Abstract
[Purpose] The short-term effects of structured exercise on the anthropometric, cardiovascular, and metabolic parameters of non-overweight women diagnosed with polycystic ovary syndrome were evaluated. [Subjects and Methods] Thirty women with a diagnosis of polycystic ovary syndrome were prospectively randomized to either a control group (n=16) or a training group (n=14) for a period of 8 weeks. Anthropometric, cardiovascular, and metabolic parameters and hormone levels were measured and compared before and after the intervention. [Results] Waist and hip measurements (anthropometric parameters); diastolic blood pressure; respiratory rate (cardiovascular parameters); levels of low-density lipoprotein cholesterol, total cholesterol, fasting glucose, and fasting insulin; and the homeostasis model assessment of insulin resistance index (metabolic parameters) were significantly lower in the training group after 8 weeks of exercise compared to the baseline values. After exercise, the training group had significantly higher oxygen consumption and high-density lipoprotein levels and significantly shorter menstrual cycle intervals. The corresponding values for controls did not significantly differ between the start and end of the 8-week experiment. [Conclusion] Short-term regular exercise programs can lead to improvements in anthropometric, cardiovascular, and metabolic parameters of non-overweight women with polycystic ovary syndrome.
Key words: Exercise, Non-overweight, Polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) occurs in 5–10% of women of reproductive age1). Chronic anovulation, hyperandrogenism, and insulin resistance are the main characteristics of PCOS2). These patients also have elevated triglyceride and low-density lipoprotein (LDL) cholesterol levels and low high-density lipoprotein (HDL) cholesterol levels3).
Treatment is generally focused on normalizing anovulation and reducing metabolic syndrome parameters. Diet and exercise are recommended as first-line treatments by the majority of gynecologists and endocrinologists. The aim is to reduce abdominal fat, hyperandrogenemia, and insulin resistance and to improve lipid profiles4). Published studies have demonstrated the positive effects of exercise training on maximal oxygen consumption (MaxVO2), weight, and waist circumferences in PCOS patients5). Combined aerobic and resistance exercise is more effective than either aerobic or resistance exercise alone in improving insulin sensitivity and glycemic control and in reducing abdominal fat in obese women with PCOS6, 7). However, the metabolic and clinical characteristics of PCOS patients who are not overweight, and thus the efficacy of the various forms of exercise, have not been evaluated8). For example, in overweight PCOS patients, exercise programs lasting 12 weeks tended to yield positive results5, 9), whereas benefits might be achieved more quickly by PCOS patients who are not overweight.
We hypothesized that 8 weeks of structured exercise would be sufficient to improve the anthropometric, cardiovascular, and metabolic parameters of non-overweight women with PCOS, at least over the short term. Therefore, we conducted a randomized controlled clinical trial to establish the beneficial effects of exercise on these 3 sets of parameters in non-overweight PCOS patients.
SUBJECTS AND METHODS
We evaluated PCOS patients whose body mass index (BMI) was in the normal range (< 25 kg/m2) according to the definition of obesity for Asians. All patients were admitted to our center between March 2011 and May 2014. The study was approved by the Human Research Ethics Committee of Dokuz Eylul University, Izmir, Turkey (reference no.: 03/38-09, GOA385). Written informed consent was obtained from all patients. PCOS patients were diagnosed on the basis of Rotterdam Criteria (2003), which requires the presence of two of the following:10) (1) a polycystic ovary, defined as the presence of >10 cysts 2–8 mm in diameter, an ovarian volume >10 cm3, and an echodense stroma on transvaginal or pelvic ultrasonography;11) (2) clinical hyperandrogenism (Ferriman-Gallwey12) score >8) or biochemical hyperandrogenism (serum testosterone level >3.6 pg/mL in the absence of other causes of hyperandrogenism); and (3) oligomenorrhea and/or anovulation.
