Abstract
Exposure to traffic-related particulate matter (PM) has been associated with increased risk of lung disease, cancer and cardiovascular disease especially in elderly and overweight subjects. The proposed mechanisms involve intracellular production of reactive oxygen species (ROS), inflammation and oxidation-induced DNA damage studied mainly in young normal-weight subjects. We performed a controlled cross-over, randomised, single-blinded, repeated-measure study where 60 healthy subjects (25 males and 35 females) with age 55–83 years and body mass index above 25kg/m2 were exposed for 5h to either particle-filtered or sham-filtered air from a busy street with number of concentrations and PM2.5 levels of 1800/cm3 versus 23 000/cm3 and 3 µg/m3 versus 24 µg/m3, respectively. Peripheral blood mononuclear cells (PBMCs) were collected and assayed for production of ROS with and without ex vivo exposure to nanosized carbon black as well as expression of genes related to inflammation (chemokine (C-C motif) ligand 2, interleukin-8 and tumour necrosis factor), oxidative stress response (heme oxygenase (decycling)-1) and DNA repair (oxoguanine DNA glycosylase). DNA strand breaks and oxidised purines were assayed by the alkaline comet assay. No statistically significant differences were found for any biomarker immediately after exposure to PM from urban street air although strand breaks and oxidised purines combined were significantly associated with the particle number concentration during exposure. In conclusion, 5h of controlled exposure to PM from urban traffic did not change the gene expression related to inflammation, oxidative stress or DNA repair, ROS production or oxidatively damaged DNA in PBMCs from elderly overweight human subjects.
Introduction
Recent epidemiological evidence indicates that exposure to particulate matter (PM) from air pollution increases the mortality and risk of cardiovascular and lung disease as well as lung cancer (1–6). Especially the fraction of ultrafine particles, mainly from diesel-powered vehicles, is thought to promote effects due to high alveolar deposition and a large surface area per mass with adhered toxic compounds such as polycyclic aromatic hydrocarbons, dioxin derivatives, quinones, aldehydes and metals (7,8).
The mechanisms involved in PM-generated health effects are thought to include excessive production of reactive oxygen species (ROS) and initiation of inflammatory responses (9,10). Both animal and cell culture studies have shown that exposure to PM is associated with increased levels of DNA oxidation products, including mutagenic DNA bases, and biomarker studies in exposed populations support that this is relevant for urban levels of air pollution (11,12). Furthermore, transition metals in urban air PM2.5 may promote oxidative stress and increase the level of oxidised guanine in DNA of peripheral blood mononuclear cells (PBMCs) in humans (13–16). Several enzymes are involved in the defence against oxidative stress, including heme oxygenase-1 (HMOX1) and enzymes involved in the repair of oxidatively generated DNA lesions. The repair of the mutagenic oxidised lesion 7,8-dihydro-8-oxoguanine (8-oxoGua) is initiated by 8-oxoguanine-DNA glycosylase 1 (OGG1), and the excised 8-oxoGua is subsequently excreted in urine (17,18). Despite increased production of DNA damage, the level found in cells may therefore be unchanged because of increased repair activity (19,20).
Elderly subjects, especially with metabolic co-morbidities such as Type 2 diabetes and hypertension, have been shown to be particularly susceptible to adverse cardiovascular effects of particulate air pollution (14). However, it is not known whether this susceptibility relates to mechanisms involving oxidative stress, inflammation and/or DNA damage.
The aim of this study was to assess the effects of 5h of controlled exposure to traffic-related PM at real-life levels from an urban street in elderly overweight subjects. We assessed the effect on biomarkers of oxidative stress, inflammatory response, ROS production and DNA damage in PBMCs. The level of oxidative stress and DNA repair response was assessed by gene expression of HMOX1 and OGG1. The inflammatory response was assessed as gene expression of chemokine (C-C motif) ligand 2 (CCL2), interleukin-8 (IL8) and tumour necrosis factor (TNF). Basal and nanoparticle-induced ROS production was assessed by 2,7-dichlorohydrofluoroscein (DCFH)-based fluorescence. DNA damage was assessed as strand breaks (SB) and formamidopyrimidine DNA glycosylase (FPG)-sensitive sites by means of the alkaline comet assay modified to detect oxidatively generated DNA lesions. Previous reports from this exposure study showed impairment of the vasomotor function and reduced heart rate variability in the subjects after the controlled exposure to urban street air PM (21).
Materials and Methods
Study population
Twenty-five male and 35 female non-smokers (>55 years) were recruited for the study as described in more detail in the Supplementary Information and elsewhere (21). The subjects were included in the study if they were overweight (body mass index, BMI > 25kg/m2) and had no personal history of cardiovascular diseases. The study was reviewed and approved by The Committees on Health Research Ethics in the Capital Region of Denmark (H-3-2011-074) and in accordance with the Helsinki II declaration. All participants were given both oral and written information before the study and written consent was obtained from all participants.
Study design
We used a cross-over, repeated-measures study design, where participants served as their own control with a single-blinded and randomised order of exposure to particle-filtered or non-filtered outdoor air. Each participant was studied on 2 days, each including 5h in an exposure chamber with exactly 14 days between exposure days for 37 subjects, 7–13 days between exposures for 11 subjects and 15–30 days between exposures for 12 subjects. On each day of exposure, between 1 and 4 participants were exposed simultaneously. The participants were instructed to wear a highly efficient face mask (Dust Respirator 8812; 3M, St Paul, MN, USA) on the way from their home to the exposure chamber in order to prevent exposure to ambient air PM immediately before the experiments. This mask type has been shown to reduce symptoms and improve cardiovascular health measures in patients with cardiovascular disease walking in an area with high levels of air pollution in Beijing, China (22). The participants arrived fasting and were served standard continental breakfast with bread, cheese, jam and low fat yoghurt with cereals after all baseline measurements were done. During the 5-h exposure, they were only allowed to leave the chamber in order to go to the bathroom. We collected blood samples before entry into the exposure chamber and within 1h after exposure. All measurements were completed within a 7-month period, from November 2011 to end of May 2012.
