Abstract
[Purpose] The hypothalamic-pituitary-adrenal (HPA) axis in the etiopathogenesis of fibromyalgia is not clear. This study aimed to analyze the effects of a 6-week aerobic exercise program on the HPA axis in patients with fibromyalgia and to investigate the effects of this program on the disease symptoms, patients’ fitness, disability, and quality of life. [Subjects and Methods] Fifty fibromyalgia patients were randomized to Group 1 (stretching and flexibility exercises at home for 6 weeks) and Group 2 (aerobic exercise three times a week and the same at-home exercises as Group 1 for 6 weeks). Serum levels of cortisol, adrenocorticotropic hormone, insulin-like growth factor-1, and growth hormone were analyzed at baseline and at the end of, and 1 hr after an exercise stress test. [Results] Group 2 showed better improvement in morning stiffness duration and pain. Growth hormone levels significantly increased after intervention and cortisol levels significantly decreased at time-time interaction in both groups. No significant differences in adrenocorticotropic hormone and insulin-like growth factor-1 were found. [Conclusion] The results of this study seem to support the hypothesis that there is a dysregulation of the HPA axis in patients with FM, and that a six-week exercise program can influence symptoms and affect the HPA axis hormones.
Key words: Fibromyalgia, Aerobic exercise, Hypothalamic-pituitary-adrenal axis
INTRODUCTION
Fibromyalgia syndrome (FMS) is a chronic pain disorder that is associated with fatigue, tender points, sleep disturbances, cognitive dysfunction and mood disorders1). Although the exact cause of the disease is not known, several theories have been proposed. The role of hypothalamic-pituitary-adrenal (HPA) axis dysfunction in the etiopathogenesis of FMS has been discussed2,3,4). The HPA axis plays a major role in the regulation of stress responses. It has been shown that, cortisol levels switch from the normal diurnal cortisol pattern to constant time-independent levels in FMS patients, and that hormonal levels are low and that they are not suppressed by dexamethasone5). Low serum growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels in FMS patients have also been reported6). Exogenic and endogenic stresses, such as metabolic, physiological, infectious, inflammatory and emotional factors, activate the neuroendocrine system7); however studies analyzing the stress responses of HPA axis alterations in FMS patients have reported conflicting results.
The management of FMS is difficult, and multifaceted treatment modalities are frequently required. Usually, the daily activities of patients are limited; thus, their physical and aerobic performance capacities decline. Aerobic exercise (AE) has positive effects on FMS through the alleviation of FMS symptoms8, 9). The serum beta endorphin level, immunoreactivity, and euphoria increase after AE, eliciting positive emotional and mental effects, better sleep quality and decreased pain sensitivity. It is also well known that acute exercise is a potent modulator of the release of HPA hormones10). In addition to this, high intensity exercise affects the HPA axis more than low intensity exercise11). The improvements in the symptoms of FMS after an exercise program might be partially attributed to amelioration of disorder in the HPA axis12). However, the manner by which exercise can benefit patients is still unclear8, 13). Unfortunately, studies investigating the effect of exercise on HPA axis in FMS patients are scarce. Kurtais et al.14) analyzed the effects of a single exercise session on the HPA axis in patients with FMS, and their results of the study suggest that there might be a hormonal perturbation response to exercise in patients. Accordingly, the hypothesis of this study was that exercise training might improve HPA axis disturbances in patients with FMS and further ameliorate symptoms. To investigate this hypothesis, were investigated the effects of a 6-week AE program on the HPA axis in patients with FMS. Our secondary aim was to investigate the effects of this program on FMS symptoms, patients’ fitness, disability, and quality of life.
SUBJECTS AND METHODS
This was a randomized, controlled study. Fifty-six female FMS patients who were admitted to the FMS Out-patient Clinic of the Physical Medicine and Rehabilitation Department of our institution were invited to participate in the study. The subjects had been diagnosed as having fibromyalgia according to clinicians’ decisions considering both the 1990 American College of Rheumatology classification criteria15) and clinical findings. Patients were excluded if they had any of the following: an endocrine, metabolic, infectious, or neurologic disease, cancer, connective tissue disorder, a cardiac, respiratory, or orthopedic disease that might have hindered AE, hormonal dysfunction, pregnancy, menopause, or a cognitive function that might have hampered the assessments. Patients who were receiving any sort of treatment such as psychological or physical therapy for FMS in the last 3 months and the patients who were in need of medication for anxiety or depression during the study were also excluded. The demographic and clinical characteristics were recorded.
Fifty-four consecutive patients, who were eligible for the study, were randomly assigned to Group 1 or Group 2 using a sequence of random numbers. Group 1 performed home exercises consisting of flexibility and stretching exercises twice a day in ten repetitions for 6 weeks. Group 2 performed the same at-home exercise program and an additional AE program comprised of walking on a treadmill three times a week for 6 weeks.
