Abstract
We evaluated a novel plasma (1,3)-β-d-glucan (BDG) detection assay for the diagnosis of candidemia in children. The median BDG levels were 73.4 pg/ml in patients with candidemia and <10 pg/ml in patients without candidemia (P < 0.001). Receiver operating characteristic analysis revealed a cutoff point of 14 pg/ml and an area under the curve of 0.802. At these values, the assay demonstrated 68% sensitivity, 91% specificity, 66% positive predictive value, and 91% negative predictive value. Plasma BDG levels were undetectable in 18 candidemia cases.
TEXT
As the number of pediatric patients with invasive candidiasis has increased in recent years (1), rapid diagnosis of the disease has become urgently needed so that appropriate treatment can be initiated without delay. (1,3)-β-d-glucan (BDG) is a fungal cell wall polysaccharide present in a variety of fungi, including Candida species. Testing BDG in serum by Fungitell assay (Associates of Cape Cod, Inc., East Falmouth, MA, USA) has been approved by the U.S. Food and Drug Administration to aid the diagnosis of invasive fungal infections. Clinical studies have demonstrated that the Fungitell assay has a sensitivity of 75% and a specificity of 80% in the diagnosis of candidiasis in intensive care unit patients in the United States (2, 3).
The GKT assay (Gold Mountain River Tech Development Co., Ltd., Beijing, China) is a novel assay to detect BDG in plasma. The assay is manufactured by developing blood extractions from the horseshoe crab Tachypleus tridentatus into limulus amebocyte lysate (LAL). BDG can be detected in plasma via the activation of factor G in LAL, leading to a cascade of proteolytic coagulation that can be measured by a reader. The assay is also modified by removing bacterial endotoxin-sensitive factor C from the LAL, making it specific for BDG detection. It is important to recognize that the GKT assay is designed to detect BDG in the plasma; this differs from the Fungitell assay used in the United States, which detects BDG in the serum, and from the Fungitec-G assay (Seikagaku Corp., Tokyo, Japan) used in Japan that detects BDG in the serum or plasma. In addition, sample pretreatment process and test result readouts vary among these kits. Furthermore, each kit is developed from different horseshoe crab species, and the amebocytes from different horseshoe crab species may possess different affinities toward the BDG molecules. Therefore, each kit has its own cutoff, and one cannot be applied to the other. Although the GKT assay has been approved by the China State Food and Drug Administration as an adjunct test to aid in the diagnosis of deep fungal infections, little is found in the English literature about its performance characteristics for the diagnosis of candidemia, particularly in pediatric patients. In this study, we evaluated the GKT assay for the diagnosis of candidemia in pediatric patients.
Pediatric patients (0 to 14 years old) admitted to Xinhua Hospital, a 2,000-bed tertiary teaching hospital affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China, from April 2009 to February 2012 were included in the study. The hospital serves a population of 1.2 million people. This study was approved by the Xinhua Hospital institutional review board. Patient demographic information and pertinent clinical data, including history of long-term use of antibiotics, presence of indwelling central venous catheters, fever, and low birth weight, were reviewed. None of these patients included in the study received antifungal prophylaxis prior to their blood culture collection. For each patient, 4 ml of blood was drawn for blood culture. Blood culture samples were processed in pediatric bottles using the BacT/Alert system (bioMérieux, Marcy l'Etoile, France). Positive samples were subcultured onto Sabouraud dextrose agar (Oxoid, United Kingdom), and subsequent culture isolates were identified to the species level using the YST panel with the Vitek 2 compact system (bioMérieux, Marcy l'Etoile, France). Patients with candidemia were defined as isolating Candida species in blood obtained from at least two separate peripheral blood samples collected at different sites. Patients without candidemia were defined as having negative blood culture results for fungi and no clinical evidence of fungal infections (excluding neonatal intensive care unit patients).
BDG detection was performed in plasma that was collected at the same time that blood was drawn for culture. Detection of BDG in plasma was performed according to the manufacturer's instructions. Briefly, 100 μl of plasma was mixed with 900 μl of pretreatment reagents and then heated at 70°C for 10 min. After cooling down, the solution was mixed with the treatment reagents for 1 h and then transferred to a reading tube. Detection of the presence of BDG was based on the absorbance change, and the result was determined by a microbiology kinetic reader provided by the manufacturer. The limit of detection of the GKT plasma BDG detection assay was <10 pg/ml.
