Abstract
[Purpose] To investigate the effects of combined aerobic and resistance training on glycolipid metabolism and inflammation levels in type 2 diabetes mellitus patients. [Subjects and Methods] Forty-two diabetes patients were randomized to the conventional therapy group (n = 20) or intensive therapy group (n = 22). The control group contained 20 healthy people. The conventional therapy group received routine drug therapy and diet control, while the intensive therapy group additionally underwent combined aerobic and resistance training for 12 weeks. The oral glucose tolerance test and cardiopulmonary exercise testing were performed. Toll-like receptor 4 and NF-κBp65 protein and mRNA expressions were determined by qPCR and western blotting. ELISA was used to determine the expression levels of interleukin-18, interleukin-33, pentraxin-related protein 3, and human cartilage glycoprotein 39. [Results] After exercise training, the intensive therapy group had significantly lower postprandial blood glucose, postprandial insulin, and glycated hemoglobin level and insulin resistance index than the conventional therapy group. The intensive therapy group had significantly lower toll-like receptor 4 and NF-κBp65 protein and mRNA expressions, and serum interleukin-18 levels but significantly higher serum interleukin-33 levels. [Conclusion] Combined aerobic and resistance training can improve glycolipid metabolism and reduce low-grade inflammation in patients with diabetes mellitus patients.
Key words: Type 2 diabetes mellitus, Exercise therapy, Inflammation
INTRODUCTION
Late-stage diabetes is always associated with multiple chronic complications that lead to extremely high rates of mortality and disability. Therefore, understanding the pathogenic mechanism of diabetes mellitus is essential. Diabetes mellitus is usually associated with hereditary and environmental factors. The disease has recently been regarded as a chronic low-grade inflammatory disease whose onset is characterized with persistent low-grade inflammation1). The latest studies confirm the significant elevation of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, C-reactive protein (CRP), etc. in diabetes2, 3). In addition, diabetes patients exhibit abnormal expression of adipokines such as leptin, resistin, and adiponectin, indicating chronic low-grade inflammation3). Furthermore, inflammatory factors involved in the genesis of insulin resistance (IR) also contribute to the pathogenesis and progression of diabetes. Those findings strongly indicate a close association between low-grade inflammations and type 2 diabetes mellitus (T2DM).
Exercise therapy is recommended for the treatment of diabetes. Regular exercise improves glycolipid metabolism and IR, thereby reducing diabetes-associated complications. Furthermore, exercise improves chronic inflammation4). Exercise can reduce systemic low-grade inflammation and thus effectively improve chronic inflammatory diseases such as obesity, metabolic syndrome, diabetes mellitus, ageing, etc.5, 6). It can decrease the levels of inflammatory factors such as TNF-α, IL-6, CRP7, 8), and leptin9) while increasing the levels of anti-inflammatory factors such as IL-4, IL-10, and adiponectin in diabetes patients10, 11). Exercise also reduces the inflammation level and improves IR by enhancing anti-stress and anti-oxidative effects. Nevertheless, the anti-inflammatory mechanism induced by exercise is complex and incompletely understood.
Toll-like receptor 4 (TLR4) is a major member of TLR family, which acts as a core mediator of innate immunity. TLR4 is an important receptor for the chronic inflammatory response because it is closely related to the inflammatory response. It substantially increases the expressions of inflammatory factors such as TNF-α, IL-6, and IL-1 by regulating the activities of transcription factors such as NF-κB12); this creates a persistent low-level chronic inflammatory response and IR in vivo. Under oxidative stress, NF-κB also activates its inhibitor and increases the serine phosphorylation of insulin receptors and insulin receptor substrate, leading to IR13). Thus, TLR4 and NF-κB play pivotal roles in generating an inflammatory response in vivo. Therefore, they might play critical roles in the pathogenesis and progression of diabetes. Accordingly, the present study investigated the long-term effects of a combination of aerobic and resistance exercises for 12 weeks on TLR4, NF-κB, and inflammatory factors in diabetes patients. Thus, we aimed to provide a theoretical basis to encourage exercise therapy for patients with diabetes.