An illustrated card depicting flexibility and stretching training programs was given to patients, and all the motions were demonstrated and described in detail by physicians before the start of the study. This card had 12 flexibility (trunk, hips, ankle, shoulders, and wrist) movement and 12 stretching (neck, lateral side, chest, shoulder, arm, triceps, quadriceps, ankle, calf, hamstring stretch) movement illustrations. Exercise compliance of all the patients was checked by telephone once a week. In Group 2, each AE session was planned to last 40–50 min, including 5 min each for the warm-up and cool-down periods. The exercise intensity was adjusted to achieve a heart rate equivalent to 60–75% of the maximum heart rate. The heart rate was monitored using a pulse oximeter.
The use of paracetamol, was allowed if necessary; however, NSAIDs utilization was prohibited during this study since they cause significant changes in the HPA axis16).
The patients were assessed for pain, tender point count, morning stiffness duration, fatigue, cardiovascular fitness, functional disability, and health-related quality of life (HRqOL). These measures were evaluated by the same examiner (AG) at baseline and at the end of the study.
Pain was assessed using a 100-mm visual analogue scale (VAS; 0 mm = no pain, 100 mm = worst possible pain). A digital tender point examination (tender point count) at the 18 sites specified in the American College of Rheumatology FMS classification criteria15) was performed. The duration of morning stiffness was recorded in minutes. The sleep quality was assessed using questions including difficulty in falling asleep (the number of nights/week in which the patient experienced difficulty in falling asleep), frequent awakening during sleep (0=none, 1=some of the nights, 2=every night) and quality of sleep (0= good, 1= moderate, 2= unrefreshing). Functional disability was evaluated using the Fibromyalgia Impact Questionnaire (FIQ)17). The Turkish adaptation of the FIQ with validity and reliability studies of this scale was conducted by Sarmer et al18). The HRqOL was evaluated using the Turkish version of the SF-3619, 20). The SF-36 assesses eight dimensions, including physical functioning, physical role problems, bodily pain, general health perception, vitality, social functioning, emotional role problems, and mental health.
An ergospirometric exercise tolerance test was used not only to evaluate cardiovascular fitness (aerobic capacity) but also to serve as a physiological stressor to evoke HPA hormonal responses14, 21). Before each test, dynamic respiratory function tests were performed (Vmax29, Sensormedics, Yorba Linda, CA), a routine procedure of our laboratory, in order to exclude any major respiratory limitation for exercise, and the forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FVC/FEV1, and vital capacity were recorded. The patients were instructed to avoid heavy exercise 24 hours before the test and to abstain from any food, coffee, tea or cigarette smoking on the morning of the test day. The ergospirometric exercise tolerance test was conducted on a treadmill using the modified Bruce protocol. Twelve-lead electrocardiography (Marquette Case I; Marquette, Milwaukee, WI), blood pressure, and breath-by-breath analysis of respiratory gases (Vmax29, Sensormedics, Yorba Linda, CA, USA) were recorded during the exercise test. The resting and maximal heart rates (HR, beats per minute; bpm), exercise duration (minutes), systolic and diastolic blood pressure (SBP, DBP, mmHg), maximal oxygen consumption during exercise (VO2max, ml/kg/min), oxygen consumption at the anaerobic threshold (AT) (ml/kg/min), and metabolic equivalent (MET) values were recorded. The ergospirometric exercise tolerance test was conducted twice for all patients. The objective of the first test was to determine the peak oxygen consumption (VO2peak) and to establish an AT in both groups so that the second exercise test could be set to a level that was sufficient to exceed the AT for provoking the HPA axis. Therefore, for all patients, the ergospirometric exercise test to volitional exhaustion (symptom-limited termination) was performed at 8.30 a.m. The AT was determined using the V-slope technique on a VO2/VCO2 plot22). The second exercise test was performed a couple of days after the first test and at the same time in the morning. Prior to the second test, blood samples were drawn to measure the fasting basal levels of GH, IGF-I, adrenocorticotropic hormone (ACTH), and cortisol. Exercise testing was performed on a treadmill with 12-lead electrocardiography and respiratory gas analysis was conducted throughout the test. The exercise intensity of each subject was set above her AT, as guided by the HR and oxygen consumption. The duration of the exercise was 15 minutes after reaching the AT, which is sufficient to provoke hormonal responses. Post-exercise blood samples were drawn to measure the levels of GH, ACTH, and cortisol immediately after the end of the exercise and at 30 and 60 minutes later. Since IGF-I is not secreted acutely after stimulation, it was only measured at 60 minutes after the end of exercise. At the end of the 6-week program period, both groups performed the two exercise tests again. All blood samples were stored at −80 °C until assayed.
Plasma ACTH, IGF-1, and GH levels were measured using immunoradiometric assays (Beckman Coulter, Immunotech, Marseille, France). Serum cortisol levels were also measured by a radioimmunoassay (Beckman Coulter, Immunotech, Prague, Czech Republic) method.