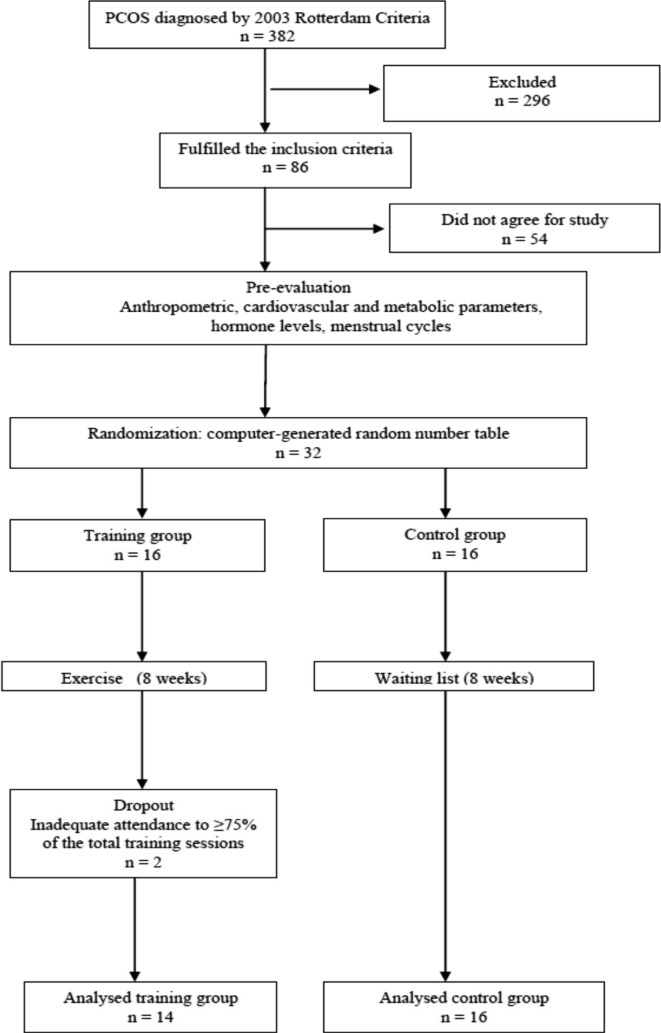
The following patients were excluded: patients with endocrinological diseases, including diabetes, thyroid, adrenal, or pituitary gland dysfunction; cardiovascular, hepatic, or pulmonary disease; a history of orthopedic or other physical symptoms that would otherwise limit exercise performance; and those who had exercised regularly within the last 6 months. The resulting 32 non-overweight women with PCOS were randomly allocated into a training group (n = 16) that participated in a structured exercise program for 8 weeks or a control group (n = 16) that did not participate in a structured exercise program. The trial flow chart is shown in Fig. 1. Randomization was carried out using a computer-generated random number table and pre-labeled, sealed envelopes. Based on the results, participants were assigned to one of the two groups.
Fig. 1.
Study flow chart
Anthropometric parameters (body mass index, waist, and hip measurements), cardiovascular parameters (systolic and diastolic blood pressure, heart rate, respiratory rate, MaxVO2), metabolic parameters (HDL cholesterol, LDL cholesterol, triglycerides, total cholesterol, fasting glucose, fasting insulin, and homeostasis model assessment [HOMA] insulin resistance index), and hormone levels (follicle stimulating hormone [FSH], luteinizing hormone [LH], estradiol, total testosterone, and free testosterone) were measured pre- and post-intervention. The dates of the patients’ menstrual cycles were also recorded. All clinical assessments were performed by the same physician.
Blood samples were obtained between 7:30 a.m. and 8:30 a.m., (after overnight fasting) during the early follicular phase (days 2–5) of a progesterone-induced menstrual cycle. Blood samples were obtained from supine patients after a 30-min resting period and collected in tubes containing ethylenediaminetetraacetic acid. The samples were immediately centrifuged at 4 °C for 20 min at 1,600 g and stored at −20 °C until assayed. Plasma LH, FSH, estradiol, and free and total testosterone levels were measured using specific radioimmunoassay. Blood insulin and glucose levels were measured with a solid-phase chemiluminescent enzyme immunoassay and the glucose oxidase method, respectively. Insulin resistance was estimated based on the HOMA index (basal glucose mmol × basal insulin mIU/mL/22.5).