To create an exposure scenario that simulates real-life exposure to traffic-related PM, air from the curb side of a street (Østersøgade) in central Copenhagen, Denmark, was introduced directly into the exposure chamber (collected ~5 m from the nearest exhaust pipe area). The traffic density on this road was 26 800 vehicles during daytime (6 am–6 pm), of which 35% were light-duty diesel-powered vehicles and 2.2% were diesel-powered heavy-duty (>3.5 tons) vehicles as assessed by traffic counts from the Copenhagen municipality. The outdoor air was continuously pumped into the exposure chamber using two KVR-100 Channel ventilators (Øland A/S Ballerup, Denmark) at 230 m3/h (pressure = 100 Pa) resulting in an air exchange of 5.3/h in the chamber. Heating devices, placed within the airstream, kept the chamber temperature constant (~25°C). To create either high or low PM exposure levels in the chamber, the outdoor air was passed through custom-built units, with or without high efficiency particulate adsorption filters (Camfil FARR HEPA filter 226002A1; Camfil A/S, Stockholm, Sweden). In both exposure set ups (with or without filtration), air flow and pressure were constant, whereas nitrogen oxides (NO, NO2), ozone and carbon monoxide levels were minimally affected by the filtration (23). The air in the chamber was continuously monitored for PM2.5 with a Dusttrak Aerosol Monitor 8520 (TSI, St Paul, MN, USA) and for the particle number concentration with a TSI 3007 condensation particle counter. In addition, PM2.5 samples were collected on filters and more extensive characterisation of the chamber air was carried out in the first half of the study as described elsewhere (21).
Collection and preparation of samples
Peripheral blood samples were collected immediately before and after the exposure. PBMCs were isolated using Vacutainer Cell Preparation Tubes (Vacutainer® CPT Becton Dickinson A/S, Brøndby, Denmark). Fresh PBMC samples were used for the assessment of intracellular ROS production. Samples of PBMCs were cryopreserved in a solution of 10% dimethylsulfoxide, 40% Roswell Park Memorial Institute cell culture medium (RPMI 1640 GibcoRBL) and 50% foetal bovine serum (GibcoRBL) at −80°C for analysis of genotoxicity. Samples of PBMCs were also suspended in TRIzol (Invitrogen, Carlsbad, CA, USA) and stored at −80°C for assessment of gene expression levels.
Intracellular ROS production
ROS production was measured in THP-1 cells and PBMCs immediately after isolation as described previously for monocytic THP-1 cells (24). The THP-1 monocytes were obtained from the American Tissue Type Culture Collection (Manassas, VA, USA) and they were cultured at 37°C and 5% CO2 in RPMI with 10% fetal bovine serum, 1.65mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 5mM sodium pyruvate and 0.05mg/ml of gentamicin as previously described (25). We measured ROS production in PBMCs and THP-1 monocytes (both cell types: 50 000 cells/well) during a 3-h exposure period to nanosized carbon black [Printex 90 from Evonik Industries, Frankfurt, Germany (primary particle size 14nm; surface area 300 m2/g)]. The ROS production method utilised intracellular trapping of dichlorohydrofluorescein diacetate (DCFH-DA), which is converted by cytosolic esterases to DCFH. The oxidation of DCFH produces the fluorescent dichlorofluoroscein (DCF). The exposure to nanosized carbon black is associated with increased ROS production in a number of cell types, including lung epithelial cells and in vascular endothelial cells (26–28).
The PBMCs and THP-1 cells were washed twice with Hanks buffered salt solution (Hank’s) and incubated with 2 µM DCFH-DA (diluted in Hank’s) for 15min at 37°C and 5% CO2. Subsequently, the samples were washed three times in Hank’s, followed by resuspension in concentrations of 0.625, 1.25, 2.5 and 5 µg Printex 90/ml Hank’s. Immediately after addition of Printex 90, the DCF fluorescence was measured (ex: 488nm, em: 525nm). Hereafter, fluorescence was measured every 15min for a total period of 180min and a total of 13 measurements. We used the area under curve of these time-curve measurements to get a single data point for ROS production. All samples were run in triplicates and expressed as a mean of the three. In addition, the ROS production was normalised to the production in THP-1 cells, analysed in parallel in order to limit influence of day-to-day variation in the assays.
DNA damage in PBMCs
The alkaline comet assay was used to measure the levels of SB and FPG-sensitive sites in PBMCs. To summarise, PBMCs were embedded in 0.75% low-melting point agarose (Sigma–Aldrich A/S, Brøndby, Denmark) on GelBond®films (Lonza Copenhagen Aps, Vallensbæk Strand, Denmark) and lysed (1% Triton X-100, 2.5M NaCl, 100mM Na2EDTA (ethylenediaminetetraacetic acid), 10mM Tris, pH = 10) for a minimum of 1h at 4°C. The gel-embedded nuclei were digested with FPG enzyme, which was a gift from Professor Andrew Collins (University of Oslo, Norway) or buffer without enzyme for 45min at 37°C. The Gelbond films were then submerged in an alkaline solution (300mM NaOH, 1mM Na2EDTA, pH > 13) for 40min and the duration of the subsequent electrophoresis was 20min at 0.83V/cm (cathode to anode) and 300 mA. Electrophoresis and alkali treatment were both performed at low temperature (4°C). After electrophoresis, the Gelbond films were washed three times for 5min in Tris buffer (0.4M Tris–HCl, pH = 7.5), rinsed with ELGA® water (ultrapure water of resistivity of 18.2 Ω/cm, by ELGA maxima water systems) and dehydrated in 96% ethanol for a minimum of 1.5h.
