Abstract
[Purpose] The present study aimed to determine the effects of short muscle strength exercise on hepatocyte growth factor expression and satellite cell activation. [Subjects] The study included 72 2–12-week-old male Sprague-Dawley rats. [Methods] The rat plantaris muscle was contracted with a 5-min electrical stimulation of the sciatic nerve, and then, the mRNA expressions of hepatocyte growth factor and myogenic regulatory factors in the plantaris muscle were determined, and the phosphorylation of the hepatocyte growth factor receptor (c-Met) was examined. [Results] The mRNA expressions of hepatocyte growth factor and myogenic regulatory factors increased after a short muscle contraction compared to that un-contraction. Immunofluorescence analysis showed the expression of hepatocyte growth factor protein and the possibility that downstream biological changes occurred in the hepatocyte growth factor-bound c-Met. [Conclusion] Our results demonstrated that activation of satellite cells induced hepatocyte growth factor expression during muscle contraction with a short 5-min electrical stimulation, which simulates short muscle strength exercise in physical therapy. The present study provides evidence for the use of short muscle strength exercise in physical therapy.
Key words: Muscle contraction, Myogenic regulatory factors, Satellite cell
INTRODUCTION
Muscle strength exercise is an important therapeutic technique in physical therapy, which is often performed for recovery from disuse atrophy. Although the precise mechanism of satellite cell activation induced by hepatocyte growth factor (HGF) is unclear, it is one of the key events in this recovery process1,2,3,4,5). In fact, previous studies using experimental animal models have demonstrated that satellite cell activation and HGF production are induced by activities that cause continuous muscle contraction, such as high-intensity running for 30 min, synergist muscle ablation, contralateral continuous overloading by ablation of a sciatic nerve, and reloading6,7,8,9,10,11,12,13,14). However, in clinical physical therapy, it is difficult to perform high-intensity exercise for 30 min or longer. Therefore, in this study, we examined the effects of short muscle strength exercise on HGF expression and satellite cell activation for clinical application.
SUBJECTS AND METHODS
To examine the changes during postnatal growth, 20 male Sprague-Dawley rats (2–12 weeks old; Charles River Japan, Shizuoka, Japan) were used. The rats were housed in a temperature-controlled room (20–24 °C) with a 12-h light/dark cycle and ad libitum access to laboratory chow and water. Under anesthesia with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g body weight), 2-, 4-, 8-, and 12-week-old rats were perfused with ice-cold phosphate buffered saline (PBS, pH 7.2), and the right plantaris muscle was extracted and trimmed of excess fat. For real-time polymerase chain reaction (PCR), the center of the muscle was stored at −80 °C until use.
To investigate the behavior of satellite cells, 61 male Sprague-Dawley rats (8 weeks old; initial body weight 293–361 g; Charles River Japan) were used. In all rats, the right ankle was fixed to a metal plate by wrapping the foot with a strap. Then, the right sciatic nerve was exposed, and a silver electrode was placed on the nerve. The rats were randomly allocated to either the Stimulation (Stim) group or the Non-stim group. In the Stim group, isometric contractions of the right plantaris muscle were induced by stimulating the sciatic nerve with 150-ms supramaximal single square pulses (0.05 ms, 100 Hz, 5 V) once every second for 5 min, using an electronic stimulator (Nihon Kohoden, Tokyo, Japan). The Non-stim group was used as the control group. The rats were perfused with ice-cold PBS under anesthesia on days 1, 3, and 7, and the plantaris muscle of the right hindlimb was removed and trimmed of excess fat. For real-time PCR, the center of the muscle was stored at −80 °C until use. Additionally, for immunofluorescence analysis, the center of the muscle was placed in Tissue-Tek O.C.T. compound (Sakura Finetek Japan, Tokyo, Japan), snap-frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C until use.
All animal care and treatment procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Kanazawa University, and all protocols were approved by the Committee on Animal Experimentation of Kanazawa University.