Patient demographic and clinical characteristics were summarized using descriptive statistics, i.e., counts and median (range) numbers. Nonparametric analysis methods were chosen due to the non-Gaussian distribution of BDG values. The Mann-Whitney U test was used to determine the statistical difference between levels of BDG in patients with candidemia and in those without the disease. The Kruskal-Wallis test was used to test the statistical difference of BDG levels in cases of candidemia caused by different Candida species. Receiver operating characteristic (ROC) curves were plotted to determine the best cutoff value for the plasma BDG assay to diagnose candidemia. Sensitivity, specificity, and exact binomial 95% confidence intervals (CIs) for each were calculated. Analyses were performed using IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA) and MedCalc software (version 11.6.1.0; Mariakerke, Belgium). All tests were two sided and were considered significant at a P value of of <0.05.
Fifty-six patients with culture-confirmed candidemia were identified over the study period from a total of 2,256 patients tested (2.5% prevalence of the infection). The median (range) age was 2 months (20 days to 11 years); 28 patients (50%) were male. Thirty-eight patients had premature birth, 10 had gastrointestinal abnormalities, 6 had congenital heart diseases, and 3 had neurological abnormalities. To identify patients without candidemia, a total of 210 patients with negative fungal cultures and without evidence of fungal infections were randomly selected from 13 different wards (8 to 22 patients per ward) over the same study period. The median (range) age was 2 months (10 days to 11 years); 103 paitents (49%) were male.
The median (range) value of plasma BDG in the 56 patients with candidemia was 73.5 pg/ml (<10 to 3,895 pg/ml), which was higher than that in 210 patients without candidemia (median, <10 pg/ml; range, <10 to 1,000 pg/ml) (P = 0.001) (Table 1 ). Candida parapsilosis (39%) was the most common cause of candidemia in our pediatric patients, followed by Candida guilliermondii (21%), Candida albicans (18%), and Candida famata (18%) (Table 1). We have further broken down our pediatric patients into neonate (≤28 days old) and nonneonate (>28 days to 12 years) groups. We found that plasma BDG levels varied among candidemia patients caused by different Candida species: the highest BDG levels were seen in candidemia cases caused by C. albicans, followed by C. guilliermondii, C. papapsilosis, and C. famata (Table 1). These findings were not affected by age groups (P = 0.024 versus 0.029). Our findings were different from previous reports using the Fungitell assay to assess BDG levels in serum from adult candidemia patients. Del Bono et al. has reported that no differences were found when comparing median BDG values in patients with C. albicans candidemia versus non-C. albicans candidemia (4). A similar observation was also reported by Odabasi et al. (2).
TABLE 1.
Plasma BDG levels in pediatric patients with or without candidemia
| Patient | No. of patients | BDG level (pg/ml) (median [range]) | P | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Candidemiaa | |||||
| Age, ≤28 days | 8 | 0.024b | |||
| C. albicans | 3 | 3,378.0 (1,797.0–3,895.0) | 100 | 91 | |
| C. parapsilosis | 5 | 41.4 (<10.0–74.3) | 60 | 91 | |
| Age, >28 days to 12 years | 48 | 0.029b | |||
| C. albicans | 7 | 453.0.4 (14.8–3,434.0) | 100 | 91 | |
| C. guilliermondii | 12 | 139.1 (<10.0–636.0) | 83 | 91 | |
| C. parapsilosis | 17 | 70.8 (<10.0–983.3) | 65 | 91 | |
| C. famata | 10 | <10.0 (<10.0–498.1) | 30 | 91 | |
| C. albicans and C. parapsilosis | 1 | <10.0 | |||
| C. lusitaniae | 1 | 18.9 | |||
| Subtotal | 56 | 73.5 (<10.0–3,895.0) | 0.001c | 68 | 91 |
| Noncandidemia | 210 | <10.0 (<10.0–1,000.0) |
Candidemia cases were divided with each age subgroup based on the different causal Candida species.
Plasma BDG levels were statistically different among candidemia cases caused by different Candida species.
The median (range) value of plasma BDG in the 56 patients with candidemia was significantly higher than that in 210 patients without candidemia.
ROC analysis for plasma BDG values demonstrated a cutoff point of 14 pg/ml, with an area under the curve of 0.802 (Fig. 1). At this value, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the diagnosis of candidemia in our pediatric patients were 68% (95% CI, 50% to 80%), 91% (95% CI, 86% to 94%), 66% (95% CI, 52% to 78%), and 91% (95% CI, 87% to 95%), respectively. Our cutoff value is different from the one established with the Fungitell assay (60 pg/ml) and the one used with the Fungitec-G assay (20 pg/ml).
FIG 1.