SUBJECTS AND METHODS
From March 2012 to March 2014, 42 patients with non-insulin-dependent T2DM were selected from among the outpatients visiting the Department of Cardiovascular Rehabilitation, Xiangya Hospital, Central South University, Changsha, China. The patients were randomized to the conventional therapy group (CT group, n = 20) or the intensive therapy group (IT group, n = 22). The CT group received regular drug treatment and diet guidance, while the IT group additionally performed aerobic and resistance exercises for 12 weeks. Furthermore, 20 healthy people were included as a control group. The inclusion criteria were as follows: (1) meeting the diagnostic criteria proposed by the World Health Organization in 1999, patients who presented diabetes symptoms, serum glucose level ≥ 11.1 mmol/L when detected randomly, fasting blood glucose ≥ 7.0 mmol/L, and oral glucose tolerance test (OGTT) showing a 2-hour postprandial blood glucose (PBG) ≥ 11.1 mmol/L; (2) no severe complications such as acute infectious diseases, numbness of limbs, decreased visual acuity, and diabetic nephropathy, retinopathy, or foot; (3) no severe cardiovascular or cerebrovascular diseases such as severe arrhythmia, or acute myocardial infarction or stroke, or severe joint disease leading to poor exercise tolerance; and (4) no frequent exercise (exercise frequency less than twice per week for less than 15 minutes). We obtained patients’ written informed consent to participant in this study. The study procedures were approved by the Institutional Review Board of Central South University, Changsha, China. All procedures conformed to the standards set by the Declaration of Helsinki.
Cardiopulmonary Exercise Testing (CPET) was performed on all participants before the intervention in a laboratory with 50–55% humidity and at a temperature of 24–25°C. The maximum oxygen consumption (VO2max) was defined as the highest value of oxygen consumption measured during the exercise period. Using an incremental and symptom-limited protocol, participants made their efforts to their maximal capacity on a cycle ergometer (SCHILLER CS-200, Switzerland). During the exercise, the cadence was held at a constant speed of 60 rep/min. The initial workload was 10–20 W, and then increased by 10–20 W/min. Finally, the test was terminated when any of the following symptoms occured, such as fatigue, dyspnea, angina, adverse changes in blood pressure, musculoskeletal complaint, and electrocardiographic evidence of ischemia or arrhythmia. Blood pressure and ECG were monitored throughout the whole testing. The test usually lasted 6–12 minutes. Participants were also asked to self report the intensity of exercise by the Borg scale of rate of perceived exertion (RPE)14).
All the subjects in the IT group received a combined home-based exercise program and supervised exercise training at the Department of Cardiovascular Rehabilitation, Xiangya Hospital, Central South University. These subjects took part in at least 3–10 supervised training sessions at hospital at the beginning of the program. Each subject received in-person instructions on how to exercise at a moderate-intensity level, how to monitor heart rate, how to use the resistant band, and how to acquire an individualized home-based exercise program. The intervention was gradually shifted to home-based exercise for the rest time of total 12 weeks, as the subjects were familiar with the whole exercise procedure. The exercise program mainly consisted of the aerobic exercise and the resistance training. Aerobic exercise was conducted for a total of 40–60 minutes per session, 3 sessions per week. The exercise intensity for each patient set at 40–60% of his/her VO2 max. The exercise was started at a low intensity of 40%VO2max for 10 minutes. As the weeks progressed, the exercise intensity was increased to 60%VO2max, while the duration was increased to 30 minutes over the period of 1 month. The aerobic exercise session generally consisted of a 10-minute warm-up period and a 15-minute cool-down period. Pulse palpation method was performed to monitor heart rate15). RPE (12–13 on the Borg scale) was also used to assist in estimating the moderate exercsie intensity16). The patients also performed a moderate-intensity (i.e., 50–60% of the one repetition maximum, 1RM) resistance training program 2–3 times per week. 1RM was roughly estimated by several Load-Repetition tests17). The subjects performed all resistance exercises with 8–10 repetitions in two sets by using an elastic band (Thera-band; Hygenic Corporation, Akron, OH, USA). Resistance training intensity commenced at 50% of 1RM and increased to 60% in the fourth week of the program. In addition, the RPE (11–14 on the Borg scale) was also used to monitor the training intensity. The patients were instructed to avoid the Valsalva maneuver during the resistance training program. Weight and blood pressure were checked regularly during the exercise training. In addition to the training, routine diet guidance and psychological counseling were provided. All the subjects had to visit the hospital to make sure the right home-based exercise program every month. Furthermore, the telephone consultation was applied to enhance the compliance of these subjects and check out the individual feedback during the exercise procedure. The telephone consultation lasted for 5–10 minutes and was offered once a week for 3 months18).
