Abstract
Exophiala species are capable of causing cutaneous and subcutaneous infections in immunocompromised patients. An Exophiala isolate was cultured from a biopsy specimen of a lesion on the forearm of a patient with myasthenia gravis. The patient also had lesions on the palm and distal aspects of the hand, which were successfully treated with a long-term course of itraconazole. A detailed morphological and molecular characterization of the isolate was undertaken. Phylogenetic analysis of the internal transcribed spacer region and portions of the β-tubulin and translation elongation factor 1-alpha genes indicated that the isolate was a novel species closely related to but genetically distinct from species within the Exophiala spinifera clade; the name Exophiala polymorpha sp. nov. is proposed. Morphologically, E. polymorpha most closely resembles E. xenobiotica but it differs in possessing phialides bearing prominent, wide collarettes, and it does not produce chlamydospores.
INTRODUCTION
Exophiala species are phaeoid, conidial fungi that are widely distributed in decaying wood, soil, plants, and water. They are capable of causing phaeohyphomycosis, including localized infections following traumatic implantation to disseminated disease (1, 2). Although cutaneous/subcutaneous phaeohyphomycosis incited by Exophiala species is relatively uncommon, several species of black yeasts identified by molecular means have been reported as etiologic agents. In the largest study to date in which 188 clinical isolates were characterized by internal transcribed spacer (ITS) sequencing and phenotypic features (3), 38.3% and 0.5% of the study isolates were from cutaneous and superficial sites, respectively. Subcutaneous isolates represented 12% of the strains and included Exophiala bergeri, E. dermatitidis, E. jeanselmei, E. lecanii-corni, E. mesophila, E oligosperma, E. phaeomuriformis, E. spinifera, and E. xenobiotica. While limited clinical information precluded documentation of these isolates as etiologic agents, their recovery and characterization highlighted the diversity of species seen in the clinical setting. A sampling of literature reports shows some of the same species seen in the above study, including E. oligosperma as an agent of olecranon bursitis (4), disseminated subcutaneous disease in a patient with systemic lupus erythematosus (5), and a patient with Wegener's granulomatosis (6), multiple reports of E. spinifera cutaneous/subcutaneous disease (7, 8), and E. xenobiotica in a patient with non-Hodgkin lymphoma (9). We report here subcutaneous sporotrichoid lesions by Exophiala polymorpha, sp. nov. in a patient with myasthenia gravis.
CASE REPORT
The patient is a 68-year-old African-American woman who complained of a right palmar painless nodule of about 6 months' duration prior to presentation. She reported that the lesion started like a callus in the middle of her right palm. This lesion gradually enlarged, and she started having sporotrichoid nodular lesions developing on the dorsum of her hand, wrist, forearm, and antecubital area. She did not have a fever or other systemic symptoms, nor did she recall any puncture wound or trauma to the hand.
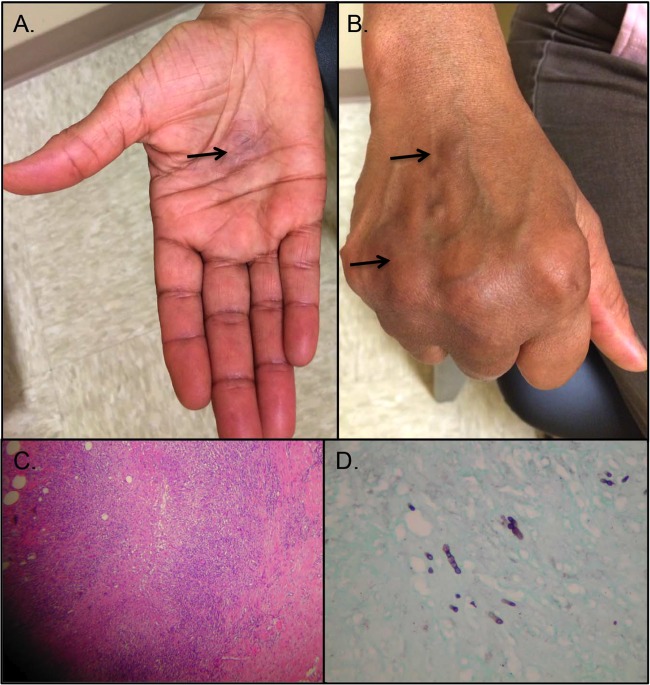
The patient had myasthenia gravis for which she was receiving 20 mg/day prednisone and pyridostigmine. She frequently did yard work around her house but did not have any pets and denied any animal contact. No travel history and no toxic habits were noted. On physical examination, there was a nodular lesion, 2 cm in diameter, at the palmar aspect of the mid-third to fourth metacarpal soft tissue (Fig. 1A). There was no significant rubor or calor at the lesion, which was firm and nontender. There were firm cutaneous nodules noted at the dorsal aspect of the third metacarpal soft tissues, as well as overlying the fourth metacarpophalangeal joint (Fig. 1B). Similar nodules were palpated at the medial wrist, volar forearm, and medial antecubital area. Her blood count and basic metabolic panel were within normal limits. Incision and drainage were performed at the palmar lesion, and she received oral antibacterial therapy. The palmar wound did not heal, and the lesion at the ventral aspect of the forearm became larger. An excisional biopsy was performed on the forearm lesion, and histopathological examination showed a granulomatous inflammatory process with some caseation in some of the granulomas (Fig. 1C). Hyphal and oval budding fungi were observed (Fig. 1D), and a culture of the tissue specimen grew an Exophiala species.
