Abstract
Herbaspirillum spp. are Gram-negative bacteria that inhabit soil and water. Infections caused by these organisms have been reported in immunocompromised hosts. We describe severe community-acquired pneumonia and bacteremia caused by Herbaspirillum aquaticum or H. huttiense in an immunocompetent adult male.
CASE REPORT
In early September 2014, a 46-year-old white male presented to a referring facility with fever, fatigue, and shortness of breath. His illness began 5 days prior to admission to our facility after he was drenched in rain during a fishing trip. He had a fever of 40°C, despite use of antipyretics, with chills, night sweats, anorexia, myalgia, and headache. Three days prior to admission, he had a transient period of dry cough for half a day that resolved spontaneously. The next day he developed sharp right-sided pleuritic chest pain, worsening dyspnea, and severe fatigue. When he presented to the referring facility, clinical findings were remarkable for a respiratory rate (RR) of 28/min with an oxygen saturation (SpO2) level of 77% on room air which improved to 91% on 4 liters/min of oxygen via nasal cannula. His white blood cell (WBC) count was 7.7 × 10−3/μl with 55% bands, and bilateral alveolar infiltrates were noted on a chest X-ray. Analysis of his arterial blood gas revealed a pH level of 7.46, partial pressure of CO2 (pCO2) of 34 mm Hg, pO2 of 53 mm Hg, SpO2 of 89% on 4 liters/min of oxygen via nasal cannula, and HCO3 of 24 meq/liter. Blood cultures were drawn, and he was treated with intravenous vancomycin, ceftriaxone, and azithromycin. His condition worsened and he was transferred to our university teaching hospital.
The patient's past medical history was unremarkable, except for childhood asthma, atypical pneumonia as a teenager, and tonsillectomy. He was on no home medications. He lived on a farm in rural Missouri with his wife and had close contact with cattle and turkeys (he had birthed calves 6 months earlier and handled 15,000 baby turkeys 3 weeks prior to the onset of illness). A few weeks prior to admission, the patient returned from work with about 20 loosely attached ticks. He also cleaned a grain bin in his barn (contained mold and possible rat excreta) a week prior to presentation. He smoked cigarettes for 30 years and also consumed alcohol regularly. He denied sick contacts, animal bites or scratches, and recent travel.
The results of a physical examination conducted on presentation to our facility were remarkable for temperature (38°C), heart rate (HR) (98 beats/min), blood pressure (BP) (141/88 mm Hg), and RR (30/min with use of accessory muscles). There was bilateral lower chest wall tenderness, coarse inspiratory crepitations were heard in left axilla and left lower chest, and diminished breath sounds were noted in the right axilla and right lower chest. The rest of the exam was unremarkable. Laboratory measurements on admission revealed a WBC count of 9.0 × 10−3/μl, granulocytes at 89.6% and no bands on automated differential determinations, hemoglobin at 13.4 g/dl, and a platelet count of 187 × 10−3/μl. Results of serum chemistry, renal function, and liver function tests were within normal limits, except for a mild elevation in the serum total bilirubin level (1.5 mg/dl). Cardiac enzyme and troponin analysis results were normal. The serum N-terminal pro B-type natriuretic peptide (NT-proBNP) level was 499.90 pg/ml. A chest X-ray showed bilateral mid- to lower zone consolidations with small pleural effusions (Fig. 1). Vancomycin and ceftriaxone treatment was continued; azithromycin was changed to doxycycline. Within 48 h of admission, because of a rapid decline in respiratory status, he was intubated and mechanically ventilated. A computed tomography (CT) scan of the chest with intravenous contrast showed bilateral multilobar