DNA damage was measured by visual inspections in an Olympus fluorescence microscope at ×40 magnifications after staining with YOYO-1 (Molecular Probes, Eugene, OR, USA).
Samples from each subject were coded and analysed in the same experiment in order to minimise inter-assay variation. We analysed 100 comets (nucleoids) per slide and there were two slides per sample, corresponding to a total number of 200 analysed nuclei per sample, and we used the average of the two slides to represent one sample. The comets were scored by visual classification based on a five-class scoring system (arbitrary score range: 0–400). In each electrophoresis, we included THP-1 cells that were either unexposed or treated with photosensitiser Ro19-8022 and exposed to white light (generates FPG-sensitive sites) as negative and positive control samples, respectively. The Ro19-8022 photosensitiser was a gift from F. Hoffmann-La Roche (Basel, Switzerland). The number of FPG-sensitive sites was obtained as the difference in scores of parallel slides incubated with or without FPG enzyme. These scores were transformed to lesions per 106 bp by means of a calibration curve based on induction of SB by ionising radiation, which has a known yield. We used a conversion factor of 0.0273 lesions/106 bp per score in 0–100 range, based on the assumption that an average molecular weight of a DNA bp is 650Da and 1 Gy yields 0.29 breaks per 109 Da DNA (29).
Expression of mRNA in PBMCs
The gene expression of inflammation markers (CCL2, IL8 and TNF), oxidative stress-related enzyme (HMOX1) and DNA repair enzyme (OGG1) was measured in PBMCs using RT-PCR (real-time reverse transcription polymerase chain reaction) with appropriate primers and probes, as described previously (30–32). We used target-specific TaqMan gene expression assays for the quantitative analysis of specific gene expression levels. Change in expression of the target gene was normalised to 18S RNA as reference by using the comparative 2−ΔCt method.
Statistical analysis
We used Stata/IC software (version 13.0) linear mixed effect models (xtmixed) to perform the statistical analyses. Gene expression levels were adjusted for baseline level (before entering the exposure chamber) in order to account for day-to-day variation within an individual as well as BMI, age and sex because of a few missing measurements from some subjects. Oxidatively damaged DNA and ROS production were only measured in the samples collected after exposure to either filtered or non-filtered air and the analysis were thus adjusted for BMI, age and sex, but not baseline adjusted. The relative response to exposure was calculated as the percentage change with 95% confidence interval (CI) from the regression coefficient with air filtration as categorical variable in the mixed effects model. In addition, mixed effects models with DNA damage as outcome and particle number concentrations as continuous predictor variable with adjustment for BMI, age and sex as well as models stratified for sex were also applied. Statistical significance was accepted at P < 0.05. A total of 60 participants were recruited in order to have sufficient power for adjustment for baseline values measured in the morning before exposure based on power calculations described elsewhere (21).
Results
Exposure characterisation
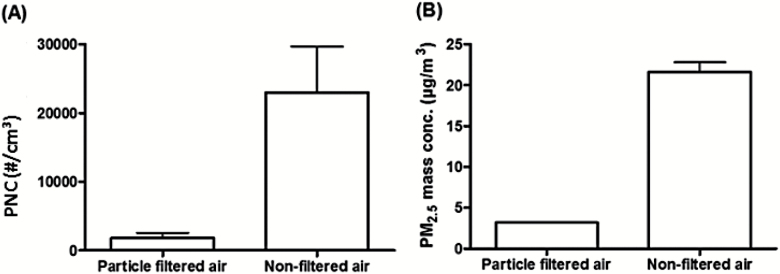
The number, mass and size distribution, mass levels and chemical composition of the particles in the exposure chamber are provided in more detail in the Supplementary Information and some of these data have recently been reported (21). Participants were without air filtration exposed to an average particle concentration of 24 µg/m3 in terms of PM2.5 mass and ~23 000/cm3 (>7nm) in terms of number concentration as opposed to 3 µg/m3 and 1800/cm3 with air filtration, respectively (Figure 1). Filter-based measurement of PM2.5 on 16 exposure days without air filtration and 8 exposure days with air filtration showed mass levels of 18 and 1.5 µg/m3 with corresponding black carbon levels of 3.9 and 0.3 µg/m3, respectively. NO levels were very similar in both exposure scenarios, 31 µg/m3 versus 33 µg/m3, whereas NO2 was higher in air without filtration (45 µg/m3) than in air with filtration (26 µg/m3). The particle number concentration in the exposure chamber during exposure days with unfiltered air was nearly identical to the ambient outdoor levels on the curb side of the street and around half of the particle numbers corresponded to fresh soot particles emitted from local traffic (33). The order of the composition of the PM mass with diameter < 1 µm was as follows: organic material > soot > sulphate > nitrate > ammonia > chlorine (21).
Figure 1.
(A) Particle number concentration (PNC) and (B) PM2.5 mass (mean with SD) in the exposure chamber with and without high-efficiency particle adsorption filtration of the inlet air from an urban street.