FIG 1.
Physical presentation and histopathology. A 2-cm-diameter nodular lesion at the palmar aspect of the mid-third to fourth metacarpal soft tissue (A, arrow) and firm cutaneous nodules noted at the dorsal aspect of third metacarpal soft tissues (B, arrow) and overlying the fourth metacarpophalangeal joint (arrow). Histopathological examination of the specimen obtained from an excisional biopsy showed granulomatous inflammatory process with some caseation in some of the granulomas by hematoxylin and eosin staining (C) and moniliform fungal elements by Gomori methenamine silver staining (D).
The isolate was forwarded to the Fungus Testing Laboratory in the Department of Pathology at the University of Texas Health Science Center at San Antonio (UTHSCSA) for species identification and antifungal susceptibility testing, where it was accessioned in the culture collection as UTHSCSA DI14-255. There the isolate was characterized by combined assessment of morphological characteristics and DNA sequence analysis. Microscopic features noted after 10 days of incubation at 25°C on a potato flake agar slide culture plate, prepared in-house, included both annellidic as well as phialidic conidiogenesis, with phialides displaying prominent collarettes and periclinal thickenings; physiologic tests demonstrated a positive nitrate assimilation test, and growth occurred at 37°C but no growth occurred at 40°C. For DNA extraction, a portion of the cultured isolate was suspended in buffer G2 (Qiagen, Valencia, CA) and lysed in a bead beater instrument (BioSpec Products, Inc., Barlesville, OK). Proteinase K was then added, and the samples were incubated at 56°C for 1 h. After incubation, the DNA was extracted from the sample using an EZ1 DNA tissue kit on a BioRobot EZ1 instrument (Qiagen) according to the manufacturer's instructions. Extracted DNA was used for PCR amplification of the ITS and D1/D2 ribosomal DNA (rDNA) regions as described previously with a slight modification (10). PCR products were then sequenced using the ITS1, ITS4, NL1, and NL4 primers at the UTHSCSA Molecular Diagnostics Laboratory (11). Sequences were assembled and analyzed using DNAStar SeqMan Pro version 9.1 (DNAStar, Inc., Madison, WI) and queried in GenBank using the BLASTn algorithm at the NCBI site (www.ncbi.nlm.nih.gov). The sequences were also compared to those available in the CBS-KNAW Fungal Biodiversity Centre database (www.cbs.knaw.nl). Sequence analysis of the D1/D2 rDNA showed the highest matches to several Exophiala spp. within the CBS-KNAW database, including E. dermatitidis (CBS 134003), E. exophialae (CBS 668.76), E. jeanselmei (CBS 507.90), E. moniliae (CBS 520.76), E. nishimurae (CBS 101538), E. oligosperma (CBS 265.49), and E. spinifera (CBS 101542), with the percent identity to each ranging from 97.1% to 98.4%. However, the ITS sequence results were not informative, as all matches were 91% or lower. Antifungal susceptibility testing was also performed according to the methods described in the Clinical and Laboratory Standards Institute (CLSI) M38-A2 standard (12). The MICs of itraconazole, posaconazole, and voriconazole against this isolate were 0.5 μg/ml for each, and the MIC was 16 μg/ml for fluconazole. The patient was treated with itraconazole (200 mg orally twice a day) for 9 months with resolution of the nodular lesions. As the isolate did not match any of the previously described Exophiala spp. by phenotypic features or ITS and D1/D2 rDNA sequence analysis, it was forward to the Universitat Rovira i Virgili in Reus, Spain, for further characterization under their accession number FMR 13569. These methods and results are described below.