Statistical analyses were performed using SPSS 11.5. The frequency (percent) was used as a descriptive statistic for categorical variables, and the mean ± standard deviation [median (minimum-maximum)] was used for metric variables. To compare the metric variables of two independent groups, Student’s t test or the Mann-Whitney U test was used. The χ2 test was used to evaluate the differences between groups in categorical variables. To compare repeated measurements of independent groups, a two-way repeated measures ANOVA/linear mixed effects model was used to test the effects of time(s), group and their interactions. When the interaction between time and group was found to be statistically significant, a Bonferroni corrected one-way repeated measures ANOVA was used for each group and values of p<0.025 were considered as statistically significant. The linear mixed effects model analysis was performed for the analysis of nested longitudinal data with independent groups. In general, p<0.05 was considered as statistically significant.
This study was approved by the Ethical Committee of our institution. All patients provided their informed consent and the study was carried out in compliance with the Declaration of Helsinki.
RESULTS
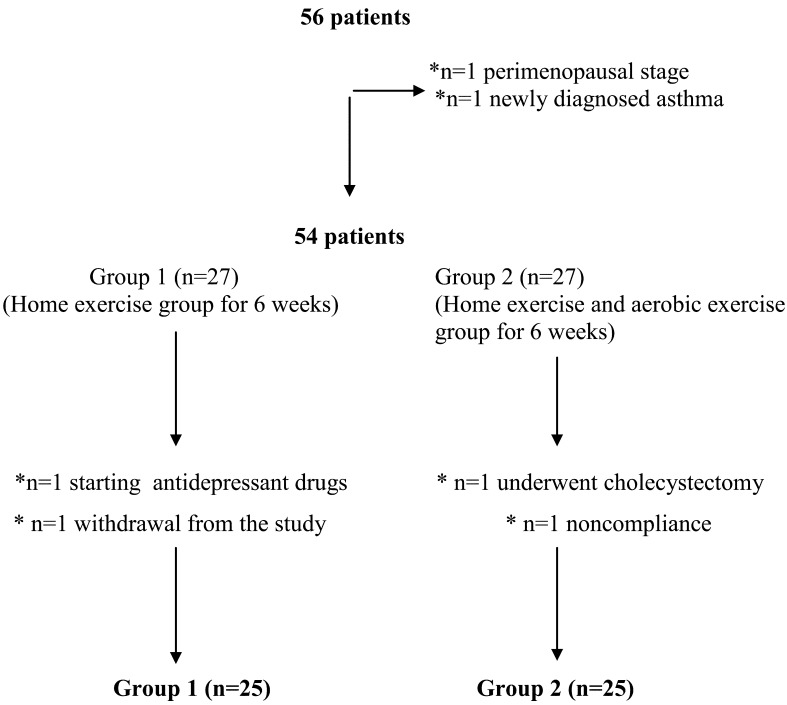
Fifty-four patients who met the inclusion criteria were recruited for this study. During the study, two patients from both groups were excluded (Fig. 1). The mean ages of the patients in Group 1 and Group 2 were 36.9 years and 35.1 years, respectively. The mean durations of the disease in Group 1 and Group 2 were 62.2 months and 67.4 months, respectively. There was no significant difference between the two groups with respect to age (p=0.427), duration of disease (p=0.617), or the presence of comorbid conditions (p=0.463).
Fig. 1.
Flow chart
*The reasons for exclusion from the study
To evaluate pain a visual analog scale was used at the beginning and end of the 6 week exercise program. Pain decreased significantly in both groups (p=0.008<0.025 for Group 1, p<0.001 for Group 2), and there was a more significant improvement in Group 2 (p=0.011). The duration of morning stiffness significantly decreased only in Group 2 (p=0.112>0.025 for Group 1, p=0.001<0.025 for Group 2). The number of tender points, FIQ and sleep scores were significantly lower in both groups at the end of the study, but there were no statistically significant differences between the groups (Table 1).
Table 1. Clinical parameters for FMS.
| Factors | Group 1 (n=25) | Group 2 (n=25) | |||||
|---|---|---|---|---|---|---|---|
| Group | Time | Group *Time interaction | BE | AE | BE | AE | |
| VAS (0–100 mm) | 0.382 | <0.001 | 0.011 | 73.96±23.47 | 63.96±19.01 | 76.04±20.07 | 55.56±20.09 |
| [80 (10–100)] | [65 (18–95)] | [79 (35–100)] | [52.2 (14–100)] | ||||
| Morning stiffness (minute) | 0.693 | <0.001 | 0.038 | 47.80±45.53 | 39.80±36.41 | 61.80±59.07 | 35.60±40.35 |
| [30 (0–180)] | [30 (0–120)] | [60 (0–240)] | [30 (0–150)] | ||||
| Number of tender points (0–18) | 0.506 | <0.001 | 0.066 | 15.36±2.56 | 13.48±2.99 | 15.68±2.17 | 12.36±2.22 |
| [16 (11–18)] | [14 (6–18)] | [16 (11–18)] | [12 (7–17)] | ||||
| FIQ score (0–100) | 0.916 | <0.001 | 0.077 | 63.21±25.21 | 57.26±18.03 | 66.37±22.37 | 52.94±16.05 |
| [68 (12–100)] | [60.1 (14–82.1)] | [61.3 (31.1–100)] | [53.6 (25.2–76.34)] | ||||
| Sleep score (0–11) | 0.732 | 0.037 | 0.081 | 8.46±3.51 | 8.02±2.91 | 9.41±2.34 | 8.24±3.37 |
| [11 (3–11)] | [11 (0–11)] | [11 (3–11)] | [8 (2–11)] | ||||
Results are expressed as the mean±SD [median (minimum–maximum)]. BE: before exercise; AE: after exercise; VAS: visual analog scale; FIQ: Fibromyalgia impact questionnaire. The “group” column shows the main effect of group (home exercise vs. aerobic exercise), the “time” column shows the main effect of time (before exercise vs. after exercise), and the “group*time interaction” column shows the interaction between the groups and the time points.