Effects on biomarkers
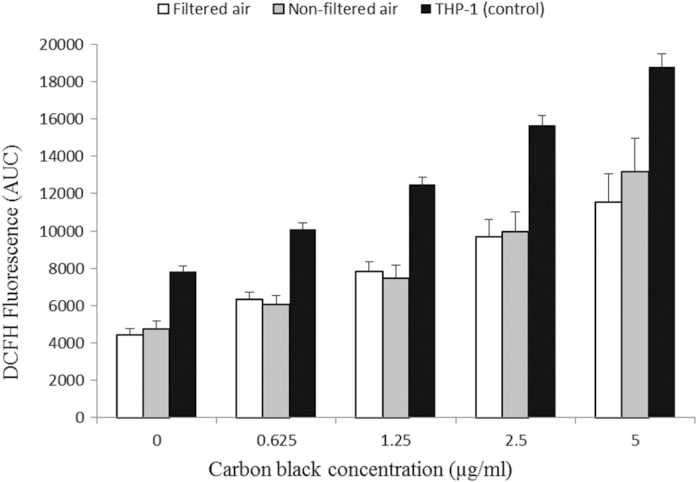
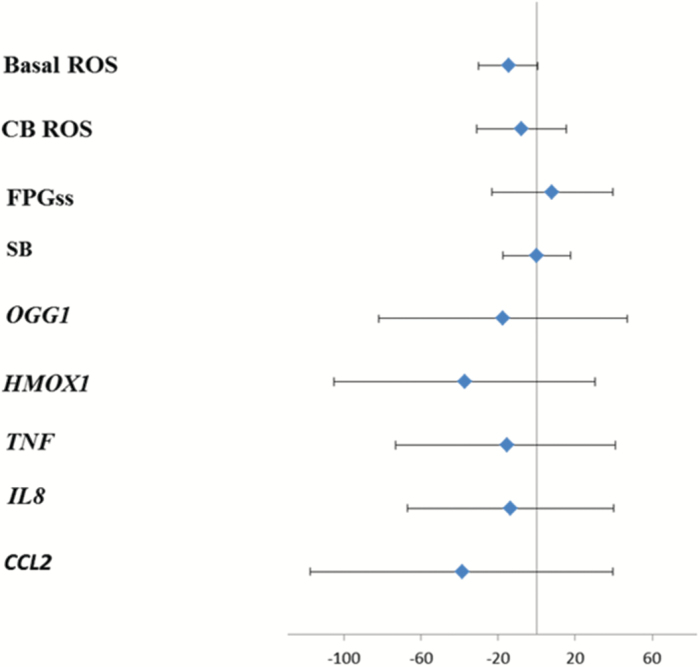
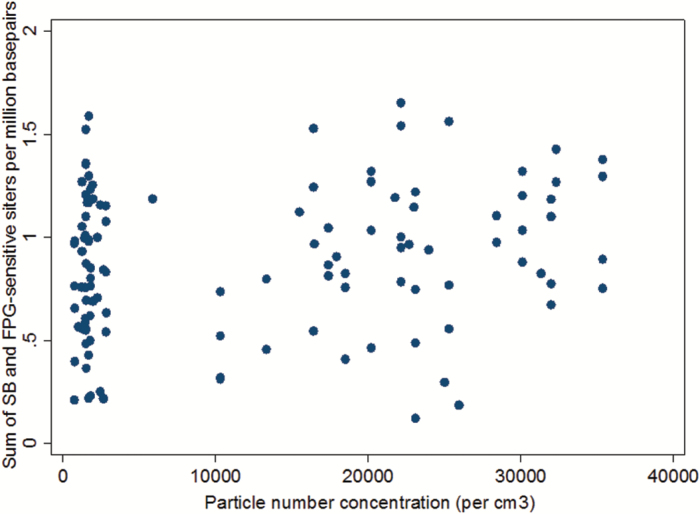
The data on levels of DNA damage and ROS production in PBMCs did not indicate any difference between the exposure conditions (Table 1). As shown in Figure 2, the PBMCs were capable of increasing their ROS production in a concentration-dependent manner upon ex vivo exposure to carbon black and to a similar extent as seen in THP-1 control cells. Similarly, gene expression levels of CCL2, HMOX1, IL8, TNF and OGG1 in PBMCs were unaltered (Table 2). Figure 3 depicts the effect size and 95% CI for relative change in biomarker level following exposure to urban air PM after adjustment for BMI, age and sex. Quite remarkably, only the level of FPG-sensitive sites showed a positive association with exposure (8% increased; 95% CI: −23 to 40), although this was far from being statistically significant. None of the measured biomarkers showed any significant change related to the exposure to filtered or non-filtered air in the exposure chamber (Figure 2). Analyses stratified for sex showed no sign of differences in susceptibility (data not shown). However, Figure 4 depicts a plot of the total level of DNA damage in terms of the sum of SB and FPG-sensitive sites on the particle number concentration in the exposure chamber and a corresponding mixed effects analysis showed a significant positive association between these two variables (P = 0.016).
Table 1.