The OGTT was performed before and after the intervention, and the results were recorded in detail. All blood samples were drawn from an antecubital vein. Plasma glucose concentrations were analyzed by glucose oxidase method, and insulin levels were measured using RIA kit (Beijing North Institute, Beijing, China). Serum glycosylated hemoglobin (HbAlc) was measured by affinity chromatography. Serum total cholesterol (TC), low-density lipoprotein (LDL), high density lipoprotein (HDL), and triglyceride (TG) concentrations were determined by using a Cobas Fara robot (Roche, Switzerland). Insulin resistance index (IRI) was calculated using the method of homeostatic model assessment (HOMA)19). The specific formula was: IRI=[FBG(mmol/L)×FIN(µIU/mL)]/22.5. Venous blood (15 mL) was drawn from each participant and subjected to Ficoll-Hypaque density gradient centrifugation in order to separate mononuclear cells; 5 mL venous blood was used for quantitative polymerase chain reaction (qPCR) to detect the expression levels of TLR4 and NF-κB mRNA, while the remaining 10 mL was used to detect the expression levels of TLR4 and NF-κB proteins. Sera were analyzed by ELISA to determine the expression levels of IL-18, IL-33, PTX3, and YKL40.
The mononuclear cells were treated with lysis buffer, and 30 µg total protein was loaded and separated on a 10% SDS-PAGE gel before transfer to a nitrocellulose membrane. Immunoblots were incubated with primary antibodies as per the indicated dilution. β-actin was used as a loading control. After incubating with the recommended horseradish peroxidase secondary antibodies, signals were detected using enhanced chemiluminescence. Chemiluminescence fluorescence image analysis system was used to scan fluorescence images and various optical density value (OD value) was calculated by Bandscan image analysis software. The relative expression of target genes was determined by the the ratio of OD value of the target genes to that of internal reference gene.
Total RNA was extracted from the mononuclear cells using TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. The expressions of TLR4 and NF-κBp65 mRNA were detected by SYBR green qPCR assay (Bio-Rad, Hercules, CA, USA). The specific primers were as follows: TLR4 forward: 5′- TGGGTAAGGAATGAGCTAGTAAA -3′, reverse:5′- GCTGGGACACCACAACAA -3′; β-actin forward: 5′- CATCCTGCGTCTGGACCTGG-3′, reverse: 5′-TAATGTCACGCACGATTTCC-3′; NF-κBp65 forward:5′- -CCCATCGGGTTCCCATAAAG-3′, reverse: 5′-GCCTGAAGCAAATGTTGGCGTA-3′. All reactions were performed in triplicate. β-actin was used as an endogenous control for TLR4 and NF-κBp65. The 2−ΔΔCT method was used to process the data.
The biochemical analyses of serum IL-18, IL-33, PTX3, and YKL40 were performed by ELISA (Abcam, MA, USA); human IL-18, IL-33, PTX3, and YKL40 were detected quantitatively. All factors were quantified using polyclonal antibodies that recognized native human IL-18, IL-33, PTX3, or YKL40 and a series of plates containing wells coated with predetermined amounts of recombinant human IL-18, IL-33, PTX3, or YKL40.
All statistical analyses were performed using SPSS version 19.0. Continuous variables are expressed as mean ± standard deviation and were compared using the Student-Newman-Keuls test. The insulin resistance index (IRI) showed a skewed distribution and a natural logarithmic transformation was performed befor statistical analysis. The level of significance was set at p < 0.05.
RESULTS
The diabetes groups did not differ significantly from the normal control group with respect to age (52.59 ± 11.43 vs. 51.20 ± 11.34 years) or gender (F30/M12 vs. F13/M7). Compared to the CT group, Fasting Blood Glucose (FBG), Postprandial Blood Glucose (PBG), Fasting Plasma Insulin (FIN), Postprandial Plasma Insulin (PIN), HbA1c, ln HOMA-IR, TG, TC, HDL, and LDL levels were not significantly different between the CT and IT groups (all p > 0.05, Table 1).
Table 1. Changes of serum glucose, insulin, HOMA-IR, and blood lipids.
