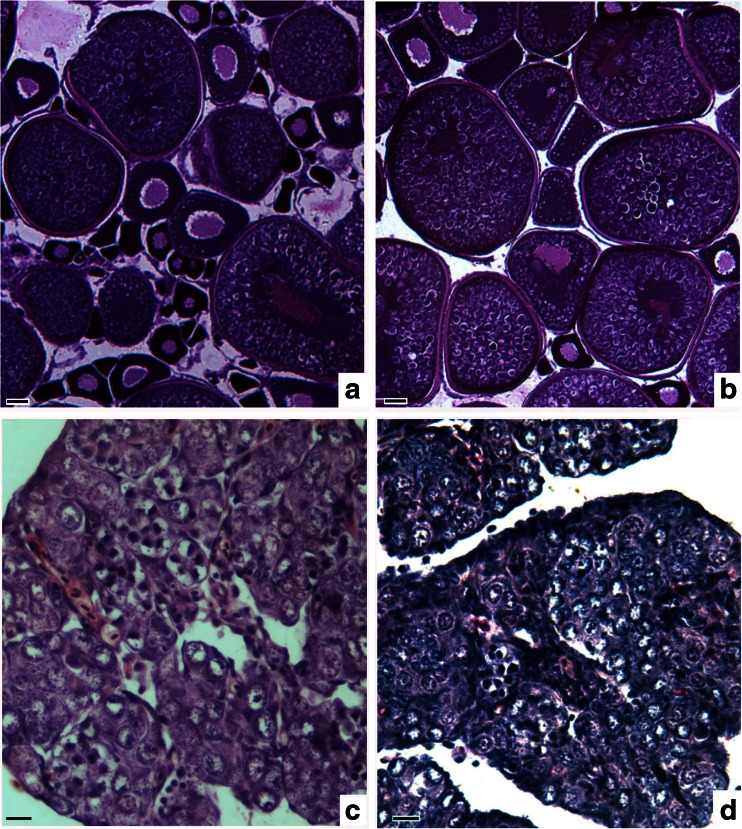
RCC, G1, and G2 reached sexual maturity at age 1 year, but 1-year-old 4nF1 did not produce mature eggs or sperm. Gonad development was observed in 40 G1, and showed that 90 % G1 possessed normal ovary structure and 10 % G1 possess abnormal. Testicular tissues were not found.
Figure 5 shows the microstructure of the ovaries RCC, 4nF1 and G1. The ovaries of RCC and G1 at 9 months old were normally developed and are mainly contained oocytes in phase III (Fig. 5a, b). However, at 9 months old, the ovaries of 4nF1 were occupied by many oogonia and very few oocytes (Fig. 5c). Oogonia proliferated in the abnormal ovaries of G1 but did not develop into oocytes, i.e., gonadal development was delayed (Fig. 5d)
Fig. 5.
Gonadal microstructure in RCC, 4nF1, and G1. a The ovary of RCC, showing normally developed oocytes in phase III; b The ovary of G1, showing normally developed oocytes in phase III; c The ovary of 4nF1 is occupied by many oogonia; d Abnormal ovary of G1, showing proliferating oogonia that did not develop into oocytes. Bar in a–d, 50 μm
Aquaculture Performance
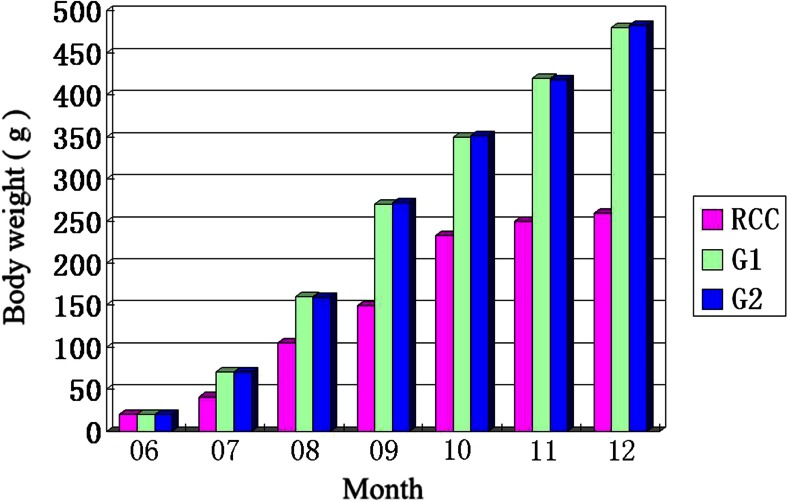
The survival rates of RCC, G1, and G2 ranged from 80.1 to 83.5 % during the fry stage and from 85.7 to 87.2 % during the adult grow-out stage; there were no significant differences among the taxa (P > 0.01) (Table 6). The mean body weight of RCC in December was 260 g, which was significantly smaller than that of G1 (480 g), and of G2 (482 g) (P < 0.01). Figure 6 presents the growth rates of RCC, G1, and G2 from June to December 2012. The growth rates of G1 and G2 were higher, and obviously increased, compared with those of RCC over the entire period.