Levels of DNA damage and ROS production in PBMCs from 60 overweight elderly subjects after 5-h exposure to particles in urban street air or filtered air from the same site
| Particle-filtered air | Non-filtered air | Positive control | |
|---|---|---|---|
| DNA damage | |||
| SB (lesions per 106 bp) | 0.57±0.04 | 0.59±0.05 | 0.98±0.11 |
| FPGss (lesions per 106 bp) | 0.32±0.04 | 0.35±0.04 | 0.58±0.22 |
| ROS production | |||
| Baseline (relative to THP-1) | 0.66±0.05 | 0.56±0.05 | NA |
| CB response (relative to THP-1) | 1.26±0.16 | 1.16±0.18 | NA |
DNA damage was assessed as SB and formamidopyrimidine DNA glycosylase sensitive sites (FPGss) by means of the comet assay. PBMCs exposed to the photosensitiser Ro19-8022 and white light were included as positive control in all assay batches. ROS production was measured immediately after blood collection as DCFH-induced fluorescence directly (baseline) and the maximum fluorescence response above baseline induced by co-incubation with carbon black 14nm particles (CB) 0.625, 1.25, 2.5 and 5 µg/ml. The fluorescence was normalised to the DCFH-induced fluorescence obtained in THP-1 monocytic cells included in every set of measurements. The data are mean ± SEM. NA, not applicable.
Figure 2.
Production of ROS induced by incubation with 14-nm black carbon nanoparticles (0–5 µg/ml) in PBMCs isolated from 60 overweight elderly subjects after 5-h exposure to filtered air (white) or non-filtered air (grey) from an urban street, whereas the black bars are ROS production in THP-1 assayed in parallel (positive control) with black carbon nanoparticles. The data are mean ± SEM in arbitrary units.
Table 2.
Gene expression levels of CCL2, IL8, TNF, OGG1 and HMOX1 in PBMCs from 60 overweight elderly subjects after 5h of exposure to particles from an urban street or filtered air
| Particle-filtered air | Non-filtered air | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| CCL2 | 0.23±0.06 | 0.37±0.10 | 0.29±0.07 | 0.28±0.09 |
| IL-8 | 32.0±7.0 | 34.6±8.0 | 32.0±7.0 | 31.2±8.0 |
| TNF | 2.0±0.3 | 4.7±1.7 | 2.6±0.6 | 2.9±0.5 |
| OGG1 | 8.3±1.7 | 14.6±3.2 | 12.8±2.7 | 10±2.9 |
| HMOX1 | 9.3±1.7 | 12.7±2.6 | 11.9±2.3 | 10.2±1.9 |
The data are mean ±SEM per 106 18S mRNA.
Figure 3.
Percentage change (with 95% CI) in ROS production, DNA damage and gene expression in PBMCs from 60 overweight elderly subjects related to 5-h exposure to particles in urban street air as compared exposure to filtered air from the same site. DNA damage was assessed as SB and FPG-sensitive sites by means of the comet assay. ROS production was measured immediately after blood collection as DCFH-induced fluorescence directly (baseline) and the maximum fluorescence response above baseline induced by co-incubation with carbon black 14nm particles (CB) 0.625, 1.25, 2.5 and 5 µg/ml. Gene expression levels of CCL2, IL8, TNF, OGG1 and HMOX1 were determined by RT-PCR with 18S RNA as reference.
Figure 4.
DNA damage as the sum of SB and FPG-sensitive sites in PBMCs from 60 subjects aged 55–83 years and the particle number concentration in the exposure chamber where they spend 5h prior to blood collection on two occasions: one with air filtration (700–6000/cm3) and one without filtration (10 000–35 000/cm3). There was a statistically significant association between these two variables.
Discussion
In this human exposure study, we observed no statistically significant effects after 5h of controlled exposure to real-life levels of PM from urban street air, on markers of systemic oxidative stress in terms of DNA damage and ROS production and gene expression levels of markers of inflammation (CCL2, IL8, TNF), DNA repair (OGG1) and oxidative stress (HMOX-1) in PBMCs. Although each of SB and FPG-sensitive sites showed no association, the sum of them was significantly positively associated with the particle number concentration in the exposure chamber as a continuous variable, suggesting some genotoxic potential of traffic-related emissions.
Exposure to ambient air pollution has consistently been associated with high levels of oxidative stress-induced damage to DNA and lipids (11), although most studies are with cross-sectional designs with inherent problems of causal inference. In a controlled exposure chamber study with a similar design, we have previously found that urban street air from another location in Copenhagen, Denmark, was associated with significantly increased levels of SB and FPG-sensitive sites, by around 50% and 40%, respectively (23). This effect size was found after both 6 and 24h in the exposure chamber. The present finding of an 8% increase, with 95% CI ranging from −23 to 40 in FPG-sensitive sites and the association of the sum of FPG-sensitive sites and SB with the particle number concentration, is compatible with our previous study (23). That study showed significant associations between each of SB and FPG-sensitive sites and the particle number concentrations in the exposure chamber. However, there were also a number of differences between the two exposure studies. In contrast to the present study, the subjects of our earlier study were younger (median age: 25 years) and of normal weight (mean BMI: 23kg/m2) and were also studied with and without exercise for a total of 180min at 60% of maximum VO2 during the stay in the exposure chamber (23). The effect size appeared slightly larger with exercise in the chamber, although there was no significant interaction. It is also possible that increases due to PM exposure were difficult to detect in the subjects in the present study because their levels of SB and FPG-sensitive sites might have already been elevated due to their older age and overweight, factors shown recently to be associated with high levels of FPG-sensitive sites (34). It should also be emphasised that the sum of FPG-sensitive sites and SB reported in the present study is a combined measure of different types of DNA damage derived from oxidative and non-oxidative mechanisms of action (35). The exposure levels in terms of particle number concentration (~10 000/cm3) and PM2.5 mass (~10 µg/m3) were actually lower in the exposure chamber in our previous study compared to the present study with average levels of 23 000/cm3 and 24 µg/m3, respectively. The lower levels in the earlier study were due to air collection further away from the curb site and despite a larger traffic load of 49 200 vehicles per day with 4–6% heavy-duty contribution (23). Differences might also be due to qualitative changes in PM composition over the 6 years passing. We have earlier shown that vanadium and chromium in PM2.5 personally monitored for 48h was associated with oxidatively damaged DNA in young healthy subjects in Copenhagen, Denmark (15). On the other hand, we have also studied groups of young (aged 13–19 years) subjects in the hours after controlled exposure to pure diesel exhaust or wood smoke at 300 µg/m3 PM for 3h without finding any effects on SB or FPG-sensitive sites (30,36,37). The lack of effect of these exposures is not likely to be due to too early sampling of the blood samples as such because a recent study of a whole week exposure of wood smoke at very high level in a reconstructed Viking age house also showed no change in oxidatively damaged DNA, although some markers of monocyte adhesion to the vessel wall were changed (32).