The general health score was lower in Group 1 than in Group 2 (p=0.003), but it showed no significant time differences. Likewise, there were no significant differences in the other subscales of SF-36. Significant improvements in the bodily pain (BP) and social functioning (SF) subscales of the SF-36 were found in both groups (p<0.001 for BP, p=0.039 for SF) (Table 2).
Table 2. SF-36 health survey norm-based scores.
| Factors | Group 1 (n=25) | Group 2 (n=25) | |||||
|---|---|---|---|---|---|---|---|
| Group | Time | Group *Time interaction | BE | AE | BE | AE | |
| Physical functioning | 0.580 | 0.528 | 0.856 | 69.84±24.84 | 71.15±31.45 | 73.29±23.83 | 75.68±28.62 |
| [80 (5.5–100)] | [85 (0–100)] | [72.2 (0–100)] | [85 (0–100)] | ||||
| Role physical | 0.741 | 0.077 | 0.248 | 33±44.32 | 36±44.53 | 24±34.97 | 38±38.27 |
| [0 (0–100)] | [0 (0–100)] | [0 (0–100)] | [25 (0–100)] | ||||
| Bodily pain | 0.517 | <0.001 | 0.300 | 31.74±21.1 | 38.52±21.16 | 32.52±17.48 | 44.48±20.97 |
| [41 (0–67.5)] | [31 (0–84)] | [32.5 (0–62)] | [45 (0–74)] | ||||
| General health | 0.003 | 0.103 | 0.596 | 24.84±12.98 | 30±16.68 | 42.36±23.28 | 45.48±24.74 |
| [25 (0–55)] | [25 (5–70)] | [42 (0–92)] | [40 (5–92)] | ||||
| Physical component summary | 0.993 | 0.842 | 0.102 | 42.56±18.89 | 39.95±8.91 | 39.57±8.11 | 42.89±10.18 |
| [38.7 (24.7–100)] | [38.8 (24.4–55.1)] | [40 (19.5–51.6)] | [44.1 (19.9–57.5)] | ||||
| Vitality | 0.154 | 0.063 | 0.411 | 25.4±18.93 | 27.6±18.15 | 30±12.08 | 35.60±17.99 |
| [20 (0–70)] | [25 (10–70)] | [25 (15–65)] | [40 (0–65)] | ||||
| Social functioning | 0.441 | 0.039 | 0.311 | 45.5±26.24 | 49±23.08 | 47±24.01 | 57±23.96 |
| [50 (0–100)] | [50 (0–87.5)] | [50 (12.5–100)] | [62.5 (0–100)] | ||||
| Role emotional | 0.741 | 0.167 | 0.256 | 28±43.76 | 29.32±40.04 | 25.33±35.07 | 38.67±40.47 |
| [0 (0–100)] | [0 (0–100)] | [0 (0–100)] | [33.3 (0–100)] | ||||
| Mental health | 0.191 | 0.537 | 0.723 | 40.64±24.07 | 41.12±18.46 | 46.24±14.15 | 48.01±12.27 |
| [40 (8–90)] | [40 (12–84)] | [44 (16–92)] | [48 (24–88)] | ||||
| Mental component summary | 0.479 | 0.202 | 0.154 | 30.98±13.26 | 30.80±9.35 | 31.18±8.75 | 34.35±8.47 |
| [25.8 (15.4–62)] | [27.7 (19.5–54.4)] | [29.3 (19.2–59.7)] | [33.4 (19.4–57.1)] | ||||
Results are expressed as the mean±SD [median (minimum–maximum)]. BE: before exercise; AE: after exercise The “group” column shows the main effect of group (home exercise vs. aerobic exercise), the “time” column shows the main effect of time (before exercise vs. after exercise), and the “group*time interaction” column shows the interaction between the groups and the time points.
Pulmonary function tests were within normal limits for all patients. Regarding the exercise test parameters, the overall difference between the groups was significant (mean score 13.75 and 15.33 for group 1 and 2, respectively) (p<0.001) and the duration of the exercise test after the exercise program significantly increased in both groups (p=0.002). The resting heart rate decreased after the exercise program in a statistically significant manner (p=0.006). As shown at Table 3, there were no statistically significant differences before and after the exercise program in either group in VO2max, O2 consumption at AT, maximal HR, SBP, DBP, or MET.