Hip and waist circumferences were determined using a tape measure, measuring the widest circumference of the buttocks and the smallest circumference of the waist. Blood pressure was measured with a manual sphygmomanometer from a seated patient after a 5-min resting period. Heart and respiratory rates were evaluated manually. The radial pulse was measured over a period of 10 s. MaxVO2 was calculated indirectly during a 6-min walking test13) performed as follows: patients were given a stopwatch and asked to begin walking on a flat track at their natural walking speed. At the end of 6 min, the walking distance in meters was recorded. Heart rate, blood pressure, and respiratory rate were recorded before and after the test. The following formula was used to calculate MaxVO2 at the end of the 6-min walking test:
MaxVO2 = 132.853 − (0.0769 × weight) − (0.3877 × age) + (6.315 × gender (male: 1, female: 0) − (3.2649 × time) − (0.1565 × heart rate).
At the beginning of the study, general dietary and behavioral advice, but not a structured calorie restriction program, was provided to all study participants. All patients were counseled regarding a healthy, balanced meal plan with regular food and a nutritional composition in which 50% of the calories were from carbohydrates, 25% from protein, and 25% from fat.
Patients participated in a structured exercise program 3 times per week for 8 weeks at the Physical Therapy and Rehabilitation Fitness Unit of Dokuz Eylul University. During each session (50–60 min), the patients performed aerobic and resistance exercises. Each session was preceded by a 5-min warm-up and static stretching of the upper and lower limbs, neck, and trunk muscles, followed by a 5-min cool down. The training sessions were supervised by a physiotherapist. Weekly physical activity was initially monitored using the International Physical Activity Questionnaire14). The exercise program included the following: 1. Warm-up and cool-down exercises: Walking on a treadmill at a low pace. 2. Aerobic exercise: A step was used for aerobic training. All patients started training with a 10 cm high step. Five weeks later, it was raised to a height of 15–20 cm. Depending on the exercise tolerance of the patient, the exercise duration was increased from 5–7 min to 20 min over the 8 weeks of the study. The exercise was submaximal in intensity. The Borg Scale was used to rate perceived exertion15). Daily exercise was considered 1 session, and the session intensity was equivalent to 10–15 points on the Borg Scale, thus achieving 65–70% of the maximum heart rate according to the American College of Sports Medicine. 3. Resistance exercise: Isoflex exercises utilizing an elastic band targeted the following muscles: Hip flexors, extensors, abductors, adductors, hamstrings, internal and external obliques, rectus abdominis, back extensors, scapular, and quadriceps muscles. The participants performed 15 repetitions of each exercise, with a minimum resting period of 30 s and a maximum of 1 min between each repetition. Exercise intensity was controlled individually, with the patient rating the exercise intensity according to a perceived exertion scale for resistance exercise16). The patients were advised to maintain an exercise intensity close to 5 or 6, which corresponded to a perception of “somewhat intense” exercise.
Statistical tests were performed using the SPSS (Statistical Package for the Social Sciences for Windows, version 15.0, SPSS, Chicago, IL, USA). The normal distribution of the variables was determined using the Kolmogorov-Smirnov test. Only the BMI and FSH/LH variables were distributed normally. Due to the small sample size and multiple non-normally distributed variables, differences between the training and control groups were assessed using the Mann-Whitney U test. The pre- and post-intervention values of the groups were compared using the Wilcoxon signed rank test. The results are presented as the mean ± standard error of the mean. A p value <0.05 was considered statistically significant.
RESULTS
During the 3 year period, 382 patients were identified as having PCOS. Of these, 56 fulfilled the inclusion criteria. The 32 patients who agreed to participate in the study were divided randomly into groups of 16 patients each. However, two patients in the training group were eventually excluded because of inadequate attendance (< 75% of the training sessions). Thus, the study was completed by 14 patients in the training group and 16 in the control group. All 30 patients fulfilled the Rotterdam Criteria for PCOS: 30 had polycystic ovaries and anovulation, 26 (86.7%) had clinical hyperandrogenism, and 19 (63.3%) had biochemical hyperandrogenism. The mean age of the patients was 24.45 ± 2.8 years (range: 17–34 years).