It can be speculated that the 5-h exposure period may not be sufficient to clearly reveal PM-mediated genotoxicity. However, our previous studies have shown that 3h of exposure is sufficient to generate SB and FPG-sensitive sites in cultured lung epithelial cells by diesel exhaust particles and urban air PM from Copenhagen, Denmark (38). In addition, pulmonary exposure to Printex 90 was associated with increased levels of SB in bronchoalveolar epithelial cells in mice at 3h after an intratracheal instillation (39).
Interestingly, we found decreased nitroglycerin-induced vasodilation as well as decreased high-frequency heart rate variability as adverse cardiovascular effects of the exposure in our present subjects, as reported elsewhere and in keeping with the general notion of such effects of traffic-related air pollution (1,9,21). However, this was not related to changes in oxidative stress or nitric oxide bioavailability indicated by any change in vitamin C or tetrahydrobiopterin or their oxidised forms or changes in any leukocyte counts (21). This is in keeping with the present lack of a significant change in the ROS production in PBMCs. The major ROS producing cell type of PBMCs is the monocyte; however, the prima facie effect on monocytes of exposure to traffic-related PM may be differentiation into dendritic cells, as indicated in studies on light-duty diesel engine PM effects and urban PM10 dust (40,41). Conversely, PBMCs have been reported to increase their ROS production in response to silica nanoparticle exposure (42). Monocytes have been shown to increase their ROS production in response to ZnO-nanoparticle exposure (43). Moreover, rod-shaped ZnO nanoparticles induced higher ROS production in PBMCs compared to spherical ZnO nanoparticles (44). This indicates that not only elemental composition and size but also the shape should be taken into consideration when comparing studies on nanoparticle effects. In our study, PBMCs were capable of ROS production as indicated by the increased levels after ex vivo exposure to nanosized carbon black which like traffic-generated PM is carbon based. The ex vivo carbon black concentrations were much higher than the comparative PM exposure dose from the chamber, which might be insufficient to cross a potential threshold of ROS response in PBMCs. The high carbon black concentrations may also result in a relatively increased nanoparticle internalisation through unspecific uptake pathways such as pinocytosis, which could increase the ROS production by the ingulfed nanoparticles per se. Moreover, there were no significant changes in gene expression of oxidative stress response, DNA repair and inflammation genes, although the variation was rather high and the 95% CI of the relative changes within the PBMCs was substantial. This is in keeping with our findings of no significant effect from 3h of controlled exposure to diesel exhaust at 300 µg/m3 on gene expression of HMOX1, OGG1, IL8 and TNF in PBMCs from young healthy subject (37). Similarly, 3h of controlled exposure to wood smoke at 354 µg/m3 or a week’s exposure to much higher levels in a reconstructed Viking age house showed no change in the expression of these genes (32,45), although OGG1 expression was enhanced after 4h of exposure to wood smoke at similar levels (36). We did not measure plasma levels of cytokines in the present study as the collection of blood samples would be too early to detect a response and the sensitivity of these biomarkers is likely to be low with only weak associations in long-term exposure studies and often negative results in controlled exposure studies (46).
The present study had high statistical power with 60 participants serving as their own control to limit importance of potential confounders between subjects. However, there was substantial within-subject variation in many of the biomarkers resulting in wide CIs for the relative changes and small effects might have been missed. Some of this variation might be caused by exposure before entering the exposure chamber, although the immediate effects should have been avoided by having the subjects wearing a protective face mask en route from their home.
Conclusions
In conclusion, 5h of controlled exposure to real-life levels of traffic-related PM from urban street air did not increase the level of DNA damage, ROS production or expression of oxidative stress, DNA repair or inflammation genes in PBMCs isolated from healthy elderly overweight human subjects.
Supplementary data
Supplementary Information is available at Mutagenesis Online.
Funding
The study was supported by the Danish Research Council for Health and Disease (12-126262).
Supplementary Material
Acknowledgements
We would like to thank all test subjects for their participation. Special thanks to lab technicians Lisbeth S. Carlsen and Julie Hansen. We very much appreciate the detailed aerosol characterisation provided by Jenny Rissler, Staffan Sjogren, Axel C. Eriksson, Aneta Wierzbicka, Mia Frosch, Patrik T. Nilsson, Joakim H. Pagels, Jakob Löndahl, Erik Z. Nordin, Erik Swietlicki and Birgitta Svenningsson from the Aerosol Group at Lund University.
Conflict of interest statement: None declared.