Table 3. Values of exercise test parameters by group.
| Factors | Group 1 (n=25) | Group 2 (n=25) | |||||
|---|---|---|---|---|---|---|---|
| Group | Time | Group *Time interaction | BE | AE | BE | AE | |
| The duration of exercise (minute) | <0.001 (Means for group 1: 13.75 group 2: 15.33) |
0.002 | 0.421 | 13.39±1.64 | 14.1±1.23 | 14.83±1.61 | 16.03±1.56 |
| [13.3 (9.7–16.4)] | [14.2 (11.3–15.9)] | [15.4 (11.3–17.2)] | [15.8 (12.5–20)] | ||||
| VO2maximum (ml/kg/min) | <0.001 (Means for group 1: 24.19 group 2: 29.26) |
0.704 | 0.583 | 24.53±3.72 | 24.09±4.87 | 30.11±3.90 | 29.14±8.12 |
| [24.8 (14.7–31.1)] | [23.7 (12.4–32.6)] | [29.5 (22.1–40.3)] | [28 (15.6–53.5)] | ||||
| O2 consumption in AT (L/min) | 0.727 | 0.283 | 0.320 | 1.02±0.36 | 1.02±0.22 | 1.09±0.20 | 1.02±0.19 |
| [1 (0.3–1.6)] | [1 (0.7–1.5)] | [1.1 (0.6–1.5)] | [1 (0.7–1.5)] | ||||
| Resting heart rate (BPM) | 0.843 | 0.006 | 0.441 | 95.25±19.20 | 91.55±20.61 | 95.79±16.19 | 88.42±13.03 |
| [94.5 (63–158)] | [86.5 (62–153)] | [99 (73–121)] | [83.5 (69–115)] | ||||
| Maximal heart rate (BPM) | 0.509 | 0.136 | 0.117 | 158.8±20.23 | 157.5±20.15 | 165.79±15.05 | 156.63±21.88 |
| [165 (124–189)] | [155.5 (123–196)] | [164 (134–188)] | [162.5 (109–192)] | ||||
| Systolic blood pressure (mmHg) | 0.037 (Means for group 1: 115 group 2: 109.1) |
0.692 | 0.883 | 114.5±12.76 | 114.5±8.87 | 108.54±11.18 | 109.58±11.6 |
| [115 (100–130)] | [115 (100–130)] | [110 (90–140)] | [110 (90–130)] | ||||
| Diastolic blood pressure (mmHg) | 0.076 | 0.855 | 0.503 | 76.25±8.87 | 77.5±6.59 | 73.75±7.7 | 72.5±9.78 |
| [80 (60–90)] | [80 (70–90)] | [70 (60–90)] | [70 (60–90)] | ||||
| MET | 0.027 (Means for group 1: 9.12 group 2: 10.28) | 0.390 | 0.739 | 9.13±1.88 | 9.31±2.1 | 10.21±1.55 | 10.56±2.59 |
| [10.1 (4.4–11.6)] | [9.5 (4.4–12)] | [9.8 (6.9–12.1)] | [11.8 (4.1–14.8)] | ||||
Results are expressed as the mean±SD [median (minimum-maximum)]. BE: before exercise; AE: after exercise; AT: anaerobic threshold; BPM: beats per minute; MET: metabolic equivalent. The “group” column shows the main effect of group (home exercise vs. aerobic exercise), the “time” column shows the main effect of time (before exercise vs. after exercise), and the “group*time interaction” column shows the interaction between the groups and the time points.
The time effect for before and after the exercise program (p=0.025) and the time-time interaction (before and after interaction with basal, 1st and 2nd measurements) (p=0.005) were statistically significant for the GH levels of both groups. The post-hoc comparisons showed that only the difference between the basal and 2nd measurements in the time period of before the exercise program was significant (p=0.003). The mean of the 2nd measurement was higher than the basal measurement (Table 4). For the cortisol levels, there were significant differences for the time effect of the basal, 1st and 2nd measurements (p=0.014) in both groups. Post-hoc comparisons indicated that the basal measurements (mean±SD: 158.22±35.76 nmol/l) were higher than the 1st measurements (mean±SD: 141.17±29.46 nmol/l) (p=0.012). There were no significant differences in ACTH and IGF-1 regarding either the time effect or between-group effects.
Table 4. Growth hormone (mIU/L) levels.