Total RNA was isolated from muscle samples using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Tokyo, Japan). Genomic DNA was degraded with DNase I. The purity of the extracted RNA was determined by measuring optical density (Taitec, Tokyo, Japan) at 260 and 280 nm, where a 260/280 nm ratio of 1.8–2.0 was the optimal result. RNA concentration was measured at 260 nm, and the muscle total RNA concentration was calculated on the basis of total RNA.
First-strand cDNA was reverse-transcribed for each muscle sample using the PrimeScript First Strand cDNA Synthesis Kit (Takara, Tokyo, Japan) according to the manufacturer’s protocol. One microgram of total RNA, random primers, and a dNTP mixture in a 10-μL total reaction volume were incubated at 65 °C for 5 min, followed by quick-cooling on ice. Then, 5× PrimeScript buffer, RNase inhibitor, and PrimeScript RTase were added to a 20-μL total reaction volume, which was incubated at 30 °C for 10 min, followed by 42 °C for 60 min. Finally, the reverse transcript reaction mixture was heated to 95 °C for 5 min to stop the reaction.
Real-time PCR was performed with a LightCycler ST300 (Roche Diagnostics, Tokyo, Japan) using the SYBR green intercalator method. Amplification was performed in a 20-μL total reaction volume using 2 µL of each RT reaction mixture with the following primers: HGF (sense: 5′-CTTAAACATTTCCCAGCTAGTC-3′; antisense: 5′-CTCGTAATAAACCATCTGCGT-3′); HGF receptor (c-Met) (sense: 5′-TAGGATTCGGTCTTCAAGTAG-3′; antisense: 5′-AAATCAGCAACCTTGACAGT-3′); myogenic differentiation 1 (MyoD) (sense: 5′-ACTACAGCGGCGACTCAGAC-3′; antisense: 5′-ACTGTAGTAGGCGGCGTCGT-3′); myogenin (sense: 5′-TGAATGCAACTCCCACAGC-3′; antisense: 5′-CAGACATATCCTCCACCGTG-3′); and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (sense: 5′-AACGGGAAACCCATCACCA-3′; antisense: 5′-CGGAGATGATGACCCTTTTG-3′). The real-time PCR samples were initially denatured for 10 s at 95 °C. PCR was then carried out for 40 cycles with a denaturation step of 5 s at 95 °C, and an annealing and extension step of 20 s at 60 °C, followed by melting curve analysis. The reaction conditions were optimized to improve the efficiency of the standard curve analysis. For each primer set, PCR specificity was judged based on the presence of a single product at the end of the 40 cycles, as determined by melting curve analysis that showed a single peak at the melting temperature of the product. The mRNA expressions of HGF, c-Met, MyoD, and myogenin were normalized against that of Gapdh. The relative content of the respective mRNA in the 2-week-old rats was compared with that in 4-, 8-, and 12-week-old rats. Additionally, the relative content of the respective mRNA in the stimulated muscle was compared with that in the non-stimulated muscle.
For immunofluorescence analysis, 9 muscle samples from day 1, 3, 7 on Stim group were cut into 10-μm-thick transverse sections using a cryostat. The transverse sections were then fixed in 70% ethanol for 30 min at 4 °C. Non-specific binding sites were blocked with 5% normal goat serum in PBS for 30 min at room temperature. The sections were incubated with 1:20 rabbit polyclonal anti-HGF alpha antibody (sc-7949; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or 1:100 mouse monoclonal anti-phospho-tyrosine antibody (#9411; Cell Signaling, Tokyo, Japan) in blocking buffer overnight at 4 °C. The sections were then incubated with a 1:600 dilution of anti-rabbit Alexa Fluor 488 (Life Technologies Japan, Tokyo, Japan) and Alexa Flour 546 (Life Technologies Japan) in blocking buffer for 20 min at room temperature, and mounted with Prolong Gold Antifade Reagent with 4′6-diaminino-2-phenylindole (DAPI; Life Technologies Japan). Fluorescent signals were captured under fluorescence microscopy (Biozero BZ-8100; Keyence, Osaka, Japan).