MATERIALS AND METHODS
Phenotypic studies.
The isolate was grown on potato dextrose agar (PDA) (Pronadisa, Spain), malt extract agar (MEA) (30 g of malt extract, 5 g of peptone, 15 g of agar, 1 liter of distilled water), and oatmeal agar (OA) (30 g of filtered oat flakes, 20 g of agar), and colony features and growth rates were determined at 7, 14, and 28 days of incubation at different temperatures (5, 15, 25, 35, 37, and 40°C). Color notations used in the descriptions were from Kornerup and Wanscher (13). Morphological features were examined on MEA after 28 days of incubation at 25°C with mounting in 85% lactic acid using light microscopy. Photomicrographs were obtained with a Zeiss Axio-Imager M1 light microscope, using Nomarski differential interference optics.
DNA extraction, amplification, and sequencing.
Total genomic DNA was extracted from mycelia scraped from colonies grown on MEA for 7 days at 25°C using FastPrep (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer's protocol. DNA was quantified using a NanoDrop 3000 instrument (Thermo Scientific, Madrid, Spain). Three nuclear DNA regions were amplified using the following primer pairs: ITS4/ITS5 for a fragment spanning the ITS-1, ITS-2, and the 5.8S rDNA gene (ITS) (11), BT2a/BT2b for a fragment of the β-tubulin gene (Tub) (14), and EF-728F/EF-986R for a fragment of the translation elongation factor 1-alpha gene (Tef) (15). Amplicons were sequenced in both directions with the same primer pair used for amplification at Macrogen Europe (Macrogen Inc., Amsterdam, The Netherlands). Consensus sequences were obtained using SeqMan version 7.0.0 (DNAStar Lasergene, Madison, WI, USA).
Phylogenetic analyses.
Sequences for each locus were aligned with MEGA version 5.05 using ClustalW and manually improved when necessary (16, 17). Phylogenetic reconstructions of each data set were made using maximum likelihood (ML) with Mega 5.05 and Bayesian inference (BI) with MrBayes version 3.1.2 (18). For ML analysis, nearest neighbor interchange was used as the heuristic method, gaps were treated as partial deletions with a 95% site coverage cutoff, the branch robustness was estimated using a 1,000 ML bootstrapped set of data; bootstrap values (bs) of >70% were considered significant. For the BI analysis, two parallel runs of four incrementally heated Markov chains were performed for 3 million generations with a sample frequency of 100 generations. The 50% majority rule consensus tree and Bayesian posterior probability values (pp) were calculated from 22,500 samples after removal of the first 25% for burn-in; pp values of >0.95 were considered significant. For each data partition, the best nucleotide substitution model (GTR + G + I for ITS, K2 + G + I for Tub, and K2 + G for Tef) was estimated using the Find Best DNA/Protein tool implemented in Mega 5.05. Ninety-one sequences of type and reference Exophiala species strains retrieved from GenBank or the National Biological Resource Centre (NBRC) were included in the analyses. The resulting topologies for each locus were compared visually using a 70% bootstrap cutoff. Since no incongruence was found, the three data sets were combined into a single combined analysis.
Accession numbers.
Sequences generated in this study were deposited in GenBank under accession numbers KP070763, LN794350 and LN794351 for ITS, Tef, and Tub, respectively. The scientific name and description of the novel species were deposited in MycoBank under accession number MB 811792.