| BE | AE | |||||
|---|---|---|---|---|---|---|
| Basal | 1st measurement | 2nd measurement | Basal | 1st measurement | 2nd measurement | |
| Group 1 | 2.81±6.37 | 2.89±4.73 | 6.79±8.33 | 9.61±12.41 | 5.64±13.7 | 5.41±11.38 |
| [0.6 (0–28.2)] | [1.3 (0–21.6)] | [2.9 (0.2–32.4)] | [4 (0.3–56.3)] | [0.8 (0–65.8)] | [2 (0–56.7)] | |
| Group 2 | 1.27±2.29 | 4.9±8.66 | 6.49±8.22 | 8.64±10.27 | 3.89±6.97 | 6.06±8.96 |
| [0.7 (0–11.1)] | [0.7 (0.1–31.4)] | [1.7 (0.3–26.1)] | [4.4 (0.2–30.9)] | [0.7 (0–22.2)] | [1.5 (0.1–30.8)] | |
| Overall | 2.04±4.8 | 3.89±6.98 | 6.64±8.19 | 9.13±11.28 | 4.77±10.79 | 5.74±10.14 |
| [0.6 (0–28.2)] | [0.8 (0–31.4)] | [2.3 (0.2–32.4)] | [4.1 (0.2–56.3)] | [0.7 (0–65.8)] | [2 (0–56.7)] | |
Results are expressed as the mean±SD [median (minimum–maximum)]. BE: before exercise; AE: after exercise
DISCUSSION
Exercise has been fully recognized as an important part of FMS treatment following the European League against Rheumatism (EULAR) recommendations for the management of FMS syndrome23). Exercise at moderate-to-high intensity results in better improvements in functional capacity and in level of activity limitations24). The effects of aerobic, progressive-resistance stretching and flexibility exercises on FMS patients have been analyzed by several studies. Some of these studies demonstrated that AE has positive effects on sleep, quality of life, self-esteem, depression, pain, and some exercise test parameters12, 24). Bircan et al.25) compared the effects of AE with a muscle-strengthening program, and found that both exercise modalities were similarly effective at improving symptoms, tender point count, fitness, depression, and quality of life. On the other hand, Mengshoel et al.26) found that AE did not show any advantages over no exercise treatment for the physical functions of FMS patients. In the present study, AE was determined to be superior to the stretching and flexibility exercises in relation to pain and morning stiffness duration. Although stretching and flexibility exercise was performed daily, it was well tolerated by both groups. This may have been because exercises were divided into two daily sessions with low numbers of repetitions. All clinical parameters of FMS except morning stiffness duration (number of tender points, FIQ score, pain levels, and length of exercise), the BP and SF subscales of the SF-36, and resting heart rate improved similarly with both exercise programs.
Approximately one third of FMS patients are estimated to suffer from GH deficiency. This condition can lead to reduced IGF-16). Ross et al. suggested that GH dysfunction was associated with increased pain and poor muscle performance in FMS patients27). Nearly fifty percent of GH secretion occurs during the third and fourth non-REM sleep stages28), and FMS patients have non-REM sleep disturbances29). This may be one of the reasons why there are low levels of these hormones in FMS patients. Furthermore, there is not always a correlation between GH and IGF-1 release. It is well known that an exercise intensity of 25% VO2max increases GH levels10). Exercise is believed to stimulate GH release via cholinergic activation, and in acute stress conditions, exercise first enhances growth hormone releasing hormone (GHRH), which then triggers an increase in GH levels30). On the other hand, some authors have reported there are no significant differences in GH and IGF-1 values between FMS patients and healthy control groups31, 32). The reasons for these discrepancies might be due to the pulsatile release of GH and/or differences in the duration of disease and/or chronic stress level14). As for IGF-1, regular exercise is expected to increase the resting level of the hormone in healthy individuals33), but Jones et al.34) reported there was no increase in patients with FMS who exercised regularly for a long time. In our study, GH levels increased in all patients after a 6-week exercise program and AE was not superior to flexibility and stretching exercises regarding the changes in GH levels. Moreover; IGF-1 levels were not changed by the exercise program, and there was no correlation between GH and IGF-1 levels. This finding was in agreement with previous studies. Lack of changes in IGF-1 levels with exercise suggests the existence of a disorder in the hypothalamus-GH-IGF-1 axis in FMS patients.
If exercise intensity reaches to 30–40% of VO2max, or if an individual exercises over the anaerobic threshold, ACTH also increases, and after a 10-minute interval, the cortisol level also increases. The critical exercise level that triggers cortisol release is 60% of VO2max. Low-intensity exercises may lead to a decrease in blood cortisol levels by increasing clearance10). Several stimulating agents such as GHRH, luteinizing hormone releasing homone, corticotropin releasing hormone, clonidine, levodopa, metyrapone, exercise, or several tests such as the dexamethasone suppression test, or the insulin tolerance test have been used to analyze hormonal changes in FMS patients by many studies4, 14, 27, 33,34,35,36,37,38). The ACTH and cortisol responses to these stimulants are somewhat conflicting. Increased basal cortisol levels were reported by one study39), whereas other studies reported decreased 24-hour urinary free cortisol and low morning cortisol release in fibromyalgia2, 40). Normal 24-hour cortisol and diurnal patterns of ACTH and cortisol secretion have been reported as well38, 41, 42). In the present study, while no significant difference was detected in the ACTH levels after the exercise program, cortisol levels significantly decreased with only a time effect. Therefore, the proposed this discordancy in the HPA axis may be associated with insufficient exercise intensity and/or duration.
The main limitation of this study was short exercise duration, since it has been reported that the positive effects of aerobic exercise become apparent from the 6th week of exercise43). Extension of the exercise period to 8–12 weeks would possibly allow better monitoring of the effects of exercise. The lack of increase in VO2max levels after the 6-week exercise program in both groups is possibly a result of insufficient exercise intensity during the intervention period. There may be several reasons for this such as: insufficient pain relief, short exercise time, and/or mild-moderate exercise intensity. Furthermore, it is known that patients with FMS cannot perform high intensity exercises due to pain and tiredness. Only the use of paracetamol was allowed during this study because NSAIDs utilization can cause significant changes in the HPA axis. Insufficient pain relief may have resulted in decreased exercise tolerance and discordance in HPA axis hormones. To relieve pain and increase exercise capacity pharmacological agents, physical treatment, biofeedback and cognitive behavioral therapy should be started before all treatments, and exercise programs should be started afterwards to soothe pain and fatigue.