Table 6.
Comparison of growth performance between RCC, G1, and G2
| Fish type | Survival rate of fries (%) | Survival rate of adults (%) | The average body weight in December (g) |
|---|---|---|---|
| RCC | 80.1 ± 4.5 | 85.7 ± 4.1 | 260 ± 69 |
| G1 | 82.1 ± 3.8 | 87.2 ± 5.6 | 480 ± 55 |
| G2 | 83.5 ± 4.2 | 86.3 ± 4.2 | 482 ± 74 |
Fig. 6.
Growth of RCC, G1, and G2 from June to December
Discussion
In fish, sterile triploid individuals potentially minimize risks associated with accidental releases and are also expected to exhibit higher feeding conversion ratios (FCR) and, consequently, faster growth rates (Foresti et al. 1996). Distant hybridization can result in genome-level alterations including the occurrence of allotriploids and allotetraploids, e.g., F1 hybrids of Ctenopharyngodon idella × Hypophthalmichthys nobilis (Marian and Kraszai 1978) and F1 hybrids of C. auratus red var. (♀) × M. amblycephala (♂) (Liu et al. 2007). The allopolyploids contain both parental genomes, which undergo bivalent pairing at meiosis because only the homologous chromosomes pair (Soltis and Soltis 2000; Wu et al. 2001). Importantly, a diploid-like pairing system prevents meiotic irregularities and improves the efficiency of gamete production in allopolyploid species (Sybenga 1996). However, several studies have documented that many polyploid or diploid individuals of the hybrid progeny of plants show abnormal chromosome behavior during mitosis and meiosis and product gametes with a complete set of paternal or maternal chromosomes (Kasha and Kao 1970; Li et al. 1998; Riera-Lizarazu et al. 2000; Li and Ge 2007). In the present study, following activation by UV-irradiated BSB sperm, the fertilized eggs of 4nF1 developed into normal live gynogenetic offspring without chromosome doubling treatment. Some of these gynogenetic offspring were autotriploids with three sets of chromosomes derived from RCC (3n = 150). This indicated that 4nF1 can generated triploid homogametes (3n = 150) with three sets of RCC-derived chromosomes. We speculate that the generative cells undergone genomic doubling by premeiotic endoreduplication, endomitosis, or fusion of oogonial germ cells in 4nF1 (Liu et al. 2007). Some showed complete separation of the parental genomes during meiosis, which then developed into gametes with one or more RCC-derived chromosome sets. Indeed, besides autotriploid gamete (3n = 150, AAA), haploid (n = 50, A), autodiploid (2n = 100, AA) and allotetraploids gamete (4n = 148, AABB) were also found in 4nF1 (Qin et al. 2014a), and that we have artificially established an autotetraploid fish line (F2–F8) by fertilization of the autodiploidy diploid eggs and diploid sperm from the females and males of 4nF1 (Qin et al. 2014b)
Autopolyploids generally exhibit random (non-preferential) pairing of chromosomes. Each chromosome has more than one potential partner, which may result in the formation of multivalency during meiosis, and sterility. In nature, gibel carp (C. auratus gibelio) is a triploid subspecies of diploid crucian carp, which is able to produce unreduced triploid eggs at the age of 1 year (Vrijenhoek 1994; Gui 1989). The reproductive characteristics of G1 are similar to those of gibel carp. G1 is autotriploid and can also produce unreduced (3n) eggs at the age of 1 year. Thus, following activation by UV-irradiated BSB sperm and without chromosome doubling treatment, the fertilized eggs of G1 are able to develop into a second generation of autotriploid gynogenetic offspring (3n = 150, G2). We speculate that BSB genetic material has been removed in the autotriploid (G1 and G2), that can effectively reduced incompatibility and improve autotriploid fertility. Thus, autotriploid reached sexual maturity at 1 year of age, earlier than 4nF1 (sexual maturity at 2 year of age) (Liu et al. 2007). Indeed, gibel carp have the dual reproductive modes of sexual reproduction and gynogenesis, which has been demonstrated using heterologous sperm from other fish species to activate egg and embryo development (Zhou et al. 2000). Our data do not conclusively demonstrate that heterologous sperm (e.g., common carp) activates egg and embryo development of G1 as in gibel carp (gynogenesis or hybridogenesis). This is an important aspect and should be investigated in the future.
In many species of cultured fish, females exhibit higher growth rates than males and attain larger sizes (Luo et al. 2011). In addition, the distant crossing or gynogenetic progeny has advantages in growth rates and resistibility. The all-female triploids (G1 and G2) were obtained by distant hybridization and gynogenesis, which also exhibited higher growth rates than RCC over the entire period. Therefore, these autotriploid provide an important source of gametes for the production of all-female triploids and for the establishment of autotriploid gynogenetic lines. Although autotriploid fish possess three sets of the RCC-derived chromosomes, phenotypic changes obviously occurred. There were clear differences in morphological traits between G1 and RCC. The body color of RCC is red while G1 are green-brown. Additionally, the ratios of the measurable traits WL/BL, BL/BW, BL/HL, and TL/TW and the countable traits, including the number of lateral line scales, abdominal fins rays, and anal fins rays also differs significantly between G1 and RCC, suggesting that this phenotypic variability resulted from genome duplication in the autotriploid fish. Importantly, genomic variation could quickly augment the divergence between homologous chromosomes and then prevent the formation of multivalency during meiosis in G1. An interesting question for future study is the mechanism by which unreduced eggs are produced in the autotriploid gynogenetic offspring.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No.31201987, 31430088, and 31210103918), the Doctoral Fund of Ministry of Education of China (Grant No.: 20124306120006), the Natural Science Foundation of Hunan Province (Grant No. 14JJ6008), Training Program of the Major Research Plan of the National Natural Science Foundation of China (Grant No. 91331105, the National Key Basic Research Program of China (Grant No. 2012CB722305), the National High Technology Research and Development Program of China (Grant No.2011AA100403), Cooperative innovation center of engineering and new products for developmental biology (20134486), and the construct program of the key discipline in Hunan province and China.
Footnotes
Qinbo Qin and Juan Wang contributed equally to this work.