RESULTS
The Exophiala sp. UTHSCSA DI14-255 isolate produced olive to blackish-green colonies, at first membranous and formed mostly by oval to ellipsoidal budding yeast-like cells. At maturity, the colonies turned velvety to slightly felty in appearance, given the formation of abundant mycelia while no diffusible pigment was observed. Microscopically, annellidic conidiogenesis coexisted with phialidic conidiogenous cells.
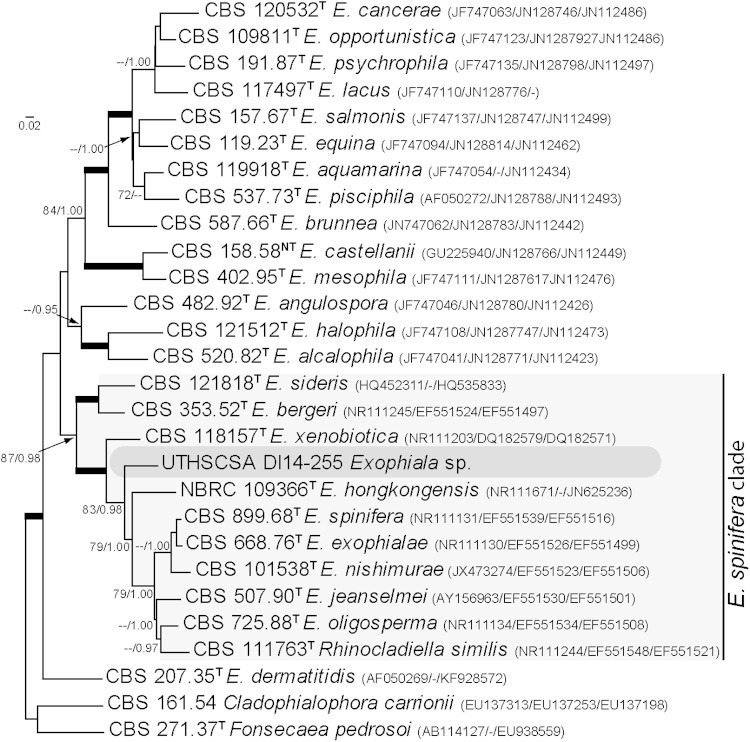
The combined phylogeny (Fig. 2) showed that isolate UTHSCSA DI14-255 formed a well-supported monotypic lineage among the members of the spinifera clade of Exophiala (19), close to the type strains of Exophiala exophialae (CBS 668.76), E. hongkongensis (NBRC 109366), E. spinifera (CBS 899.68), and E. xenobiotica (CBS 118157), although with a high genetic difference (91.1, 90.4, 90.4, and 90.6% sequence similarity, respectively). Given the isolate's striking morphological characteristics and genetic divergence we concluded that it belongs to an undescribed species here described as Exophiala polymorpha.
FIG 2.
Maximum likelihood trees inferred from ITS, Tub, and Tef sequences of Exophiala spp. and related genera. The branch lengths are proportional to the phylogenetic distance. ML bootstrap support values of ≥70% and posterior probability values of ≥0.95 are shown above the branches. Cladophialophora carrionii and Fonsecaea pedrosoi were used as outgroups. T, type strain; CBS, CBS-KNAW Fungal Biodiversity Centre culture collection, The Netherlands; NBRC, National Biological Resource Centre, Japan. Sequence accession numbers are indicated in parentheses.