The findings of the present study, especially the absence of correlation between GH and IGF-1, and between ACTH and cortisol, seem to support our hypothesis of a perturbation of the HPA axis resulting from exercise by FMS patients; however, they do not exactly support our assumption that this program would provide specific effects on the HPA axis, other than improvement of FMS symptoms. The increase in GH observed in this study might indicate a possible link between them. Nonetheless, the findings do verify our hypothesis that exercise can decrease FMS symptoms. Confirmation of this hypothesis was our secondary objective. AE programs with stretching and flexibility exercises were found to be similarly effective with regard to many of the clinical parameters assessed.
It is our belief that more accurate findings can be obtained by randomized controlled studies of larger samples and if possible more prolonged or higher intensity exercise.
Acknowledgments
We thank our laboratory staff Serpil Okyar, PT, for her valuable help in testing study subjects.
REFERENCES
- 1.Bennett RM, Jones J, Turk DC, et al. : An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord, 2007, 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crofford LJ, Pillemer SR, Kalogeras KT, et al. : Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum, 1994, 37: 1583–1592. [DOI] [PubMed] [Google Scholar]
- 3.Adler GK, Kinsley BT, Hurwitz S, et al. : Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med, 1999, 106: 534–543. [DOI] [PubMed] [Google Scholar]
- 4.Wingenfeld K, Nutzinger D, Kauth J, et al. : Salivary cortisol release and hypothalamic pituitary adrenal axis feedback sensitivity in fibromyalgia is associated with depression but not with pain. J Pain, 2010, 11: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 5.Riva R, Mork PJ, Westgaard RH, et al. : Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med, 2010, 17: 223–233. [DOI] [PubMed] [Google Scholar]
- 6.Cuatrecasas G, Gonzalez MJ, Alegre C, et al. : High prevalence of growth hormone deficiency in severe fibromyalgia syndromes. J Clin Endocrinol Metab, 2010, 95: 4331–4337. [DOI] [PubMed] [Google Scholar]
- 7.Crofford LJ, Demitrack MA: Evidence that abnormalities of central neurohormonal systems are key to understanding fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am, 1996, 22: 267–284. [DOI] [PubMed] [Google Scholar]
- 8.Thomas EN, Blotman F: Aerobic exercise in fibromyalgia: a practical review. Rheumatol Int, 2010, 30: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 9.Vural M, Berkol TD, Erdogdu Z, et al. : Evaluation of the effectiveness of an aerobic exercise program and the personality characteristics of patients with fibromyalgia syndrome: a pilot study. J Phys Ther Sci, 2014, 26: 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjaer M, Dela FM: Endocrine responses to exercise. In: Goetz-Hoffman L, ed. Exercise and immune function. Florida: CRC Press, 1996, pp 1–19. [Google Scholar]
- 11.Hill EE, Zack E, Battaglini C, et al. : Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest, 2008, 31: 587–591. [DOI] [PubMed] [Google Scholar]
- 12.Valim V, Oliveira L, Suda A, et al. : Aerobic fitness effects in fibromyalgia. J Rheumatol, 2003, 30: 1060–1069. [PubMed] [Google Scholar]
- 13.Ortega E, García JJ, Bote ME, et al. : Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc Immunol Rev, 2009, 15: 42–65. [PubMed] [Google Scholar]
- 14.Gürsel Y, Ergin S, Ulus Y, et al. : Hormonal responses to exercise stress test in patients with fibromyalgia syndrome. Clin Rheumatol, 2001, 20: 401–405. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Smythe HA, Yunus MB, et al. : The American College of Rheumatology 1990 criteria for the classification of Fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum, 1990, 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 16.Aloisi AM, Buonocore M, Merlo L, et al. : Chronic pain therapy and hypothalamic-pituitary-adrenal axis impairment. Psychoneuroendocrinology, 2011, 36: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 17.Burckhardt CS, Clark SR, Bennett RM: The fibromyalgia impact questionnaire: development and validation. J Rheumatol, 1991, 18: 728–733. [PubMed] [Google Scholar]
- 18.Sarmer S, Ergin S, Yavuzer G: The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheumatol Int, 2000, 20: 9–12. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr: SF-36 health survey update. Spine, 2000, 25: 3130–3139. [DOI] [PubMed] [Google Scholar]
- 20.Koçyiğit H, Aydemir Ö, Fişek G: Kısa form-36 (KF-36)’nın Türkçe versiyonunun güvenirliliği ve geçerliliği. İlaç ve Tedavi Dergisi, 1999,. 12: 102–106. [Google Scholar]