| Variables | CT group (n = 20) | IT group (n = 22) | Control group (n = 20) | ||
|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| FBG (mmol/L) | 7.14 ± 1.97 | 6.37 ± 1.40** | 6.84 ± 1.42 | 5.78 ± 0.48** | 5.65 ± 0.41 |
| PBG (mmol/L) | 11.08 ± 2.63 | 9.00 ± 1.91** | 11.30 ± 2.17 | 7.30 ± 0.98** ΔΔ | 6.26 ± 1.04 |
| FIN (μU/mL) | 12.81 ± 6.16 | 11.65 ± 5.26 | 13.40 ± 5.11 | 9.04 ± 3.19** | 9.19 ± 3.73 |
| PIN (μU/mL) | 94.54 ± 46.05 | 69.67 ± 31.30** | 83.66 ± 30.75 | 36.16 ± 18.26**Δ | 34.50 ± 11.74 |
| HbA1c (%) | 6.88 ± 0.76 | 6.44 ± 0.65** | 6.85 ± 1.14 | 5.89 ± 0.61** ΔΔ | 5.73 ± 0.31 |
| HOMA-IR | 4.23 ± 2.99 | 3.32 ± 1.73 | 4.11 ± 1.98 | 2.33 ± 0.92 | 2.30 ± 0.94 |
| Ln HOMA-IR | 1.26 ± 0.60 | 1.08 ± 0.50 | 1.33 ± 0.40 | 0.77 ± 0.40**Δ | 0.74 ± 0.43 |
| TG (mmol/L) | 1.99 ± 1.07 | 1.75 ± 0.76 | 2.20 ± 1.27 | 1.17 ± 0.35** Δ | 1.13 ± 0.31 |
| TC (mmol/L) | 4.62 ± 1.31 | 4.45 ± 1.12 | 5.53 ± 1.65 | 4.77 ± 1.03* | 3.91 ± 0.56 |
| HDL (mmol/L) | 1.27 ± 0.31 | 1.31 ± 0.30 | 1.38 ± 0.29 | 1.43 ± 0.26 | 1.38 ± 0.19 |
| LDL (mmol/L) | 2.58 ± 1.00 | 2.46 ± 1.02 | 3.22 ± 1.47 | 2.54 ± 1.22* | 2.08 ± 0.38 |
Data are mean ± SD. *p < 0.05, **p < 0.01 vs. pre-treatment; Δp < 0.05, ΔΔp < 0.01 vs. conventional therapy group. FBG: fasting blood glucose; FIN: fasting insulin; HbA1c: glycated hemoglobin; HOMA-IR: the index for homeostasis model assessment of insulin resistance; LDL: low-density lipoprotein; PBG: postprandial blood glucose (2-h); PIN: postprandial insulin (2-h); TC: total cholesterol; TG: triglycerides
In the CT group, FBG, PBG, PIN, and HbA1c decreased significantly after treatment (p <0.05, <0.01). Meanwhile, FBG, PBG, FIN, PIN, HbA1c, and ln HOMA-IR decreased significantly in the IT group, (all p < 0.01). PBG, PIN, HbA1c, and ln HOMA-IR were significantly lower in the IT group than the CT group (p <0.01, <0.05, Table 1).
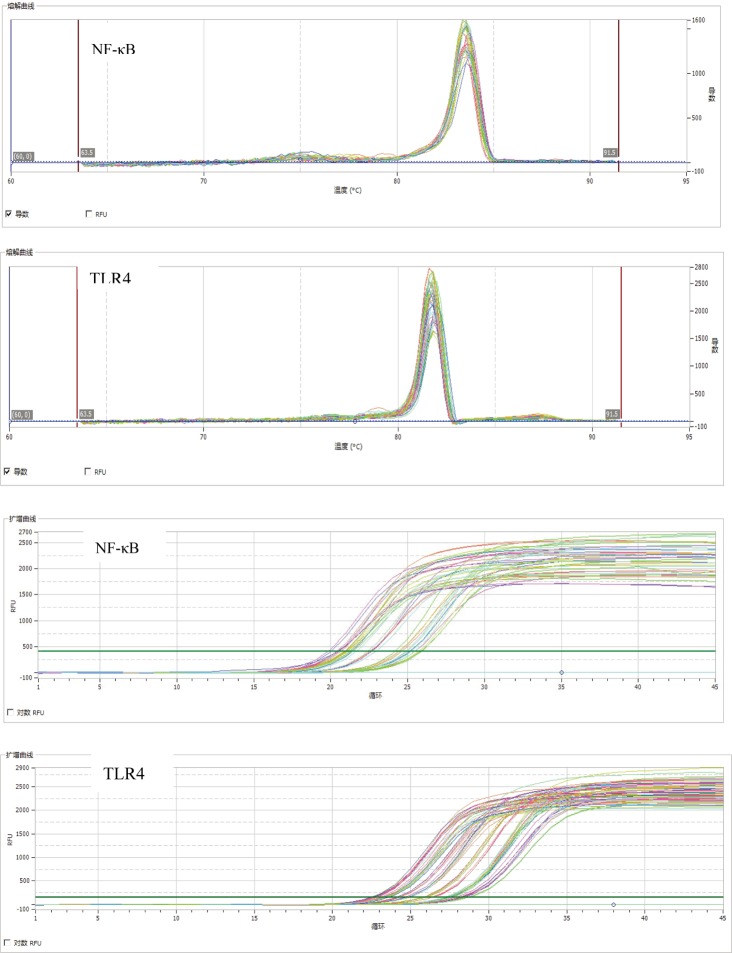
Before treatment, the mRNA and protein expression levels of TLR4 and NF-κBp65, and serum IL-18 level were significantly higher in the CT and IT groups than the control group, while IL-33 level were significantly lower in the CT and IT groups (p <0.05, <0.01). After the exercise intervention, the mRNA and protein expressions of TLR4 and NF-κBp65 were significantly lower in the IT group than the CT group (p <0.05, <0.01). The melting and amplification curves of TLR4 and NF-κB mRNA detected by qPCR are shown in Fig. 1. Serum IL-18 level was significantly lower in the IT group than the CT group (p < 0.05). Meanwhile, the serum IL-33 level was significantly higher in the IT group than the CT group but lower than that in the control group (p < 0.05). There was no significant difference in the expression levels of PTX3 or YKL40 among the three groups (Table 2 and Fig. 2).