TAXONOMY
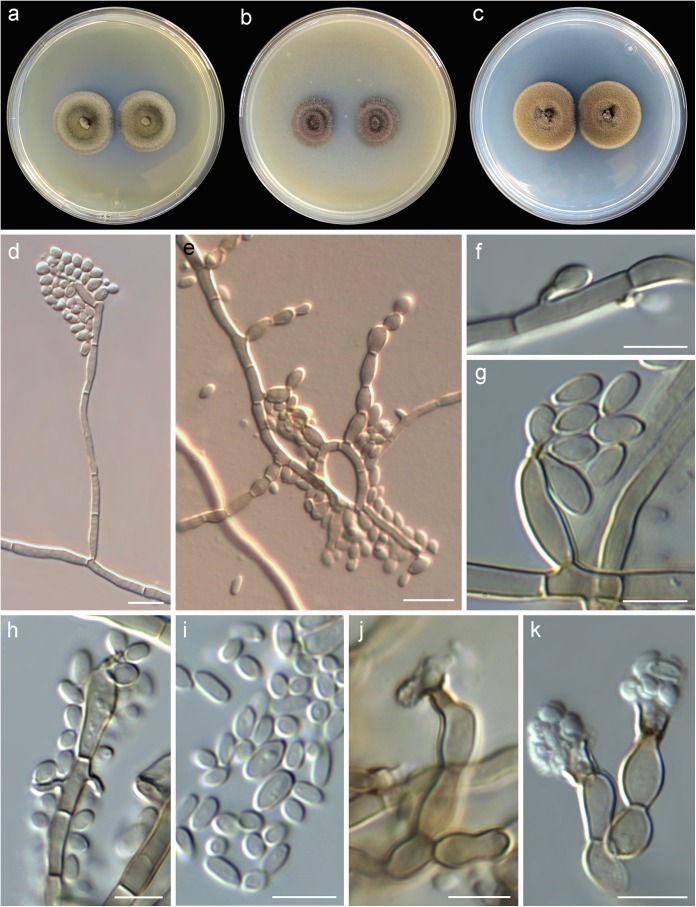
Exophiala polymorpha sp. nov. J. Guarro, M. Sandoval-Denis, Deanna A. Sutton, Nathan P. Wiederhold, MycoBank accession no. MB 811792. Etymology: from the Greek poly (many) and morphe (form), given the variable conidiogenous patterns. Diagnosis: the new species closely resembles E. xenobiotica, although it can be differentiated by the presence of phialides bearing prominent, wide collarettes and by the absence of chlamydospores. Colonies restricted, at first flat and slimy on all culture media tested (Fig. 3a to c). On PDA at 25°C, attaining 23 to 25 mm diameter in 28 days, flat or slightly raised and folded at the center, velvety to slightly granular toward the periphery, olive to olive-gray (2F3-F2) with entire margins; reverse dark green to greenish-gray (30F-F3). On MEA at 25°C attaining 19 to 25 mm diameter in 28 days, flat with central elevation, velvety, dark green to greenish-gray (30F2-F3) with entire margins; reverse dark green to greenish-gray (30F2-F3). On OA at 25°C attaining 12 to 15 mm diameter in 28 days, raised or crateriform, velvety to felty, olive gray (2F2), with entire margins; reverse dark green (30F3) to black. No diffusible pigment produced on any medium. Budding cells abundant in young cultures (up to day 7), oval to ellipsoidal, light olivaceous-green, 4 to 6 by 2.5 to 4 μm, often showing a short annellated zone, without capsule in India ink. Torulose mycelium present or absent. Hyphae greenish-brown, 1.5 to 3 μm wide, thick walled. Conidiophores poorly differentiated, short, erect, cylindrical, greenish-brown, smooth and thin walled, rising at right angles from the aerial hyphae and bearing clusters of conidia (Fig. 3d and e). Conidiogenous cells terminal or intercalary, cylindrical and tapering toward an apical annellated zone or borne solitary on aerial hyphae in the form or short annellated pegs and often forming next to a septum (Fig. 3f to h). Conidia one-celled, light green, oval, broadly ellipsoidal or nearly clavate 3.5 to 4.0 by 1.5 to 2.5 μm, smooth and thin walled often with a prominent basal scar (Fig. 3f to i). Chlamydospores absent. Phialophora-like conidial state present in mature cultures (28 days) (Fig. 3j and k). Conidiophores simple, often reduced to single conidiogenous cells borne laterally or terminally directly from the aerial hyphae, solitary or forming dense clusters. Conidiogenous cells flask shaped 6 to 9 by 3 to 4 μm, light greenish-brown distinctly swollen at the base and showing a conspicuous collarette and periclinal thickening, vasiform or irregularly cylindrical 2 to 5 by 2 to 3 μm, opening gradually toward the apex. Conidia obovoidal to nearly clavate 2 to 3 by 1.0 to 1.5 μm, subhyaline, smooth and thin walled. Cardinal temperatures for growth: optimum, 25°C; maximum, 37°C; minimum, 15°C. Holotype: USA, from human subcutaneous lesion, 2014, D. A. Sutton (CBS H-22004; ex-type cultures CBS 138920 = UTHSCSA DI14-255 = FMR 13569).