Data are presented as mean ± standard deviation. Differences of variance between the 2-week-old rats and the 4-, 8-, and 12-week-old rat in the postnatal growth experiment were analyzed by using Dunnett’s multiple comparison test, and differences of variance between the Stim and Non-stim groups were examined by using the Student’s t-test or Welch’s test, followed by the F-test. For all tests, values of p < 0.05 were considered statistically significant.
RESULTS
In the growth phase, the plantaris muscle wet weight to body weight significantly increased between 2 and 8 weeks of age compared to that at 2 weeks of age, and then plateaued thereafter (Table 1). The mRNA expression of HGF was significantly high at 2 weeks of age, and then decreased thereafter (Table 1). The mRNA expression of MyoD was significantly lower at week 8 than at week 2 (Table 1).
Table 1. Morphological and molecular biological changes during postnatal growth.
| Postnatal weeks | ||||
|---|---|---|---|---|
| 2 wk | 4 wk | 8 wk | 12 wk | |
| n = 5 | n = 5 | n = 5 | n = 5 | |
| Body weight (g) | 32.8 ± 1.1 | 88.4 ± 2.3 | 278.4 ± 6.2 | 419.0 ± 7.6 |
| Plantaris muscle wet weight (mg) | 16.2 ± 0.6 | 71.7 ± 7.3 | 298.6 ± 17.7 | 464.5 ± 15.9 |
| Muscle wet weight/body weight (mg/g) | 0.99 ± 0.03 | 1.62 ± 0.14* | 2.14 ± 0.10*† | 2.21 ± 0.09*† |
| HGF mRNA | 1.00 ± 0.33 | 0.57 ± 0.13* | 0.44 ± 0.25* | 0.35 ± 0.09* |
| MyoD mRNA | 1.00 ± 0.14 | 0.86 ± 0.06 | 0.59 ± 0.19* | 0.73 ± 0.20 |
Data are expressed as mean ± standard deviation. *p < 0.01, compared with 2 wk. †p < 0.01, compared with 4 wk
The mRNA expressions of MyoD, myogenin and c-Met significantly increased in the stimulated muscles compared to the expressions in the non-stimulated muscles at day 1, and the high expression levels continued up to day 7 (Table 2). The mRNA expression of HGF was significantly high only at day 7 (Table 2). Furthermore, immunofluorescence analysis showed positive signals of HGF and phospho-tyrosine in the same locations from day 1 to day 7 (Fig. 1).
Table 2. mRNA expressions in the plantaris muscle contracted with a 5-min electrical stimulation.
| Days after stimulation | ||||||
|---|---|---|---|---|---|---|
| 1 | 3 | 7 | ||||
| Non-stim group | Stim group | Non-stim group | Stim group | Non-stim group | Stim group | |
| n = 8 | n = 8 | n = 9 | n = 9 | n = 9 | n = 9 | |
| HGF | 1.00 ± 0.44 | 1.02 ± 0.39 | 1.00 ± 0.60 | 1.73 ± 0.90 | 1.00 ± 0.39 | 1.65 ± 0.69* |
| c-Met | 1.00 ± 0.30 | 1.30 ± 0.11* | 1.00 ± 0.39 | 2.42 ± 0.97** | 1.00 ± 0.33 | 2.39 ± 1.18** |
| MyoD | 1.00 ± 0.31 | 2.06 ± 0.64** | 1.00 ± 0.27 | 4.94 ± 2.31** | 1.00 ± 0.12 | 3.01 ± 1.26** |
| myogenin | 1.00 ± 0.16 | 2.12 ± 1.31* | 1.00 ± 0.29 | 20.51 ± 12.5** | 1.00 ± 0.29 | 2.66 ± 1.65* |
Data are expressed as mean ± standard deviation. *p < 0.05, compared with the Non-stim group for the same number of days. **p < 0.01, compared with the Non-stim group for the same number of days.
Fig. 1.