Fig.1.
Melting and amplification curves of TLR4 mRNA and NF-κB mRNA detected by qPCR. NF-κB: Nuclear factor-κB; TLR4: Toll-like receptor 4
Table 2. Effects of exercise intervention on the inflammatory factors in diabetes patients.
| Items | CT group (n = 20) | IT group (n = 22) | Control group (n = 20) | ||
|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| TLR4 mRNA | 1.68 ± 0.29** | 0.77 ± 0.18 | 1.85 ± 0.31** | 0.54 ± 0.63**Δ | 1.05 ± 0.18 |
| NF-κBp65mRNA | 1.28 ± 0.26** | 1.01 ± 0.11 | 1.41 ± 1.77** | 0.86 ± 0.66Δ | 0.86 ± 0.12 |
| TLR4 protein | 0.95 ± 0.34** | 0.67 ± 0.33* | 0.86 ± 0.25** | 0.41 ± 0.16Δ | 0. 45 ± 0.17 |
| NF-κBp65 protein | 0.88 ± 0.36** | 0.64 ± 0.25* | 0.85 ± 0.24** | 0.49 ± 0.21ΔΔ | 0.45 ± 0.21 |
| YKL40 | 0.41 ± 0.11 | 0.34 ± 0.21 | 0.31 ± 0.14 | 0. 28 ± 0.09 | 0.27 ± 0.11 |
| IL-18 | 0.80 ± 0.12** | 0.72 ± 0.12* | 0.81 ± 0.09** | 0.51 ± 0.10Δ | 0.59 ± 0.31 |
| PTX3 | 0.63 ± 0.13 | 0.63 ± 0.14 | 0.64 ± 0.15 | 0.58 ± 0.08 | 0.54 ± 0.78 |
| IL-33 | 0.40 ± 0.09** | 0.65 ± 0.12* | 0.42 ± 0.14** | 0.85 ± 0.14*ΔΔ | 1.38 ± 0.19 |
Data are mean ± SD, *p < 0.05, **p < 0.01 vs. control group; Δp < 0.05, ΔΔp < 0.01 vs. conventional therapy group. CT group: the conventional therapy group; IT group: the intensive therapy group; IL-18: interleukin-18; IL-33: interleukin-33; NF-κBp65: Nuclear factor-κBp65; PTX3: pentraxin-related protein 3; TLR4: Toll-like receptor 4; YKL40: human cartilage glycoprotein 39
Fig. 2.
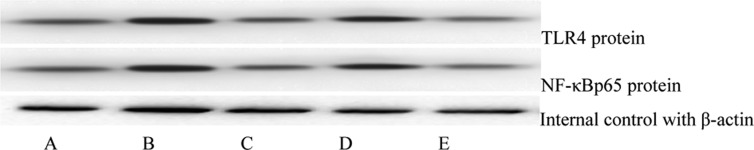
Changes in the protein expressions of TLR4 and NF-κBp65 before and after the intervention. A: control group; B: conventional therapy group before intervention; C: conventional therapy group after intervention; D: intensive-therapy group before intervention; E: intensive therapy group after intervention. NF-κBp65: Nuclear factor-κBp65; LR4: Toll-like receptor 4
DISCUSSION
Exercise therapy is an effective method for treating diabetes mellitus. Many studies show aerobic exercise including home-based exercise decreases blood glucose, lipid, and HbA1c levels as well as improves insulin sensitivity, hypercoagulability state, quality of life, and physical condition in T2DM patients20,21,22,23,24,25). In the present study, T2DM patients performed a combination of aerobic exercises and resistance training for 12 weeks. The results show significant improvements in the fasting and postprandial blood glucose, HbA1c, TG, TC, LDL cholesterol, and IR in the intensive-therapy group after 12 weeks of therapy compared to conventional therapy. Balducci et al.26) report that compared to conventional therapies, a 1 year exercise intervention significantly reduced the blood glucose, TG, TC, and LDL cholesterol levels of patients with T2DM while significantly increasing HDL cholesterol level. Tokmakidis et al.27) report that a 4-month exercise intervention significantly reduced the fasting and (2-hour) postprandial blood glucose, insulin, and HbA1c levels of diabetes patients. Thus, the present results are concordant with those of Balducci et al. and Tokmakidis et al. Among diabetes patients, regular exercise has a better blood glucose-controlling effect, because it improves insulin sensitivity. Moreover, regular exercise enhances blood pressure and blood lipid control, thus reducing the risks of large vascular and microvascular complications.