FIG 3.
Exophiala polymorpha sp. nov. (UTHSCSA DI14-255). (a to c) Colonies on MEA, OA, and PDA, respectively, after 7 days at 25°C; (d, e) conidiophores; (f to i) conidiogenous cells and conidia; (j, k) phialophora-like conidial state, phialides and conidia. Bars, = 10 μm (d, e) and 5 μm (f to k).
DISCUSSION
Most Exophiala species are highly pleomorphic, showing mostly variable features that are difficult to differentiate by means of morphological comparisons (20). Since the introduction of molecular tools, species recognition within and taxonomy of the genus have been improved considerably (21). However, conidiogenous structures and other morphological features still remain important for species recognition in Exophiala. Species in the E. spinifera clade are characterized by mostly annellidic conidiogenesis coexisting with sympodial or phialidic conidiogenous cells and lacking sexual known cycles (19, 22). In E. polymorpha, annellidic and phialidic conidiogenous patterns were observed. Moreover, a phialidic phialophora-like conidial state was observed in mature cultures. Phialidic conidiogenesis has been observed in other clinically important Exophiala species like E. jeanselmei. However, in E. polymorpha, phialides were quite distinct, formed in clusters, and possessed wide subhyaline collarettes.
As has been demonstrated before, phylogenetic analyses of ITS, Tef, and Tub loci showed different levels of resolution (21). The three genes accurately discriminated E. polymorpha from its closest relatives with Tef and Tub showing the highest genetic difference. However, given the genetic variability, availability of reference sequences, and relatively easy alignment of the ITS region, the ITS sequence target has proven to be an efficient tool for species discrimination in medically important black yeast.
Exophiala species are capable of causing both cutaneous/subcutaneous infections as well as deep mycoses. For example, E. dermatitidis has been shown to cause central nervous system infections in immunocompetent individuals in Asia and cutaneous and subcutaneous infections in immunocompromised individuals, as well as catheter-associated fungemia, endocarditis, lymphadenitis, peritonitis, and pneumonia and both invasive disease and colonization of the airways of cystic fibrosis patients (23–31). Other species that are associated with cutaneous and subcutaneous infections include E. xenobiotica, which was the most frequently detected black yeast from cutaneous sources in one report (3), and E. oligosperma (3, 5). These species are now recognized as emerging causes of cutaneous and subcutaneous tissue infections, especially in immunocompromised patients. As in the patient in this case report, subcutaneous phaeohyphomycosis usually presents with asymptomatic nodules found on the extremities. Our patient was at risk for fungal infection due to the daily use of prednisone for the treatment of myasthenia gravis. Although previous case reports of infections due to Exophiala species in patients with myasthenia gravis were not found in a search of the literature, there are reports of invasive fungal infections in patients with this disease. Some of these include dermatophytosis secondary to Trichophyton rubrum (6), Candida esophagitis (32), various cases of cryptococcal meningitis and cryptococcal cellulitis (33–35), and one case of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella (36).
In summary, this report characterizes a new species of Exophiala, E. polymorpha, with the potential to cause subcutaneous infections in immunocompromised patients. The patient in this case received extended treatment with itraconazole and responded well. Treatment of infections caused by Exophiala species often involves the use of triazoles, such as itraconazole, posaconazole, and voriconazole, which are sometimes combined with amphotericin B or surgical incision if possible (5, 31). However, the optimal treatment is unknown, as no comparative clinical trials have been conducted. Although success with voriconazole was reported in one case of a soft tissue infection due to E. oligosperma, this drug may not have been an option in the current patient, as this triazole has been reported to cause an exacerbation of myasthenia gravis, which was speculated to be due to blockage of nicotinic acetylcholine receptors by voriconazole (37).