The anthropometric and cardiovascular parameters of the two groups are listed in Table 1, and the metabolic parameters are shown in Table 2. Patients in the control and training groups did not significantly differ in their baseline parameters. In the training group, several anthropometric and cardiovascular parameters improved significantly after the exercise program; they showed significant decreases in waist circumference (p = 0.02), hip circumference (p = 0.01), diastolic blood pressure (p = 0.04), and respiratory rate (p = 0.04) and a significant increase in MaxVO2 (p = 0.001). In the control group, anthropometric and cardiovascular parameters were not significantly different after 8 weeks compared to the baseline values. The differences in hip circumference (p = 0.04) and MaxVO2 (p < 0.001) after 8 weeks were significantly higher in the training group than in controls (Table 1).
Table 1. Comparison of anthropometric and cardiovascular parameters between and within groups.
| Variable | Training group (n = 14) | Control group (n = 16) | ||||
|---|---|---|---|---|---|---|
| Baseline | 8 w later | Difference | Baseline | 8 w later | Difference | |
| BMI (kg/m2) | 21.8±1.0 | 21.7±1.1 | 0.1±0.1 | 21.9±1.1 | 21.7±1.1 | 0.04±0.1 |
| Waist measurement (cm) | 68.8±2.1 | 67.6±1.9 | 1.18±0.4* | 68.4±3.9 | 68.6±4.0 | 0.31±0.1 |
| Hip measurement (cm) | 99.8±4.7 | 97.7±4.6 | 2.06±0.6* | 100±2.6 | 100.1±2.6 | 0.31±0.1** |
| SBP (mmHg) | 120.5±1.6 | 117.8±2.5 | 2.5±2.9 | 110.6±3.4 | 108.1±1 | 2.6±1.4 |
| DBP (mmHg) | 75.3±1.0 | 72±2.5 | 3.1±3.1* | 70.6±2.6 | 67.5±1.7 | 3.3±1.8 |
| HR (beats/min) | 84.8±1.6 | 81.2±2.2 | 3.62±2.7 | 84.5±2.0 | 88.1±2.8 | 3.5±1.7 |
| RR (breaths/min) | 22.5±0.8 | 21.5±0.9 | 1.0±0.4* | 21.6±0.7 | 21.4±0.7 | 0.2±0.1 |
| MaxVO2 (ml/kg/min) | 587.9±21.7 | 664.9±23.1 | 76.1±10.8* | 588.3±41.1 | 591.7±36 | 3.4±1.3** |
Results are presented as the mean ± standard error of the mean. *p < 0.05, between baseline and after exercise values, by Mann-Whitney U test. **p < 0.05, between difference scores of training and control groups, by Mann-Whitney U test. BMI: body mass index; DBP: diastolic blood pressure; HR: heart rate; max VO2: maximal oxygen consumption; SBP: systolic blood pressure; RR: respiratory rate
Table 2. Comparison of hormone levels and metabolic parameters between and within groups.