Despite the attention of many researchers for a long time, the underlying pathogenic mechanism of diabetes mellitus remains unclear. The latest studies indicate a close association between the pathogenesis of diabetes mellitus and a chronic low-grade inflammatory response. In the present study, the mRNA and protein expression levels of TLR4 and NF-κB in mononuclear cells as well as serum levels of the inflammatory factor IL-18 were higher in T2DM patients than healthy controls. However, the expression level of the protective cytokine IL-33 was lower in T2DM patients than control group. Meanwhile, the serum inflammatory factors PTX3 and YKL40 did not differ significantly between T2DM patients and controls. These results indicate TLR4, NF-κB, IL-18, and IL-33 may play important roles in the pathogenesis of diabetes mellitus. Previous studies report that patients with diabetes mellitus have higher TLR4 mRNA and protein expression in mononuclear cells and that these changes are closely associated with blood glucose and HbA1c levels28). Another study reports the same findings in patients with metabolic syndrome29). TLR4 activates several cell signaling pathways, particularly the TLR4/NF-κB pathway. NF-κB subsequently activates a series of inflammatory factors including TNF-α, IL-1, IL-6, and IL-8 and induces the expressions of several adherence factors and chemokines by mononuclear macrophages and endothelial cells30). This cascade ultimately results in IR and secretary dysfunction of insulin in the body. IL-18 is mainly secreted by mononuclear macrophages and may be important for generating an inflammatory response31). Some studies report diabetes patients have elevated plasma IL-18 expression, which is closely associated with fasting blood glucose and HbA1c levels32). In addition, elevated IL-18 expression is reported to be an independent risk factor for the development of diabetes mellitus33). IL-33, a member of the IL-1 cytokine family, is released after cell damage or as a negative regulator of NF-κB gene transcription; it not only enhances cytokine production by T helper cells, but also reduces the activities of natural killer cells and the generation of oxidation products; therefore, it is recognized as a protective factor against IR, diabetes mellitus, and obesity. Elevated IL-33 expression improves fasting blood glucose and glucose tolerance34, 35). On the contrary, the removal of IL-33 may increase the cytotoxicity of natural killer cells and trigger inflammatory factors such as TNF-α, possibly resulting in IR36). These studies are consistent with the results of the present study. Although some studies show PTX3 and YKL40 are associated with the onset and progression of diabetes37, 38), we found no significant increases of their expression levels. This discrepancy is probably due to the variation in the duration and type of disease of our patients. The present results indicate diabetes patients exhibit increased expressions of some inflammatory factors and decreased expressions of protective cytokines, indicating a close relationship between diabetes mellitus and a chronic low-grade inflammatory response.
Long-term regular exercise can reduce chronic low-level inflammation in humans, and an exercise-containing lifestyle may also delay the genesis and development of T2DM3, 39). There was research also demontrated that the home-based exercise improved the metabolic asset and reduced the systemic inflammation of sedentary people40). Another study proved that home-based exercise could improve clinical features and systemic inflammation of patients with chronic obstructive pulmonary disease41). In the present study, the intensive intervention program for diabetes patients included a 12-week moderate-intensity workout program consisting of aerobic exercise and resistance training (i.e., the IT group). After exercise training, the IT group had significantly lower TLR4 and NF-κB mRNA and protein expressions as well as serum IL-18 levels than the CT group. Meanwhile, the serum level of IL-33 (a protective cytokine) was significantly higher in the IT group than the CT group but lower than that in the control group. Exercise can significantly reduce TLR4 expression on the surface of mononuclear cells in non-diabetes patients42, 43). Lambert et al. also report a similar reduction in elderly obese patients following long-term exercise therapy44). Other studies also demonstrate that exercise reduces IL-18 levels in elderly people and patients with metabolic syndrome45, 46). Furthermore, regular exercise increases the expressions of heat shock proteins and IL-33 by vascular endothelial cells. These findings are concordant with the results of the present study. The present study suggests the anti-inflammatory mechanism of long-term medium-intensity exercise may be associated with decreased mRNA and protein expressions of TLR4 and NF-κB, and the inflammatory factor IL-18 as well as elevated levels of the anti-inflammatory factor IL-33, all of which improve IR and the outcome of diabetes.