ACKNOWLEDGMENTS
We thank Dora McCarthy for performing the antifungal susceptibility testing.
We declare no conflicts of interest.
REFERENCES
- 1.de Hoog GS, Guarro J, Gene J, Figueras MJ. 2011. Atlas of clinical fungi, 3rd ed Central bureau voor Schimmelcultures and Reus, Universitat Rovira I Virgili, Utrecht, The Netherlands. [Google Scholar]
- 2.Sudduth EJ, Crumbley AJ III, Farrar WE. 1992. Phaeohyphomycosis due to Exophiala species: clinical spectrum of disease in humans. Clin Infect Dis 15:639–644. doi: 10.1093/clind/15.4.639. [DOI] [PubMed] [Google Scholar]
- 3.Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. 2007. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol 45:3713–3720. doi: 10.1128/JCM.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossler AD, Richter SS, Chavez AJ, Vogelgesang SA, Sutton DA, Grooters AM, Rinaldi MG, de Hoog GS, Pfaller MA. 2003. Exophiala oligosperma causing olecranon bursitis. J Clin Microbiol 41:4779–4782. doi: 10.1128/JCM.41.10.4779-4782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimawi BH, Rimawi RH, Mirdamadi M, Steed LL, Marchell R, Sutton DA, Thompson EH, Wiederhold NP, Lindner JR, Boger MS. 2013. A case of Exophiala oligosperma successfully treated with voriconazole. Med Mycol Case Rep 2:144–147. doi: 10.1016/j.mmcr.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki Y, Ota K, Sato K, Nara S, Yagushi T, Nakano H, Sawamura D. 2013. Deep pseudocystic dermatophytosis caused by Trichophyton rubrum in a patient with myasthenia gravis. Acta Derm Venereol 93:358–359. doi: 10.2340/00015555-1452. [DOI] [PubMed] [Google Scholar]
- 7.Harris JE, Sutton DA, Rubin A, Wickes B, De Hoog GS, Kovarik C. 2009. Exophiala spinifera as a cause of cutaneous phaeohyphomycosis: case study and review of the literature. Med Mycol 47:87–93. doi: 10.1080/13693780802412611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radhakrishnan D, Jayalakshmi G, Madhumathy A, Banu ST, Geethalakshmi S, Sumathi G. 2010. Subcutaneous phaeohyphomycosis due to Exophiala spinifera in an immunocompromised host. Indian J Med Microbiol 28:396–399. doi: 10.4103/0255-0857.71838. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama Y, Nomura M, Yamanaka S, Ogawa Y, Kitajima Y. 2009. Subcutaneous phaeohyphomycosis caused by Exophiala xenobiotica in a non-Hodgkin lymphoma patient. Med Mycol 47:95–99. doi: 10.1080/13693780802526857. [DOI] [PubMed] [Google Scholar]
- 10.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 48:741–752. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, New York. [Google Scholar]
- 12.Clinical Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Kornerup A, Wanscher JH. 1978. Methuen handbook of colour, 3rd ed Methuen, London, United Kingdom. [Google Scholar]
- 14.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556. doi: 10.2307/3761358. [DOI] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson JD, Higgins DG, Gibson TJ. 1994. Clustal-W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 19.Haase G, Sonntag L, Melzer-Krick B, de Hoog GS. 1999. Phylogenetic inference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Stud Mycol 43:80–97. [Google Scholar]
- 20.Heinrichs G, de Hoog GS, Haase G. 2012. Barcode identifiers as a practical tool for reliable species assignment of medically important black yeast species. J Clin Microbiol 50:3023–3030. doi: 10.1128/JCM.00574-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Hoog GS, Zeng JS, Harrak MJ, Sutton DA. 2006. Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie Van Leeuwenhoek 90:257–268. doi: 10.1007/s10482-006-9080-z. [DOI] [PubMed] [Google Scholar]