consolidation that was most prominent in the lower lobes (Fig. 1). On day 3, he continued to be febrile, with a further increase in levels of leukocytosis (WBC count, 14.5 × 10−3/μl), serum bilirubin (total bilirubin, 2.1 mg/dl), and liver enzymes (aspartate transaminase [AST], 72 U/liter; alanine aminotransferase [ALT], 55 U/liter; alkaline phosphatase [ALP], 194 U/liter). Ceftriaxone was switched to piperacillin-tazobactam. Sputum cultures grew normal upper respiratory flora; acid-fast and fungal stains and cultures of respiratory specimens remained negative. Results of analysis using a FilmArray (BioFire Diagnostics Inc., Salt Lake City, UT) respiratory viral panel were negative. Laboratory tests in the following categories were performed (all with negative results): Histoplasma and Legionella urine antigens, blood cultures, blood Ehrlichia PCR, Anaplasma IgG/IgM, Lyme IgG/IgM, Rocky Mountain spotted fever IgG/IgM, and Q fever titers. Bronchoscopy with bronchoalveolar lavage (BAL) fluid was performed. A Gram stain of BAL fluid was negative for organisms and showed only a few neutrophils. The result of a test for Aspergillus galactomannan antigen in BAL fluid was negative. Serum quantitative immunoglobulin levels were normal. On day 4, the referring facility contacted us and reported that their blood culture (BD-Bactec; Becton Dickinson, Sparks, MD) had grown a Gram-negative rod (GNR), identified by their automated microbial identification system (Vitek 2 Compact; bioMérieux, Inc., Durham, NC) as Burkholderia cepacia complex (BCC), which was susceptible to piperacillin-tazobactam, imipenem, cefepime, ceftazidime, levofloxacin, tobramycin, and gentamicin and not resistant to any other antibiotic. On day 5, BAL fluid cultures from our facility grew GNR (see a Gram stain of the isolate in Fig. 2). This GNR was identified by our automated microbial identification system (Vitek 2; bioMérieux, Inc., Hazelwood, MO) as BCC, but the isolate failed to produce yellow colonies on oxidation/fermentation-polymyxin-bacitracin-lactose (OFPBL) medium. The sample was subsequently sent to a reference laboratory (ARUP Laboratories, Salt Lake City, UT). After 12 days of treatment, the patient improved and was extubated. ARUP Laboratories used matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) to identify the GNR as Herbaspirillum aquaticum or Herbaspirillum huttiense (susceptible to amikacin, cefepime, ceftazidime, ciprofloxacin, levofloxacin, meropenem, piperacillin-tazobactam, tobramycin, and trimethoprim/sulfamethoxazole and resistant to gentamicin). By this time, the patient had completed a 14-day course of doxycycline and piperacillin-tazobactam and was discharged home after 15 days. Upon our request, the referring facility also sent their sample to ARUP Laboratories, and the GNR isolated from blood was also identified as Herbaspirillum aquaticum or Herbaspirillum huttiense by MALDI-TOF MS. He missed his follow-up appointment at 2 weeks, but in a telephone conversation after a month he was noted to be in good health.
FIG 1.
Chest X-ray and computed tomography images demonstrating bilateral consolidation.
FIG 2.
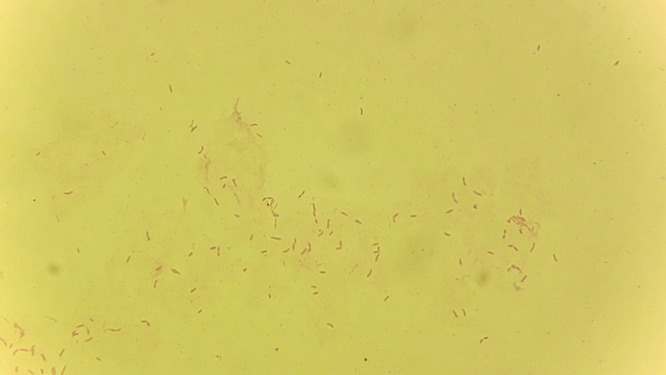
Gram stain of BAL fluid isolate showing the Gram-negative rods identified as Herbaspirillum aquaticum or Herbaspirillum huttiense.
Herbaspirillum species are nitrogen-fixing bacteria that inhabit the roots of plants in the rhizosphere and have been found in wells and other ground water (1). They are Gram-negative curved bacilli, strict aerobes, motile with polar flagella, and urease, oxidase, and catalase positive. These species do not ferment lactose or grow in nitrogen-free medium (1, 2). A number of Herbaspirillum species have been identified and studied, but only a few species have been reported to be human pathogens (3).