References
- Cherfas NB, Gomelsky B, Ben-Dom N, Peretz Y, Hulata G. Assessment of triploid common carp (Cyprinus carpio L.) for culture. Aquaculture. 1994;127:11–18. doi: 10.1016/0044-8486(94)90187-2. [DOI] [Google Scholar]
- Foresti F, Toledo-Filho SA, Almeida-Toledo LF. Biotecnologia genética aplicada à piscicultura. Cad Ictiogenética. 1996;3:59. [Google Scholar]
- Gomelsky BL, Cherfas NB, Gissis A, Hulata G. Induced diploid gynogenesis in white bass. Prog Fish-Cult. 1998;60:288–292. doi: 10.1577/1548-8640(1998)060<0288:IDGIWB>2.0.CO;2. [DOI] [Google Scholar]
- Gui JF. Evolutionary genetics of unisexual vertebrates. Nat J (Shanghai) 1989;12:116–122. [Google Scholar]
- He WG, Qin QB, Liu SJ, Li TL, Wang J, Xiao J, Xie LH, Zhang C, Liu Y. Organization and variation analysis of 5S rDNA in different ploidy-level hybrids of red crucian carp × topmouth culter. PLoS One. 2012;7(6):e38976. doi: 10.1371/journal.pone.0038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulata G. A review of genetic-improvement of the common carp (Cyprinus carpio L.) and other cyprinids by crossbreeding, hybridization and selection. Aquaculture. 1995;129:143–155. doi: 10.1016/0044-8486(94)00244-I. [DOI] [Google Scholar]
- Hulata G. Genetic manipulations in aquaculture: a review of stock improvement by classical and modern technologies. Genetica. 2001;111:155–173. doi: 10.1023/A:1013776931796. [DOI] [PubMed] [Google Scholar]
- Hulata G, Karplus I, Harpaz S. Evaluation of some red tilapia strains for aquaculture: growth and colour segregation in hybrid progeny. Aquac Res. 2008;26(10):765–771. doi: 10.1111/j.1365-2109.1995.tb00869.x. [DOI] [Google Scholar]
- Kasha KJ, Kao KN. High frequency haploid production in barley (Hordeum vulga re L.) Nature. 1970;225:874–876. doi: 10.1038/225874a0. [DOI] [PubMed] [Google Scholar]
- Kavumpurath S, Pandian TJ. Induction of heterozygous and homozygous diploid gynogenesis in Bettasplendens (Regan) using hydrostatic pressure. Aquac Fish Manag. 1994;25:133–142. [Google Scholar]
- Li ZY, Ge XH. Unique chromosome behavior and genetic control in Brassica × Orychophragmus wide hybrids: a review. Plant Cell Rep. 2007;26:701–710. doi: 10.1007/s00299-006-0290-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Heneen WK. Production and cytogenetics of intergeneric hybrids between the three cultivated Brassica diploids and Orychophragmusviolaceus. Theor Appl Genet. 1999;99(3–4):694–704. doi: 10.1007/s001220051286. [DOI] [PubMed] [Google Scholar]
- Li Z, Wu JG, Liu Y, Liu HL, Heneen WK. Production and cytogenetics of the intergeneric hybrids Brassica juncea × Orychophragmus violaceus and B. carinata × O. violaceus. Theor Appl Genet. 1998;96(2):251–265. doi: 10.1007/s001220050734. [DOI] [Google Scholar]
- Linhart O, Haffray P, Ozouf-Costaz C, Vandeputte MF. Comparison of methods for hatchery-scale triploidization of European catfish (Siluris glanis) J Appl Ichthyol. 2001;17:247–255. doi: 10.1046/j.1439-0426.2001.00299.x. [DOI] [Google Scholar]
- Liu Y. Germ cells of fish. Propagation physiology of main cultivated fish in China. Beijing: Agricultural Publishing House; 1993. pp. 22–30. [Google Scholar]
- Liu SJ. Distant hybridization leads to different ploidy fishes. Sci China Ser C Life Sci. 2010;53(4):416–425. doi: 10.1007/s11427-010-0057-9. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Liu Y, Zhou GJ, Zhang XJ, Luo C, Feng H, He XX, Zhu GH, Yang H. The formation of tetraploid stocks of red crucian carp × common carp hybrids as an effect of interspecic hybridization [J] Aquaculture. 2001;192(2–4):171–186. doi: 10.1016/S0044-8486(00)00451-8. [DOI] [Google Scholar]