- 22.Zeng JS, De Hoog GS. 2008. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med Mycol 46:193–208. doi: 10.1080/13693780701799217. [DOI] [PubMed] [Google Scholar]
- 23.Chang CL, Kim DS, Park DJ, Kim HJ, Lee CH, Shin JH. 2000. Acute cerebral phaeohyphomycosis due to Wangiella dermatitidis accompanied by cerebrospinal fluid eosinophilia. J Clin Microbiol 38:1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto T, Nishimoto K, Kimura K, Padhye AA, Ajello L, McGinnis MR. 1984. Phaeohyphomycosis caused by Exophiala moniliae. Sabouraudia 22:17–26. doi: 10.1080/00362178485380051. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Padhye AA, Ajello L, Standard PG, McGinnis MR. 1984. Critical review of human isolates of Wangiella dermatitidis. Mycologia 76:232–249. doi: 10.2307/3793099. [DOI] [Google Scholar]
- 26.Alabaz D, Kibar F, Arikan S, Sancak B, Celik U, Aksaray N, Turgut M. 2009. Systemic phaeohyphomycosis due to Exophiala (Wangiella) in an immunocompetent child. Med Mycol 47:653–657. doi: 10.1080/13693780802715815. [DOI] [PubMed] [Google Scholar]
- 27.Hohl PE, Holley HP Jr, Prevost E, Ajello L, Padhye AA. 1983. Infections due to Wangiella dermatitidis in humans: report of the first documented case from the United States and a review of the literature. Rev Infect Dis 5:854–864. doi: 10.1093/clinids/5.5.854. [DOI] [PubMed] [Google Scholar]
- 28.Pihet M, Carrere J, Cimon B, Chabasse D, Delhaes L, Symoens F, Bouchara JP. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med Mycol 47:387–397. doi: 10.1080/13693780802609604. [DOI] [PubMed] [Google Scholar]
- 29.Haase G, Skopnik H, Groten T, Kusenbach G, Posselt HG. 1991. Long-term fungal cultures from sputum of patients with cystic fibrosis. Mycoses 34:373–376. [DOI] [PubMed] [Google Scholar]
- 30.Horre R, Schaal KP, Siekmeier R, Sterzik B, de Hoog GS, Schnitzler N. 2004. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration 71:360–366. doi: 10.1159/000079640. [DOI] [PubMed] [Google Scholar]
- 31.Patel AK, Patel KK, Darji P, Singh R, Shivaprakash MR, Chakrabarti A. 2013. Exophiala dermatitidis endocarditis on native aortic valve in a postrenal transplant patient and review of literature on E. dermatitidis infections. Mycoses 56:365–372. doi: 10.1111/myc.12009. [DOI] [PubMed] [Google Scholar]
- 32.Ebert S, Schweiger KP, Nau R. 2011. Candida esophagitis as the cause of swallowing disturbances in an 85-year-old patient with myasthenia gravis. Z Gerontol Geriatr 44:268–269. doi: 10.1007/s00391-011-0213-2. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzoni PJ, Scola RH, Kay CS, Almeida SM, Muro MD, Burigo IP, Carraro H Jr, Werneck LC. 2011. Myasthenia gravis complicated with cryptococcal meningitis after thymectomy and long-term immunosuppressive therapy. Arq Neuropsiquiatr 69:410–411. doi: 10.1590/S0004-282X2011000300031. [DOI] [PubMed] [Google Scholar]
- 34.Lafleur L, Beaty S, Colome-Grimmer MI, LaForte RA, Dotson AD. 2004. Cryptococcal cellulitis in a patient on prednisone monotherapy for myasthenia gravis. Cutis 74:165–170. [PubMed] [Google Scholar]
- 35.Rowland LP, Griffiths CO, Kabat EA. 1965. Myasthenia gravis, thymoma and cryptococcal meningitis. N Engl J Med 273:620–627. doi: 10.1056/NEJM196509162731202. [DOI] [PubMed] [Google Scholar]
- 36.Baker JG, Salkin IF, Forgacs P, Haines JH, Kemna ME. 1987. First report of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella. J Clin Microbiol 25:2395–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzam R, Shaikh AG, Serra A, Katirji B. 2013. Exacerbation of myasthenia gravis with voriconazole. Muscle Nerve 47:928–930. doi: 10.1002/mus.23751. [DOI] [PubMed] [Google Scholar]