The members of the diverse group of Gram-negative, nonfermenting bacilli pose challenges for definitive genus and species identification. Due to phylogenetic and phenotypic similarities, Herbaspirillum bacilli are often identified as BCC by automated microbial identification systems (4, 5). The Vitek 2 microbial identification system with its expanded identification database (Advanced Expert System) is the automated system used by our Clinical Microbiology Laboratory to identify bacteria according to their biochemical profiles. In our case, Vitek 2 identified the isolate as BCC, but it subsequently failed to produce typical yellow colonies on the OFPBL medium, in which polymyxin inhibits the growth of common Gram negatives and bacitracin inhibits Gram positives and which has excellent sensitivity and reasonable specificity to identify BCC (6).
Molecular identification is one of the most useful techniques for identifying organisms, and its use is becoming more widespread in diagnostic microbiology laboratories. 16S rRNA gene sequencing is independent of microbial metabolism and provides reliable results, although it may not provide identification to the species level. For identification of close relatives, consensus sequences are compared with 16S rRNA gene sequences in a sequence database such as GenBank (4, 7–9). MALDI-TOF MS is a spectroscopic method that requires a reliable and complete database. The prompt (less than 1 h) identification and high discriminatory power of MALDI-TOF make it a useful tool for the characterization of microorganisms that are difficult to identify using routine methods. Unlike publicly available sequence databases such as GenBank, MALDI-TOF databases are proprietary (9–11). MALDI-TOF can identify BCC and bacterial species that are rarely reported as human pathogens compared with conventional phenotypic and molecular identifications (9, 12, 13). ARUP used a Brucker Daltronics MALDI-TOF system with MicroFlex and Biotyper and database version MBT DB 5627 to identify our patient isolate. They used 16S rRNA gene sequencing to validate the MALDI-TOF results before adding to the MALDI-TOF database. The ARUP MALDI-TOF identification result was H. huttiense. However, this organism had not yet been added to the MALDI-TOF database. Therefore, since 16S rRNA sequencing could not differentiate between H. huttiense and H. aquaticum, it was reported as “H. aquaticum/huttiense” (ARUP, private communication). MALDI-TOF has its strengths and weaknesses but may replace biochemical identification in the near future (14).
Human disease caused by Herbaspirillum has been previously described in cystic fibrosis patients and as an opportunistic pathogen in patients with cirrhosis and cancer. The reported manifestations included skin and soft tissue infections, bacteremia, and pneumonia (4, 5, 14–16). In our search of the available literature, we found no documented cases of Herbaspirillum infections in immunocompetent adults. This is the first reported case of severe community-acquired pneumonia and bacteremia caused by H. huttiense or H. aquaticum in an immunocompetent adult. That our patient was a farmer with significant recent soil, water, and livestock contact suggests an acquisition from one or more of these environmental sources. Antimicrobial susceptibility can serve as a clue for differentiating Herbaspirillum spp. from BCC species because the latter are usually multidrug resistant whereas the former are not (14). With institution of appropriate antimicrobial therapy, the outcome seems favorable. The increased use and availability of newer molecular methods (e.g., MALDI-TOF MS) should allow laboratories to correctly identify this organism, considering the known issue of misidentification by established microbial identification systems (e.g., Vitek). Perhaps Herbaspirillum species may be more prevalent pathogens than previously known.
ACKNOWLEDGMENT
We declare that we have no potential conflicts of interest.