References
- 1. Brook R. D., Rajagopalan S., Pope C. A., III, et al. (2010) Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation, 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- 2. Dockery D. W. (2009) Health effects of particulate air pollution. Ann. Epidemiol., 19, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IARC and Monographs on the Evaluation of Carcinogenic Risk to Humans (2013) Diesel and Gasoline Engine Exhausts and Some Nitroarenes, Vol. 105 IARC, Lyon, France. [Google Scholar]
- 4. Ibald-Mulli A., Wichmann H. E., Kreyling W., Peters A. (2002) Epidemiological evidence on health effects of ultrafine particles. J. Aerosol Med., 15, 189–201. [DOI] [PubMed] [Google Scholar]
- 5. Loomis D., Grosse Y., Lauby-Secretan B., et al. (2014) The carcinogenicity of outdoor air pollution. Lancet Oncol., 14, 1262–1263. [DOI] [PubMed] [Google Scholar]
- 6. Pope C. A., III, Burnett R. T., Thun M. J., Calle E. E., Krewski D., Ito K., Thurston G. D. (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc., 287, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid O., Möller W., Semmler-Behnke M., Ferron G. A., Karg E., Lipka J., Schulz H., Kreyling W. G., Stoeger T. (2009) Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers, 14(Suppl. 1), 67–73. [DOI] [PubMed] [Google Scholar]
- 8. Ghio A. J., Carraway M. S., Madden M. C. (2012) Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health. B Crit. Rev., 15, 1–21. [DOI] [PubMed] [Google Scholar]
- 9. Langrish J. P., Bosson J., Unosson J., Muala A., Newby D. E., Mills N. L., Blomberg A., Sandström T. (2012) Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J. Intern. Med., 272, 224–239. [DOI] [PubMed] [Google Scholar]
- 10. Møller P., Folkmann J. K., Forchhammer L., Bräuner E. V., Danielsen P. H., Risom L., Loft S. (2008) Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett., 266, 84–97. [DOI] [PubMed] [Google Scholar]
- 11. Møller P., Loft S. (2010) Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ. Health Perspect., 118, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moller P., Jacobsen N. R., Folkmann J. K., et al. (2010) Role of oxidative damage in toxicity of particulates. Free Radic. Res., 44, 1–46. [DOI] [PubMed] [Google Scholar]
- 13. Avogbe P. H., Ayi-Fanou L., Autrup H., Loft S., Fayomi B., Sanni A., Vinzents P., Møller P. (2005) Ultrafine particulate matter and high-level benzene urban air pollution in relation to oxidative DNA damage. Carcinogenesis, 26, 613–620. [DOI] [PubMed] [Google Scholar]
- 14. Rückerl R., Schneider A., Breitner S., Cyrys J., Peters A. (2011) Health effects of particulate air pollution: a review of epidemiological evidence. Inhal. Toxicol., 23, 555–592. [DOI] [PubMed] [Google Scholar]
- 15. Sørensen M., Schins R. P., Hertel O., Loft S. (2005) Transition metals in personal samples of PM2.5 and oxidative stress in human volunteers. Cancer Epidemiol. Biomarkers Prev., 14, 1340–1343. [DOI] [PubMed] [Google Scholar]
- 16. Vinzents P. S., Møller P., Sørensen M., Knudsen L. E., Hertel O., Jensen F. P., Schibye B., Loft S. (2005) Personal exposure to ultrafine particles and oxidative DNA damage. Environ. Health Perspect., 113, 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loft S., Møller P. (2006) Oxidative DNA damage and human cancer: need for cohort studies. Antioxid. Redox Signal., 8, 1021–1031. [DOI] [PubMed] [Google Scholar]
- 18. Loft S., Danielsen P., Løhr M., Jantzen K., Hemmingsen J. G., Roursgaard M., Karotki D. G., Møller P. (2012) Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch. Biochem. Biophys., 518, 142–150. [DOI] [PubMed] [Google Scholar]
- 19. Risom L., Dybdahl M., Bornholdt J., Vogel U., Wallin H., Møller P., Loft S. (2003) Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis, 24, 1847–1852. [DOI] [PubMed] [Google Scholar]
- 20. Risom L., Møller P., Loft S. (2005) Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res., 592, 119–137. [DOI] [PubMed] [Google Scholar]
- 21. Hemmingsen J. G., Rissler J., Lykkesfeldt J., Sallsten G., Kristiansen J., Møller P., Loft S. (2015) Controlled exposure to particulate matter from urban street air is associated with decreased vasodilation and heart rate variability in overweight and older adults. Part. Fibre Toxicol. 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langrish J. P., Li X., Wang S., et al. (2012) Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ. Health Perspect., 120, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bräuner E. V., Forchhammer L., Møller P., Simonsen J., Glasius M., Wåhlin P., Raaschou-Nielsen O., Loft S. (2007) Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ. Health Perspect., 115, 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jantzen K., Roursgaard M., Desler C., Loft S., Rasmussen L. J., Møller P. (2012) Oxidative damage to DNA by diesel exhaust particle exposure in co-cultures of human lung epithelial cells and macrophages. Mutagenesis, 27, 693–701. [DOI] [PubMed] [Google Scholar]
- 25. Danielsen P. H., Moller P., Jensen K. A., et al. (2011) Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem. Res. Toxicol., 24, 168–184. [DOI] [PubMed] [Google Scholar]