| Variable | Training group (n = 14) | Control group (n = 16) | ||||
|---|---|---|---|---|---|---|
| Baseline | 8 w later | Difference | Baseline | 8 w later | Difference | |
| FSH (IU/L) | 5.4±0.23 | 5.0±0.3 | 0.4±0.2 | 5.4±0.2 | 5.3±0.2 | 0.04±0.08 |
| LH (IU/L) | 7.1±1.5 | 8.8±1.57 | 1.7±0.9 | 12.5±1.3 | 12.4±1.2 | 0.1±0.1 |
| FSH/LH | 1.0±0.1 | 1.0±0.2 | 0.02±0.2 | 0.5±0.04 | 0.5±0.05 | 0.0±0.0 |
| E2 (pmol/L) | 36.0±8.4 | 63.5±14 | 27.4±18.5 | 56.7±3.8 | 56.8±3.8 | 0.12±0.1 |
| Total testosterone (nmol/L) | 1.2±0.9 | 1.1±0.9 | 0.1±0.7 | 1.2±0.1 | 1.2±0.2 | 0.2±0.7 |
| Free testosterone (pg/ml) | 3.15±0.0 | 3.15±0.1 | 0.00±0.0 | 2.9±0.1 | 2.83±0.2 | 0.10±0.1 |
| HDL-C (mg/dl) | 44.6±0.63 | 45.2±0.6 | 0.6±0.1* | 46.1±0.9 | 46.1±1 | 0.0±0.5 |
| LDL-C (mg/dl) | 122.6±6.1 | 114±4.9 | 8.62±1.5* | 118.5±3.2 | 118.6±3.2 | 0.1±0.2** |
| Triglycerides (mg/dl) | 141.1±0.5 | 138.2±0.8 | 2.8±1.3 | 139.6±2.2 | 140.5±1.9 | 0.8±0.4** |
| Total Cholesterol (mg/dl) | 198.6±3.7 | 195.1±3 | 3.4±1.1* | 200.5±4.1 | 200.7±4.0 | 0.2±0.7** |
| Fasting glucose (mg/dl) | 98.1±2.4 | 93.6±2.3 | 4.4±2.5* | 97.7±2.0 | 97.3±2.3 | 0.4±0.3** |
| Fasting insulin (μU/ml) | 14.7±1 | 13.9±1 | 0.8±0.1* | 14.3±1 | 14.5±0.8 | 0.2±0.2** |
| HOMA index | 3.2±0.3 | 2.9±0.2 | 0.3±0.0* | 3.1±0.2 | 3.1±0.2 | 0.1±0.1** |
Results are presented as the mean ± standard error of mean. *p < 0.05, between baseline and after exercise values, by Mann-Whitney U test. **p < 0.05, between difference scores of training and control groups, by Mann-Whitney U test. E2: estradiol; FSH: follicle stimulating hormone; HDL-C: high-density lipoprotein cholesterol; HOMA: homeostatic model assessment; LDL-C: low-density lipoprotein cholesterol; LH: luteinizing hormone
The changes in hormone levels and metabolic parameters between the two groups are shown in Table 2. In the training group, the decreases in LDL cholesterol (p < 0.001), total cholesterol (p = 0.01), fasting glucose (p < 0.001), fasting insulin (p = 0.001), and HOMA (p < 0.001) after exercise were significant, as was the increase in HDL (p = 0.002). In controls, none of the hormonal or metabolic parameters were significantly different at the end of the study period. The differences in LDL cholesterol (p = 0.01), HDL (p = 0.05), triglycerides (p = 0.005), total cholesterol (p = 0.01), fasting glucose (p = 0.01), fasting insulin (p = 0.001), and HOMA index (p < 0.001) values were significantly greater in the training group than in controls at the end of the study (Table 2). The mean menstrual cycle interval did not change significantly in the control group (46.12 ± 1.3 days vs. 47.05 ± 1.2 days, p = 0.125) but decreased significantly in the training group after exercise (48.09 days vs. 27.3 days, p = 0.04).
DISCUSSION
We investigated the effects of an 8-week structured exercise program on non-overweight patients with PCOS. Many studies have reported that exercise training has beneficial effects on cardiopulmonary functional capacity and metabolic syndrome parameters in overweight patients with PCOS; however, this is the first study to evaluate the benefits of structured exercise in non-overweight PCOS patients. We found that 8 weeks of structured exercise was effective for improving anthropometric, cardiovascular, and metabolic parameters and for regulating menstrual cycles in non-overweight patients with PCOS, despite the absence of significant changes in sex hormones.