REFERENCES
- 1.Baldani JI, Pot B, Kirchhof G, Falsen E, Baldani VL, Olivares FL, Hoste B, Kersters K, Hartmann A, Gillis M, Dobereiner J. 1996. Emended description of Herbaspirillum; inclusion of [Pseudomonas] rubrisubalbicans, a milk plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Bacteriol 46:802–810. doi: 10.1099/00207713-46-3-802. [DOI] [PubMed] [Google Scholar]
- 2.Dobritsa AP, Reddy M, Samadpour M. 2010. Reclassification of Herbaspirillum putei as a later heterotypic synonym of Herbaspirillum huttiense, with the description of H. huttiense subsp. huttiense subsp. nov. and H. huttiense subsp. putei subsp. nov., comb. nov., and description of Herbaspirillum aquaticum sp. nov. Int J Syst Evol Microbiol 60(Pt 6):1418–1426. doi: 10.1099/ijs.0.009381-0. [DOI] [PubMed] [Google Scholar]
- 3.Marques AC, Paludo KS, Dallagassa CB, Surek M, Pedrosa FO, Souza EM, Cruz LM, LiPuma JJ, Zanata SM, Rego FG. 2015. Biochemical characteristics, adhesion, and cytotoxicity of environmental and clinical isolates of Herbaspirillum spp. J Clin Microbiol 53:302–308. doi: 10.1128/JCM.02192-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spilker T, Uluer AZ, Marty FM, Yeh WW, Levison JH, Vandamme P, LiPuma JJ. 2008. Recovery of Herbaspirillum species from persons with cystic fibrosis. J Clin Microbiol 46:2774–2777. doi: 10.1128/JCM.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziga ED, Druley T, Burnham C-AD. 2010. Herbaspirillum species bacteremia in a pediatric oncology patient. J Clin Microbiol 48:4320–4321. doi: 10.1128/JCM.01479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Pelt C, Verduin CM, Goessens WH, Vos MC, Tümmler B, Segonds C, Reubsaet F, Verbrugh H, van Belkum A. 1999. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J Clin Microbiol 37:2158–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Olmos A, García-Castillo M, Morosini M-I, Lamas A, Máiz L, Cantón R. 2012. MALDI-TOF MS improves routine identification of non-fermenting Gram negative isolates from cystic fibrosis patients. J Cyst Fibros 11:59–62. doi: 10.1016/j.jcf.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Marques da Silva R, Caugant DA, Eribe ERK, Aas JA, Lingaas PS, Geiran O, Tronstad L, Olsen I. 2006. Bacterial diversity in aortic aneurysms determined by 16S ribosomal RNA gene analysis. J Vasc Surg 44:1055–1060. doi: 10.1016/j.jvs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Patel R. 2015. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem 61:100–111. doi: 10.1373/clinchem.2014.221770. [DOI] [PubMed] [Google Scholar]
- 10.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier P-E, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 11.Vanlaere E, Sergeant K, Dawyndt P, Kallow W, Erhard M, Sutton H, Dare D, Devreese B, Samyn B, Vandamme P. 2008. Matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry of intact cells allows rapid identification of Burkholderia cepacia complex. J Microbiol Methods 75:279–286. doi: 10.1016/j.mimet.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Fehlberg LCC, Andrade LHS, Assis DM, Pereira RHV, Gales AC, Marques EA. 2013. Performance of MALDI-ToF MS for species identification of Burkholderia cepacia complex clinical isolates. Diagn Microbiol Infect Dis 77:126–128. doi: 10.1016/j.diagmicrobio.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Lambiase A, Del Pezzo M, Cerbone D, Raia V, Rossano F, Catania MR. 2013. Rapid identification of Burkholderia cepacia complex species recovered from cystic fibrosis patients using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Microbiol Methods 92:145–149. doi: 10.1016/j.mimet.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Chemaly RF, Dantes R, Shah DP, Shah PK, Pascoe N, Ariza-Heredia E, Perego C, Nguyen DB, Nguyen K, Modarai F, Moulton-Meissner H, Noble-Wang J, Tarrand JJ, LiPuma JJ, Guh AY, MacCannell T, Raad I, Mulanovich V. 12 September 2014, posting date. Cluster and sporadic cases of Herbaspirillum spp. infections in patients with cancer. Clin Infect Dis doi: 10.1093/cid/ciu712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan MJ, Oehler RL. 2005. Lower extremity cellulitis and bacteremia with Herbaspirillum seropedicae associated with aquatic exposure in a patient with cirrhosis. Infect Dis Clin Pract 13:277–279. doi: 10.1097/01.idc.0000170026.41994.8d. [DOI] [Google Scholar]
- 16.Chen J, Su Z, Liu Y, Sandoghchian S, Zheng D, Wang S, Xu H. 2011. Herbaspirillum species: a potential pathogenic bacteria isolated from acute lymphoblastic leukemia patient. Curr Microbiol 62:331–333. doi: 10.1007/s00284-010-9703-5. [DOI] [PubMed] [Google Scholar]