- 21.Kurtais Y, Tur BS, Elhan AH, et al. : Hypothalamic-pituitary-adrenal hormonal responses to exercise stress test in patients with rheumatoid arthritis compared to healthy controls. J Rheumatol, 2006, 33: 1530–1537. [PubMed] [Google Scholar]
- 22.Beaver WL, Wasserman K, Whipp BJ: A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1985, 1986, 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 23.Arnold B, Häuser W, Arnold M, et al. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften: [Multicomponent therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz, 2012, 26: 287–290. [DOI] [PubMed] [Google Scholar]
- 24.Mannerkorpi K, Nordeman L, Cider A, et al. : Does moderate-to-high intensity Nordic walking improve functional capacity and pain in fibromyalgia? A prospective randomized controlled trial. Arthritis Res Ther, 2010, 12: R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bircan C, Karasel SA, Akgün B, et al. : Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol Int, 2008, 28: 527–532. [DOI] [PubMed] [Google Scholar]
- 26.Mengshoel AM, Komnaes HB, Førre O: The effects of 20 weeks of physical fitness training in female patients with fibromyalgia. Clin Exp Rheumatol, 1992, 10: 345–349. [PubMed] [Google Scholar]
- 27.Ross RL, Jones KD, Bennett RM, et al. : Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J, 2010, 3: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta A, Hindmarsh PC: The use of somatropin (recombinant growth hormone) in children of short stature. Paediatr Drugs, 2002, 4: 37–47. [DOI] [PubMed] [Google Scholar]
- 29.Moldofsky H, Scarisbrick P, England R, et al. : Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med, 1975, 37: 341–351. [DOI] [PubMed] [Google Scholar]
- 30.Jones KD, Deodhar P, Lorentzen A, et al. : Growth hormone perturbations in fibromyalgia: a review. Semin Arthritis Rheum, 2007, 36: 357–379. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen S, Main K, Danneskiold-Samsøe B, et al. : A controlled study on serum insulin-like growth factor-I and urinary excretion of growth hormone in fibromyalgia. J Rheumatol, 1995, 22: 1138–1140. [PubMed] [Google Scholar]
- 32.Buchwald D, Umali J, Stene M: Insulin-like growth factor-I (somatomedin C) levels in chronic fatigue syndrome and fibromyalgia. J Rheumatol, 1996, 23: 739–742. [PubMed] [Google Scholar]
- 33.Berg U, Bang P: Exercise and circulating insulin-like growth factor I. Horm Res, 2004, 62: 50–58. [DOI] [PubMed] [Google Scholar]
- 34.Jones KD, Deodhar AA, Burckhardt CS, et al. : A combination of 6 months of treatment with pyridostigmine and triweekly exercise fails to improve insulin-like growth factor-I levels in fibromyalgia, despite improvement in the acute growth hormone response to exercise. J Rheumatol, 2007, 34: 1103–1111. [PubMed] [Google Scholar]
- 35.Kirnap M, Colak R, Eser C, et al. : A comparison between low-dose (1 microg), standard-dose (250 microg) ACTH stimulation tests and insulin tolerance test in the evaluation of hypothalamo-pituitary-adrenal axis in primary fibromyalgia syndrome. Clin Endocrinol (Oxf), 2001, 55: 455–459. [DOI] [PubMed] [Google Scholar]
- 36.Riedel W, Layka H, Neeck G: Secretory pattern of GH, TSH, thyroid hormones, ACTH, cortisol, FSH, and LH in patients with fibromyalgia syndrome following systemic injection of the relevant hypothalamic-releasing hormones. Z Rheumatol, 1998, 57: 81–87. [DOI] [PubMed] [Google Scholar]
- 37.Riedel W, Schlapp U, Leck S, et al. : Blunted ACTH and cortisol responses to systemic injection of corticotropin-releasing hormone (CRH) in fibromyalgia: role of somatostatin and CRH-binding protein. Ann N Y Acad Sci, 2002, 966: 483–490. [DOI] [PubMed] [Google Scholar]
- 38.Wingenfeld K, Heim C, Schmidt I, et al. : HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosom Med, 2008, 70: 65–72. [DOI] [PubMed] [Google Scholar]
- 39.Catley D, Kaell AT, Kirschbaum C, et al. : A naturalistic evaluation of cortisol secretion in persons with fibromyalgia and rheumatoid arthritis. Arthritis Care Res, 2000, 13: 51–61. [DOI] [PubMed] [Google Scholar]
- 40.Gur A, Cevik R, Sarac AJ, et al. : Hypothalamic-pituitary-gonadal axis and cortisol in young women with primary fibromyalgia: the potential roles of depression, fatigue, and sleep disturbance in the occurrence of hypocortisolism. Ann Rheum Dis, 2004, 63: 1504–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maes M, Lin A, Bonaccorso S, et al. : Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand, 1998, 98: 328–335. [DOI] [PubMed] [Google Scholar]
- 42.McLean SA, Williams DA, Harris RE, et al. : Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum, 2005, 52: 3660–3669. [DOI] [PubMed] [Google Scholar]
- 43.Pollock M, Gaesser GA, Butcher JD, et al. : The recommend quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc, 1998, 30: 975–991. [DOI] [PubMed] [Google Scholar]