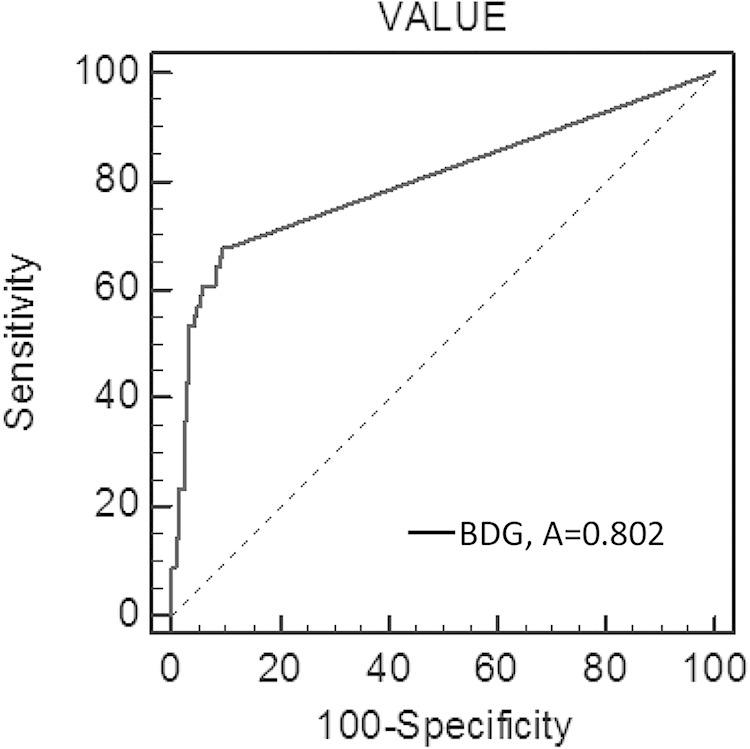
Receiver operating characteristic (ROC) curve of BDG cutoff values (pg/ml) for determining positive and negative plasma (n = 266 specimens from 56 patients with culture-confirmed candidemia and from 210 patients without candidemia). Area under the ROC curve, 0.802.
Seven candidemia patients had multiple plasma specimens collected at different time points, which, thus, allowed us to measure serial plasma BDG levels in each of them after antifungal drug treatment (Table 2). In all cases, the plasma BDG levels declined by 4 weeks. In 5 patients, BDG levels were still detectable at the time that cultures were negative.
TABLE 2.
Plasma BDG levels in candidemia patients following antifungal drug treatment
| Patient | Week | Culture result | BDG level (pg/ml) | Antifungal drug used | Clinical outcome |
|---|---|---|---|---|---|
| 1 | 0a | C. albicans | 3,434 | Fluconazole | |
| 1 | 954.3 | ||||
| 2 | 174.9 | ||||
| 3 | Negative | 102.3 | |||
| 4 | 13.87 | Survived | |||
| 2 | 0a | C. albicans | 759.7 | Fluconazole | |
| 1 | |||||
| 2 | 781.3 | Added caspofungin | |||
| 3 | Negative | 671.2 | |||
| 4 | 186.8 | Survived | |||
| 3 | 0a | C. famata | 498.1 | Fluconazole | |
| 1 | 1,817 | Added flucytosine | |||
| 2 | Negative | 384.2 | |||
| 3 | <10 | ||||
| 4 | <10 | Survived | |||
| 4 | 0a | C. parapsilosis | 207.2 | Fluconazole | |
| 1 | 295.7 | ||||
| 2 | Negative | <10 | |||
| 3 | <10 | ||||
| 4 | Survived | ||||
| 5 | 0a | C. parapsilosis | 139.9 | ||
| 1 | 1,000 | ||||
| 2 | 867.5 | Fluconazole | |||
| 3 | Negative | 728 | |||
| 4 | <10 | Survived | |||
| 6 | 0a | C. parapsilosis | <10 | ||
| 1 | <10 | Fluconazole | |||
| 2 | Negative | 259.9 | |||
| 3 | <10 | ||||
| 4 | <10 | Survived | |||
| 7 | 0a | C. parapsilosis | 72.6 | ||
| 1 | Negative | <10 | Fluconazole | ||
| 2 | <10 | ||||
| 3 | <10 | ||||
| 4 | <10 | Survived |
Time when the blood culture was positive.