- Liu SJ, Qin QB, Xiao J, Lu WT, Shen JM, Li W, Liu JF, Duan W, Zhang C, Min T, Zhao RR, Yan JP, Liu Y. The formation of the polyploidy hybrids from different subfamily fish crossing and its evolutionary significance. Genetics. 2007;176(2):1023–1034. doi: 10.1534/genetics.107.071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Qin QB, Wang YQ, Hong Z, Zhao RR, Zhang C, Wang J, Li W, Chen L, Xiao J, Luo KK, Min T, Wei D, Liu Y. Evidence for the formation of the male gynogenetic fish. Mar Biotechnol. 2010;12:160–172. doi: 10.1007/s10126-009-9219-9. [DOI] [PubMed] [Google Scholar]
- Luo KK, Xiao J, Liu SJ, Wang J, He WG, Hu J, Qin QB, Zhang C, Tao M, Liu Y. Massive production of all-female diploids and triploids in the Crucian carp. Int J Biol Sci. 2011;7(4):487–495. doi: 10.7150/ijbs.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean N, Razak AS, Hwang G, Rahman MA. Growth performance and gonadal development of growth enhanced transgenic tilapia Oreochromis niloticus (L.) following heatshock-induced triploidy. Mar Biotechnol. 1999;1:533–544. doi: 10.1007/PL00011808. [DOI] [PubMed] [Google Scholar]
- Marian T, Kraszai Z. Karyological investigation on Ctenopharyngodon idella and Hypophthalmichthys nobilis and their cross-breeding. Aquac Hung. 1978;1:44–50. [Google Scholar]
- Qin QB, Wang YD, Wang J, Dai J, Liu Y, Liu SJ. The abnormal chromosome behavior during meiosis was revealed in allotetraploid of Carassius auratus red var. (♀) × Megalobrama amblycephala (♂) BMC Genet. 2014;15:95. doi: 10.1186/s12863-014-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin QB, Wang YD, Wang J, Dai J, Xiao J, Hu FZ, Luo KK, Tao M, Zhang C, Liu Y, Liu SJ. The autotetraploid fish derived from hybridization of Carassius auratus red var. (female) × Megalobrama amblycephala (male) Biol Reprod. 2014;91(4):93,1–93,11. doi: 10.1095/biolreprod.114.122283. [DOI] [PubMed] [Google Scholar]
- Riera-Lizarazu O, Vales MI, Ananiev EV, Rines HW, Phillips RL. Production and characterization of maize chromosome 9 radiation hybrids derived from an oatmaize addition line. Genetics. 2000;156:327–339. doi: 10.1093/genetics/156.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. The role of genetics and genomic attributes in the success of polyploids. Proc Natl Acad Sci U S A. 2000;97:7051–7067. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybenga AM. Chromosome pairing affinity and quadrivalent formation in polyploids: do segmental allopolyploids exist? Genome. 1996;39:1176–1184. doi: 10.1139/g96-148. [DOI] [PubMed] [Google Scholar]
- Teskered E, Donaldson EM, Teskered Z, Solar II, McClean E. Comparison of hydrostatic pressure and thermal shocks to induce triploidy in Coho salmon (Oncorhynchus kistuch) Aquaculture. 1993;117:47–55. doi: 10.1016/0044-8486(93)90122-F. [DOI] [Google Scholar]
- Vrijenhoek RC. Unisexual fish: model system for study ecology and evolution. Annu Rev Ecol Syst. 1994;25:71–96. doi: 10.1146/annurev.es.25.110194.000443. [DOI] [Google Scholar]
- Wu RL, Gallo-Meagher M, Littell RC, Zeng ZB. A general polyploid model for analyzing gene segregation in out crossing tetraploid species. Genetics. 2001;159:869–882. doi: 10.1093/genetics/159.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang Y, Gui JF. Genetic evidence for gonochoristic reproduction in gynogenetic silver crucian carp (Carassius auratus gibelio) as revealed by RAPD assays. J Mol Evol. 2000;51:498–506. doi: 10.1007/s002390010113. [DOI] [PubMed] [Google Scholar]
- Zou SP, Fang YL, Zhou RQ. Measurement of characters. Inspection of germplasm for cultured fishes, Part 3. Ministry of agriculture of the People’s Republic of China GB/T. 2008;18654:3–2008. [Google Scholar]