- 26. Cao Y., Roursgaard M., Danielsen P. H., Møller P., Loft S. (2014) Carbon black nanoparticles promote endothelial activation and lipid accumulation in macrophages independently of intracellular ROS production. PLoS One, 9, e106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frikke-Schmidt H., Roursgaard M., Lykkesfeldt J., Loft S., Nøjgaard J. K., Møller P. (2011) Effect of vitamin C and iron chelation on diesel exhaust particle and carbon black induced oxidative damage and cell adhesion molecule expression in human endothelial cells. Toxicol. Lett., 203, 181–189. [DOI] [PubMed] [Google Scholar]
- 28. Vesterdal L. K., Mikkelsen L., Folkmann J. K., Sheykhzade M., Cao Y., Roursgaard M., Loft S., Møller P. (2012) Carbon black nanoparticles and vascular dysfunction in cultured endothelial cells and artery segments. Toxicol. Lett., 214, 19–26. [DOI] [PubMed] [Google Scholar]
- 29. Forchhammer L., Johansson C., Loft S., et al. (2010) Variation in the measurement of DNA damage by comet assay measured by the ECVAG inter-laboratory validation trial. Mutagenesis, 25, 113–123. [DOI] [PubMed] [Google Scholar]
- 30. Forchhammer L., Møller P., Riddervold I. S., Bønløkke J., Massling A., Sigsgaard T., Loft S. (2012) Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part. Fibre Toxicol., 9, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemmingsen J. G., Møller P., Nøjgaard J. K., Roursgaard M., Loft S. (2011) Oxidative stress, genotoxicity, and vascular cell adhesion molecule expression in cells exposed to particulate matter from combustion of conventional diesel and methyl ester biodiesel blends. Environ. Sci. Technol., 45, 8545–8551. [DOI] [PubMed] [Google Scholar]
- 32. Jensen A., Karottki D. G., Christensen J. M., Bønløkke J. H., Sigsgaard T., Glasius M., Loft S., Møller P. (2014) Biomarkers of oxidative stress and inflammation after wood smoke exposure in a reconstructed Viking Age house. Environ. Mol. Mutagen., 55, 652–661. [DOI] [PubMed] [Google Scholar]
- 33. Rissler J., Nordin E. Z., Eriksson A. C., et al. (2014) Effective density and mixing state of aerosol particles in a near-traffic urban environment. Environ. Sci. Technol., 48, 6300–6308. [DOI] [PubMed] [Google Scholar]
- 34. Lohr M., Jensen A., Eriksen L., Gronbaek M., Loft S., Moller P. (2015) Age and metabolic risk factors associated with oxidatively damaged DNA in human peripheral blood mononuclear cells. Oncotarget, 6, 2641–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moller P., Hemmingsen J. G., Jensen D. M., et al. (2015) Applications of the comet assay in particle toxicology: air pollution and engineered nanomaterials exposure. Mutagenesis, 30, 67–83. [DOI] [PubMed] [Google Scholar]
- 36. Danielsen P. H., Bräuner E. V., Barregard L., Sällsten G., Wallin M., Olinski R., Rozalski R., Møller P., Loft S. (2008) Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat. Res., 642, 37–42. [DOI] [PubMed] [Google Scholar]
- 37. Hemmingsen J.G., Møller P., Jantzen K., Jönsson B.A., Albin M., Wierzbicka A., Gudmundsson A., Loft S., Rissler J. Controlled exposure to diesel exhaust and traffic noise— effects on oxidative stress and activation in mononuclear blood cells. Mutation Res, in press. [DOI] [PubMed] [Google Scholar]
- 38. Danielsen P. H., Loft S., Møller P. (2008) DNA damage and cytotoxicity in type II lung epithelial (A549) cell cultures after exposure to diesel exhaust and urban street particles. Part. Fibre Toxicol., 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobsen N. R., Møller P., Jensen K. A., Vogel U., Ladefoged O., Loft S., Wallin H. (2009) Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol., 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bleck B., Tse D. B., Jaspers I., Curotto de Lafaille M. A., Reibman J. (2006) Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J. Immunol., 176, 7431–7437. [DOI] [PubMed] [Google Scholar]
- 41. Hirota J. A., Alexis N. E., Pui M., Wong S., Fung E., Hansbro P., Knight D. A., Sin D. D., Carlsten C. (2014) PM10-stimulated airway epithelial cells activate primary human dendritic cells independent of uric acid: application of an in vitro model system exposing dendritic cells to airway epithelial cell-conditioned media. Respirology, 19, 881–890. [DOI] [PubMed] [Google Scholar]
- 42. Mendoza A., Torres-Hernandez J. A., Ault J. G., Pedersen-Lane J. H., Gao D., Lawrence D. A. (2014) Silica nanoparticles induce oxidative stress and inflammation of human peripheral blood mononuclear cells. Cell Stress Chaperones, 19, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanley C., Thurber A., Hanna C., Punnoose A., Zhang J., Wingett D. G. (2009) The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett., 4, 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhattacharya D., Santra C. R., Ghosh A. N., Karmakar P. (2014) Differential toxicity of rod and spherical zinc oxide nanoparticles on human peripheral blood mononuclear cells. J. Biomed. Nanotechnol., 10, 707–716. [DOI] [PubMed] [Google Scholar]
- 45. Forchhammer L., Loft S., Roursgaard M., Cao Y., Riddervold I. S., Sigsgaard T., Møller P. (2012) Expression of adhesion molecules, monocyte interactions and oxidative stress in human endothelial cells exposed to wood smoke and diesel exhaust particulate matter. Toxicol. Lett., 209, 121–128. [DOI] [PubMed] [Google Scholar]
- 46. Møller P., Danielsen P., Karotki D. G., et al. (2014) Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res., 762, 133–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.