Plasma BDG levels were undetectable in 18 candidemia cases, of which 9 were caused by C. parapsilosis, 7 by C. famata, and 2 by C. guilliemondii. It is possible that C. parapsilosis and C. famata have smaller amounts of BDG in their cell walls, as suggested by their lower susceptibility to echinocandins (antifungals targeting BDG synthesis) (4, 5). As a result, 70% of our patients with C. famata candidemia had undetectable BDG values. Likewise, low BDG levels were observed in our patients with C. parapsilosis candidemia within both neonatal and nonneonatal pediatric populations (Table 1), which is similar to the findings reported by other investigators (3, 4, 6). These data suggest that the BDG detection may be suboptimal in the diagnosis of candidemia cases caused by these two Candida species.
BDG was detectable in plasma from 19 (9%) patients with negative blood cultures, with concentrations ranging from 11 to 1,000 pg/ml. It is true that the sensitivity of a blood culture in detecting candidemia is not high (7), but none of these patients presented with evidence of fungal infections. False-positive BDG detection may be attributable to multiple factors, such as blood dialysis, surgical gauze usage, bacterial infections (Pseudomonas aeruginosa), or receipt of albumin or immunoglobulin products (8–10). One study also showed that the baseline level of BDG in pediatric patients without candidemia was slightly higher than that of adult patients (68 pg/ml versus 48 pg/ml) using the serum BDG Fungitell assay (11). We selected patients for chart review whose plasma BDG values were >100 pg/ml. A total of five patients were identified, and none of them had evidence of fungal infections. Although we could not identify any factor directly contributing to false positives in these five cases, we found that one patient had a plasma transfusion done 1 day prior to the BDG testing, and another patient had a positive blood culture growing Klebsiella pneumoniae.
In summary, we demonstrated that the GKT plasma BDG assay has a moderate efficacy (68% sensitivity and 66% PPV) to aid the diagnosis of candidemia in pediatrics patients. The assay is useful to rule out the candidemia due to its high NPV (91%). The assay also showed prognostic value in monitoring antifungal treatment response (though this result is based on the study of a small number of available cases). Since poor sensitivity of blood culture may be exacerbated in children due to the low volume of blood collection, the plasma BDG assay that only requires 100 μl of plasma can provide early and rapid diagnostic value to aid the diagnosis of candidemia in children, thus allowing initiation of appropriate antifungal therapy without delay. However, low sensitivity of the plasma BDG assay was noticed in detecting candidemia caused by C. famata and C. parapsilosis.
ACKNOWLEDGMENT
We declare no conflicts of interest.
REFERENCES
- 1.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 2.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. 2004. β-d-Glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 3.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH. 2005. Multicenter clinical evaluation of the (1,3)-β-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis 41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 4.Del Bono V, Delfino E, Furfaro E, Mikulska M, Nicco E, Bruzzi P, Mularoni A, Bassetti M, Viscoli C. 2011. Clinical performance of the (1,3)-β-d-glucan assay in early diagnosis of nosocomial Candida bloodstream infections. Clin Vaccine Immunol 18:2113–2117. doi: 10.1128/CVI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyda ND, Chuang SH, Alam MJ, Shah DN, Ng TM, McCaskey L, Garey KW. 2013. Treatment of Candida famata bloodstream infections: case series and review of the literature. J Antimicrob Chemother 68:438–443. doi: 10.1093/jac/dks388. [DOI] [PubMed] [Google Scholar]
- 6.Abe M, Kimura M, Araoka H, Taniguchi S, Yoneyama A. 2014. Serum (1,3)-β-d-glucan is an inefficient marker of breakthrough candidemia. Med Mycol 52:835–840. doi: 10.1093/mmy/myu066. [DOI] [PubMed] [Google Scholar]
- 7.Clancy CJ, Nguyen MH. 2013. Finding the missing 50% of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 56:1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 8.Ikemura K, Tanaka H, Yoshioka T, Sugimoto T. 1990. Interference in endotoxin and fungal polysaccharide assays from blood products and antimicrobial agents. Rinsho Byori 38:87–92. (In Japanese.). [PubMed] [Google Scholar]
- 9.Mennink-Kersten MA, Ruegebrink D, Verweij PE. 2008. Pseudomonas aeruginosa as a cause of 1,3-β-d-glucan assay reactivity. Clin Infect Dis 46:1930–1931. doi: 10.1086/588563. [DOI] [PubMed] [Google Scholar]
- 10.Mennink-Kersten MA, Verweij PE. 2006. Nonculture-based diagnostics for opportunistic fungi. Infect Dis Clin North Am 20:711–727, viii. doi: 10.1016/j.idc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Smith PB, Benjamin DK Jr, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ. 2007. Quantification of 1,3-β-d-glucan levels in children: preliminary data for diagnostic use of the beta-glucan assay in a pediatric setting. Clin Vaccine Immunol 14:924–925. doi: 10.1128/CVI.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