The beneficial effects of physical fitness are related to its reduction of the risk of cardiovascular dysfunction and to the delayed onset of metabolic syndrome activity of the proteins involved in insulin signal transduction in skeletal muscles17,18,19). Therefore, by following a systematic exercise program, PCOS patients can improve their cardiopulmonary functional capacity and insulin sensitivity. Modalities other than fitness have been suggested for the treatment of PCOS. Fux Otta et al.20) reported the additive effect of metformin on diet and exercise in the improvement of hyperandrogenism and insulin resistance. Although some studies have reported additive benefits of insulin sensitizers21, 22), others have not23, 24). In contrast, all studies of PCOS have found that lifestyle changes should be considered in the management of these patients. However, there is no consensus on the basic variables of exercise training, including its type, intensity, duration, frequency, and progression. Orio et al.25) evaluated whether the effects of a training program were maintained after its cessation. They found that 12 weeks of non-training resulted in a complete loss of all favorable adaptations obtained from the exercise program. Therefore, exercise may need to be maintained throughout life for its benefits to be preserved. Although other studies have evaluated the impact of diet on the treatment of PCOS, we found that structured exercise alone was beneficial, at least in the short-term.
The role of non-pharmacological therapy in PCOS patients has been evaluated in several studies. Giallauria et al.26) assigned 124 PCOS patients to 2 groups for a 3-month exercise program. They demonstrated that exercise improved autonomic function and inflammatory patterns. In a similar study (based on 90 young women with PCOS who were randomly divided into 2 groups27)), patients who participated in a 3-month structured exercise program exhibited improvements in cardiopulmonary functional capacity. Other studies have reported greater reductions in BMI than our study28), but we did not focus on weight loss, as the patients were not overweight. Nevertheless, they experienced important changes in several cardiovascular parameters and in MaxVO2.
Among the PCOS studies in the literature, positive results have been obtained in overweight patients who exercised for 12–24 weeks26,27,28). Exactly when the beneficial effects of exercise can first be observed has not been determined. We found that in non-overweight PCOS patients with adequate (≥75%) participation in an exercise program consisting of aerobic and resistance exercise, anthropometric, metabolic, and cardiovascular parameters were already improved after 8 weeks. However, whether the same effects occur in morbidly obese patients with PCOS or whether they are apparent after a longer or shorter period of exercise remains to be determined.
Several studies have reported that diet changes, either alone or in combination with exercise, resulting in a weight loss of 5–10% could restore menstrual cycles. Weight reduction also has been shown to improve both the reproductive and the metabolic features of PCOS. Weight loss normalizes ovulation, ameliorates hyperandrogenism, and improves insulin sensitivity29). In the present study, structured exercise for 8 weeks was sufficient for non-overweight patients with PCOS to achieve significant improvements in waist and hip measurements but did not lead to significant weight loss.
Our results suggest that an 8-week exercise program improves reproductive and metabolic disorders related to metabolic syndrome in non-overweight PCOS patients. Long-term regular exercise may provide better results in metabolic parameters and improve the quality of life of these patients. The effects of exercise may occur more quickly in non-overweight than in overweight PCOS patients. The duration of exercise that achieves the maximum benefits may differ between these two groups of patients and requires further study.