This study has some limitations that should be mentioned. As the sample size was relatively small, our results may be affected by some biases. Moreover, the mechanism by which exercise intervention alters the gene expressions of inflammatory factors remains unclear. Therefore, further animal and cellular experiments must be conducted to clarify the underlying mechanism of exercise intervention.
In conclusion, the present study demonstrates the existence of low-grade chronic inflammation in diabetes patients and that long-term medium-intensity exercise can not only decrease the mRNA and protein expressions of TLR4 and NF-κB, but can also reduce the level of the inflammatory factor IL-18 and increase the level of the anti-inflammatory factor IL-33. Thus, long-term moderate-intensity exercise can resolve inflammatory response, improve IR, and achieve better blood glucose control in T2DM patients. Hence, exercise intervention can minimize the incidence of T2DM-associated complications. Therefore, exercise therapy should be widely recommended to patients with T2DM.
Acknowledgments
The authors acknowledge the contributions of all the clinical staff members of the Cardiac Rehabilitation Center of Xiangya Hospital. We also thank the Lihua Jiang, Lihue Deng, Cui Li, Fan Zheng, and Zhuo Chen, who helped with data collection.
REFERENCES
- 1.Duncan BB, Schmidt MI: The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther, 2006, 8: 7–17. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan AD, Manson JE, Rifai N, et al. : C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA, 2001, 286: 327–334. [DOI] [PubMed] [Google Scholar]
- 3.de Lemos ET, Oliveira J, Pinheiro JP, et al. : Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev, 2012, 2012: 741545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt C, Pedersen BK: The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol, 2010, 2010: 520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest, 2005, 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen AM, Pedersen BK: The anti-inflammatory effect of exercise. J Appl Physiol 1985, 2005, 98: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 7.Jae SY, Fernhall B, Heffernan KS, et al. : Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism, 2006, 55: 825–831. [DOI] [PubMed] [Google Scholar]
- 8.Beavers KM, Brinkley TE, Nicklas BJ: Effect of exercise training on chronic inflammation. Clin Chim Acta, 2010, 411: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amy EM, Rob D, Frank M, et al. : A 12-week sports-based exercise programme for inactive Indigenous Australian men improved clinical risk factors associated with type 2 diabetes mellitus. J Sci Med Sport, 2014, 06: 1052–1057. [DOI] [PubMed] [Google Scholar]
- 10.Kim DY, Seo BD, Kim DJ: Effect of walking exercise on changes in cardiorespiratory fitness, metabolic syndrome markers, and high-molecular-weight Adiponectin in obese middle-aged women. J Phys Ther Sci, 2014, 26: 1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balducci S, Zanuso S, Nicolucci A, et al. : Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis, 2010, 20: 608–617. [DOI] [PubMed] [Google Scholar]
- 12.Bouloumié A, Curat CA, Sengenès C, et al. : Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care, 2005, 8: 347–354. [DOI] [PubMed] [Google Scholar]
- 13.Romzova M, Hohenadel D, Kolostova K, et al. : NFkappaB and its inhibitor IkappaB in relation to type 2 diabetes and its microvascular and atherosclerotic complications. Hum Immunol, 2006, 67: 706–713. [DOI] [PubMed] [Google Scholar]
- 14.Borg GA: Psychophysical bases of perceived exertion. Med Sci Sports Exerc, 1982, 14: 377–381. [PubMed] [Google Scholar]
- 15.Pollock ML, Broida J, Kendrick Z: Validity of the palpation technique of heart rate determination and its estimation of training heart rate. Res Q, 1972, 43: 77–81. [PubMed] [Google Scholar]
- 16.Koltyn KF, Morgan WP: Efficacy of perceptual versus heart rate monitoring in the development of endurance. Br J Sports Med, 1992, 26: 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MA, Haskell WL, Ades PA, et al. American Heart Association Council on Clinical CardiologyAmerican Heart Association Council on Nutrition, Physical Activity, and Metabolism: Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation, 2007, 116: 572–584. [DOI] [PubMed] [Google Scholar]
- 18.Wu YT, Hwang CL, Chen CN, et al. : Home-based exercise for middle-aged Chinese at diabetic risk: a randomized controlled trial. Prev Med, 2011, 52: 337–343. [DOI] [PubMed] [Google Scholar]
- 19.Bonora E, Targher G, Alberiche M, et al. : Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care, 2000, 23: 57–63. [DOI] [PubMed] [Google Scholar]