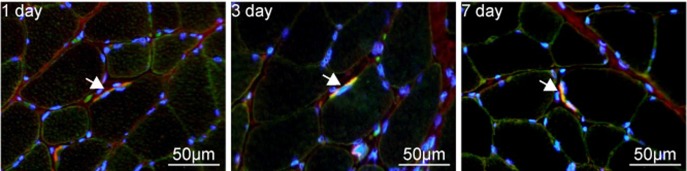
Immunofluorescence images of the localization of hepatocyte growth factor and phospho-tyrosine in the plantaris muscle contracted with a 5-min electrical stimulation. Muscle sections at 1, 3, and 7 days after 5-min electrical stimulation are stained for hepatocyte growth factor (HGF; green) and phospho-tyrosine (red). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Merged images are presented. HGF and phospho-tyrosine-immunopositive cells are indicated by arrows.
DISCUSSION
In the present study, the plantaris muscle wet weight to body weight, and the mRNA expressions of HGF and MyoD, plateaued at 8 weeks of age during postnatal growth. These results are consistent with those of previous studies using HGF enzyme-linked immunosorbent assay and immunohistochemistry11, 15, 16). Therefore, 8-week-old rats in which the effect of growth was small were used in the subsequent study.
MyoD and myogenin belong to a class of myogenic differentiation factors, which are expressed in myogenic cells during proliferation and differentiation, and in the present study, expression of these genes indicated satellite cell activation11, 17,18,19). The mRNA expressions of MyoD, myogenin, and c-Met immediately increased after the 5-min electrical stimulation. In contrast, the mRNA expression of HGF increased on day 7, and not immediately after stimulation. However, strong signals of HGF and phospho-tyrosine were observed in the same locations immediately after electrical stimulation. These findings suggest that preexisting HGF was activated. Proteolytic activation of HGF protein by the HGF-specific serine protease HGF activator, is required for binding to c-Met20, 21). The active form of HGF has been reported to increase after muscle injury3). Additionally, the active form of HGF has been reported to be released in the extracellular matrix where it undergoes enzymolysis by matrix metalloproteinases induced by mechanical stimulation such as stretch stimulation and/or muscle injury22,23,24). HGF-bound c-Met is tyrosine-phosphorylated, and mediates mitogenesis and morphogenesis25). In the present study, the HGF-α antibody recognized both the active and inactive forms of HGF, and the anti-phospho-tyrosine antibody did not recognize phospho-c-Met. Therefore, in this study, the occurrence of these activation processes is unclear. HGF expression has been reported to be induced by several growth factors and cytokines including HGF itself26, 27). The increase in the expression of HGF mRNA on day 7 may have occurred through an indirect mechanism. The results indicate that activation of satellite cells related to HGF does not only occur during continuous muscle activities, such as down-hill running for 30 min, synergist ablation, and reloading6,7,8,9,10,11,12,13,14), but also occurs after activities that induce short muscle contraction, such as a 5-min electrical stimulation. However, in this study, electrical stimulation induced tetanic contraction of the plantaris muscle, which does not easily occur in daily life activities. Additionally, muscle hypertrophy occurs upon repeated muscle strength exercise over approximately 3 weeks. Therefore, skeletal muscle hypertrophy can be achieved with repeated short muscle contractions, as shown in this study.
In physical therapy, achieving a curative effect with exercise in a limited amount of time is very important. In the present study, we demonstrated that the biological signal of muscle hypertrophy triggered by HGF was induced with a 5-min electrical stimulation that caused muscle contractions. This study provided evidence for the use of short muscle strength exercise in physical therapy.