REFERENCES
- 1.Ehrmann DA: Polycystic ovary syndrome. N Engl J Med, 2005, 352: 1223–1236. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan MT: Polycystic ovarian syndrome: diagnosis and management. Clin Med Res, 2004, 2: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, et al. : Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab, 2007, 18: 280–285. [DOI] [PubMed] [Google Scholar]
- 4.Moran LJ, Lombard CB, Lim S, et al. : Polycystic ovary syndrome and weight management. Womens Health (Lond Engl), 2010, 6: 271–283. [DOI] [PubMed] [Google Scholar]
- 5.Thomson RL, Buckley JD, Noakes M, et al. : The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab, 2008, 93: 3373–3380. [DOI] [PubMed] [Google Scholar]
- 6.Lee HC, Heo T: Effects of exercise therapy on blood lipids of obese women. J Phys Ther Sci, 2014, 26: 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura T, Kida K, Seki T, et al. : Study of the relationship between exercise therapy and diet therapy in type 2 diabetes mellitus patients. J Phys Ther Sci, 2011, 23: 485–488. [Google Scholar]
- 8.Li L, Chen X, He Z, et al. : Clinical and metabolic features of polycystic ovary syndrome among Chinese adolescents. J Pediatr Adolesc Gynecol, 2012, 25: 390–395. [DOI] [PubMed] [Google Scholar]
- 9.Thomson RL, Buckley JD, Moran LJ, et al. : Comparison of aerobic exercise capacity and muscle strength in overweight women with and without polycystic ovary syndrome. BJOG, 2009, 116: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 10.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril, 2004, 81: 19–25. [DOI] [PubMed] [Google Scholar]
- 11.Carmina E, Orio F, Palomba S, et al. : Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril, 2005, 84: 413–419. [DOI] [PubMed] [Google Scholar]
- 12.Ferriman D, Gallwey JD: Clinical assessment of body hair growth in women. J Clin Endocrinol Metab, 1961, 21: 1440–1447. [DOI] [PubMed] [Google Scholar]
- 13.Kervio G, Carre F, Ville NS: Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc, 2003, 35: 169–174. [DOI] [PubMed] [Google Scholar]
- 14.Saglam M, Arikan H, Savci S, et al. : International physical activity questionnaire: reliability and validity of the Turkish version. Percept Mot Skills, 2010, 111: 278–284. [DOI] [PubMed] [Google Scholar]
- 15.Borg G: Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med, 1970, 2: 92–98. [PubMed] [Google Scholar]
- 16.Robertson RJ, Goss FL, Rutkowski J, et al. : Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc, 2003, 35: 333–341. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, Jun I, Ju S: Impact of home exercise training on patients with acute myocardial infarction. J Phys Ther Sci, 2012, 24: 743–745. [Google Scholar]
- 18.Kim DY, Seo BD, Kim DJ: Effect of walking exercise on changes in cardiorespiratory fitness, metabolic syndrome markers, and high-molecular-weight adiponectin in obese middle-aged women. J Phys Ther Sci, 2014, 26: 1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagura C, Takamura N, Goto Y, et al. : Cardiorespiratory fitness and metabolic markers in healthy young adult men. J Phys Ther Sci, 2011, 23: 845–849. [Google Scholar]
- 20.Fux Otta C, Wior M, Iraci GS, et al. : Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double-blind, and placebo control trial. Gynecol Endocrinol, 2010, 26: 173–178. [DOI] [PubMed] [Google Scholar]
- 21.Harborne L, Fleming R, Lyall H, et al. : Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet, 2003, 361: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 22.Nestler JE: Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med, 2008, 358: 47–54. [DOI] [PubMed] [Google Scholar]
- 23.Açbay O, Gündoğdu S: Can metformin reduce insulin resistance in polycystic ovary syndrome? Fertil Steril, 1996, 65: 946–949. [PubMed] [Google Scholar]
- 24.Ehrmann DA, Cavaghan MK, Imperial J, et al. : Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab, 1997, 82: 524–530. [DOI] [PubMed] [Google Scholar]
- 25.Orio F, Giallauria F, Palomba S, et al. : Metabolic and cardiopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clin Endocrinol (Oxf), 2008, 68: 976–981. [DOI] [PubMed] [Google Scholar]
- 26.Giallauria F, Palomba S, Maresca L, et al. : Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf), 2008, 69: 792–798. [DOI] [PubMed] [Google Scholar]
- 27.Vigorito C, Giallauria F, Palomba S, et al. : Beneficial effects of a three-month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab, 2007, 92: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 28.Liao LM, Nesic J, Chadwick PM, et al. : Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: a pilot investigation. Gynecol Endocrinol, 2008, 24: 555–561. [DOI] [PubMed] [Google Scholar]
- 29.Panidis D, Farmakiotis D, Rousso D, et al. : Obesity, weight loss, and the polycystic ovary syndrome: effect of treatment with diet and orlistat for 24 weeks on insulin resistance and androgen levels. Fertil Steril, 2008, 89: 899–906. [DOI] [PubMed] [Google Scholar]