- 20.Heo M, Kim E: Effects of endurance training on lipid metabolism and glycosylated hemoglobin levels in Streptozotocin-induced type 2 diabetic rats on a high-fat diet. J Phys Ther Sci, 2013, 25: 989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dengel DR, Pratley RE, Hagberg JM, et al. : Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 1985, 1996, 81: 318–325. [DOI] [PubMed] [Google Scholar]
- 22.Tamura T, KidA K, Seki T, et al.: Study of the relationship between exercise therapy and diet therapy in type 2 diabetes mellitus patients. J Phys Ther Sci, 2011, 23: 485–488. [Google Scholar]
- 23.Lucha-López MO, Lucha-López AC, Vidal-Peracho C, et al. : Study of the relationship between exercise therapy and diet therapy in type 2 diabetes mellitus patients. J Phys Ther Sci, 2012, 24: 1299–1305. [Google Scholar]
- 24.Krousel-Wood MA, Berger L, Jiang X, et al. : Does home-based exercise improve body mass index in patients with type 2 diabetes? Results of a feasibility trial. Diabetes Res Clin Pract, 2008, 79: 230–236. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer-García JC, Sánchez López P, Pablos-Abella C, et al. : [Benefits of a home-based physical exercise program in elderly subjects with type 2 diabetes mellitus]. Endocrinol Nutr, 2011, 58: 387–394. [DOI] [PubMed] [Google Scholar]
- 26.Rathcke CN, Johansen JS, Vestergaard H: YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res, 2006, 55: 53–59. [DOI] [PubMed] [Google Scholar]
- 27.Tokmakidis SP, Touvra AM, Douda HT, et al. : Training, detraining, and retraining effects on glycemic control and physical fitness in women with type 2 diabetes. Horm Metab Res, 2014, 46: 974–979. [DOI] [PubMed] [Google Scholar]
- 28.Dasu MR, Devaraj S, Park S, et al. : Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care, 2010, 33: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jialal I, Huet BA, Kaur H, et al. : Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care, 2012, 35: 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adaikalakoteswari A, Rema M, Mohan V, et al. : Oxidative DNA damage and augmentation of poly(ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem Cell Biol, 2007, 39: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 31.Gracie JA, Robertson SE, McInnes IB: Interleukin-18. J Leukoc Biol, 2003, 73: 213–224. [DOI] [PubMed] [Google Scholar]
- 32.Moriwaki Y, Yamamoto T, Shibutani Y, et al. : Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism, 2003, 52: 605–608. [DOI] [PubMed] [Google Scholar]
- 33.Thorand B, Kolb H, Baumert J, et al. : Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes, 2005, 54: 2932–2938. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Liew FY: The IL-33/ST2 pathway—a new therapeutic target in cardiovascular disease. Pharmacol Ther, 2011, 131: 179–186. [DOI] [PubMed] [Google Scholar]
- 35.Miller AM, Asquith DL, Hueber AJ, et al. : Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res, 2010, 107: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milovanovic M, Volarevic V, Radosavljevic G, et al. : IL-33/ST2 axis in inflammation and immunopathology. Immunol Res, 2012, 52: 89–99. [DOI] [PubMed] [Google Scholar]
- 37.Yang HS, Woo JE, Lee SJ, et al. : Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci, 2014, 55: 5989–5997. [DOI] [PubMed] [Google Scholar]
- 38.Hempen M, Kopp HP, Elhenicky M, et al. : YKL-40 is elevated in morbidly obese patients and declines after weight loss. Obes Surg, 2009, 19: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 39.Bai HP, Gao QJ: Effects of swimming exercise on inflammation and oxidative stress status in diabetic mellitus type2 rats. Chin J Rehabilit Med, 2013, 28: 708–713. [Google Scholar]
- 40.Di Raimondo D, Tuttolomondo A, Buttà C, et al. : Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int J Clin Pract, 2013, 67: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 41.do Nascimento ES, Sampaio LM, Peixoto-Souza FS, et al. : Home-based pulmonary rehabilitation improves clinical features and systemic inflammation in chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis, 2015, 10: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel T, Emslander I, Sisic Z, et al. : Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol, 2012, 112: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 43.McFarlin BK, Flynn MG, Campbell WW, et al. : Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci, 2006, 61: 388–393. [DOI] [PubMed] [Google Scholar]
- 44.Lambert CP, Wright NR, Finck BN, et al. : Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 1985, 2008, 105: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohut ML, McCann DA, Russell DW, et al. : Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun, 2006, 20: 201–209. [DOI] [PubMed] [Google Scholar]
- 46.Trøseid M, Lappegård KT, Mollnes TE, et al. : The effect of exercise on serum levels of interleukin-18 and components of the metabolic syndrome. Metab Syndr Relat Disord, 2009, 7: 579–584. [DOI] [PubMed] [Google Scholar]