REFERENCES
- 1.Tatsumi R, Anderson JE, Nevoret CJ, et al. : HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol, 1998, 194: 114–128. [DOI] [PubMed] [Google Scholar]
- 2.Tatsumi R, Sheehan SM, Iwasaki H, et al. : Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res, 2001, 267: 107–114. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Yamanouchi K, Soeta C, et al. : Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochem Biophys Res Commun, 2002, 292: 709–714. [DOI] [PubMed] [Google Scholar]
- 4.Tatsumi R, Hattori A, Ikeuchi Y, et al. : Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell, 2002, 13: 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishido M, Kasuga N: In vivo real-time imaging of exogenous HGF-triggered cell migration in rat intact soleus muscles. Acta Histochem Cytochem, 2012, 45: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith HK, Maxwell L, Rodgers CD, et al. : Exercise-enhanced satellite cell proliferation and new myonuclear accretion in rat skeletal muscle. J Appl Physiol 1985, 2001, 90: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 7.Hill M, Goldspink G: Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol, 2003, 549: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi A, Ishii H, Morita I, et al. : mRNA expression of fibroblast growth factors and hepatocyte growth factor in rat plantaris muscle following denervation and compensatory overload. Pflugers Arch, 2004, 448: 539–546. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Tachino K, Kawahara E, et al. : Hepatocyte growth factor in mouse soleus muscle increases with reloading after unloading. J Phys Ther Sci, 2006, 18: 33–41. [Google Scholar]
- 10.Tanaka Y, Yamaguchi A, Fujikawa T, et al. : Expression of mRNA for specific fibroblast growth factors associates with that of the myogenic markers MyoD and proliferating cell nuclear antigen in regenerating and overloaded rat plantaris muscle. Acta Physiol (Oxf), 2008, 194: 149–159. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Miyata T, Fujita Y, et al. : Differing responses of satellite cell activity to exercise training in rat skeletal muscle. J Phys Ther Sci, 2009, 21: 141–145. [Google Scholar]
- 12.Miyata T, Tanaka S, Tachino K: MyoD and myogenin mRNA levels after single session of treadmill exercise in rat skeletal muscle. J Phys Ther Sci, 2009, 21: 81–84. [Google Scholar]
- 13.Miyata T, Tanaka S, Yamazaki T: MyoD, myogenin and myosin heavy chain mRNA expression in rat skeletal muscle after a single session of low-intensity treadmill exercise. J Phys Ther Sci, 2009, 21: 379–383. [Google Scholar]
- 14.Miyata T, Tanaka S, Yamazaki T: Effects of walking and weight-bearing exercise on soleus muscle in hindlimb-suspended rat. J Phys Ther Sci, 2011, 23: 385–389. [Google Scholar]
- 15.Tamaki T, Akatsuka A, Yoshimura S, et al. : New fiber formation in the interstitial spaces of rat skeletal muscle during postnatal growth. J Histochem Cytochem, 2002, 50: 1097–1111. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Tanaka J, Kawahara E, et al. : Expression of hepatocyte growth factor in rat skeletal muscle. J Phys Ther Sci, 2005, 17: 109–113. [Google Scholar]
- 17.Wright WE, Sassoon DA, Lin VK: Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell, 1989, 56: 607–617. [DOI] [PubMed] [Google Scholar]
- 18.Grounds MD, Garrett KL, Lai MC, et al. : Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res, 1992, 267: 99–104. [DOI] [PubMed] [Google Scholar]
- 19.Koishi K, Zhang M, McLennan IS, et al. : MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn, 1995, 202: 244–254. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura T, Ochiai M, Kondo J, et al. : A novel protease obtained from FBS-containing culture supernatant, that processes single chain form hepatocyte growth factor to two chain form in serum-free culture. Cytotechnology, 1992, 8: 219–229. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa K, Shimomura T, Naka D, et al. : Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem, 1994, 269: 8966–8970. [PubMed] [Google Scholar]
- 22.Tatsumi R, Allen RE: Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve, 2004, 30: 654–658. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Tatsumi R, Kikuiri T, et al. : Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve, 2006, 34: 313–319. [DOI] [PubMed] [Google Scholar]
- 24.Yamada M, Sankoda Y, Tatsumi R, et al. : Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol, 2008, 40: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 25.Day RM, Cioce V, Breckenridge D, et al. : Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene, 1999, 18: 3399–3406. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan SM, Tatsumi R, Temm-Grove CJ, et al. : HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve, 2000, 23: 239–245. [DOI] [PubMed] [Google Scholar]
- 27.Tatsumi R, Sankoda Y, Anderson JE, et al. : Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol, 2009, 297: C238–C252. [DOI] [PubMed] [Google Scholar]


