Abstract
We present an unusual equine endometritis case associated with Cladophialophora bantiana in a 15-year-old mare. The mare displayed infertility and uterine fluid accumulation with numerous black, hairy granules. Microscopically, the fluid revealed numerous septate, dark fungal hyphae and conidia in chains. Culture yielded C. bantiana (CBS 138271); the species was confirmed by internal transcribed spacer (ITS) sequencing. Treatment was unsuccessful. C. bantiana causes cerebral phaeohyphomycosis in humans, while animal cases are rare. Animal cases are reviewed.
CASE REPORT
A 15-year-old Standardbred mare was referred to the Production Animal Veterinary Teaching Hospital of the University of Helsinki, Finland, at the end of April 2013 because of infertility problems. The mare was born in Kentucky, USA, but was sold to a stud farm in Canada. It foaled in Canada four times during 2003 to 2007. The mare was sold pregnant to Finland in 2009. The foaling was successful in 2010, but the following parturition in 2011 was difficult and fetotomy was required. During the procedure, the mare's cervix was lacerated. Since then, the mare had not conceived, despite repeated inseminations.
At admission, on 26 April 2013, the examination revealed mild cervical damage and a partly open cervix. The mare had been inseminated 5 days prior to admission. Upon ultrasonography of the uterus, a small amount of slightly echogenic fluid was detected. No polymorphonuclear leukocytes (PMNs) were observed in cytology. Bacterial culture yielded only a few colonies of contaminant growth. To support cervical closure, therapy with the synthetic progestin alternogest was initiated.
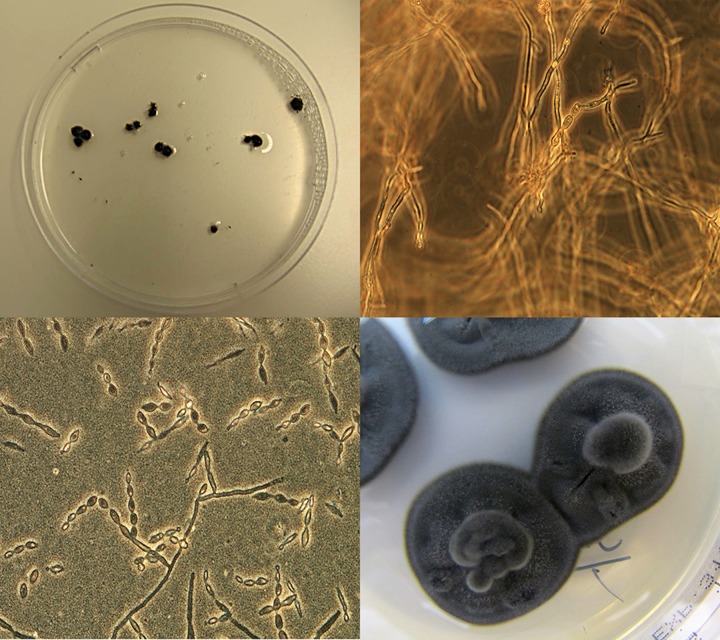
On 8 May, ultrasound examination for 14-day pregnancy was negative. A small amount of slightly echogenic fluid was observed in the uterus. A uterine biopsy specimen and a swab for bacterial culture were taken. Uterine lavage was also performed. The lavage fluid was initially cloudy, but the last lavage fluid sample contained multiple variable-sized black, hairy-looking granules (Fig. 1), the largest of which was 0.5 cm in diameter. Bacterial culture was performed at the clinic's laboratory; this time, abundant beta-hemolytic Streptococcus sp. and a few Escherichia coli colonies, as well as unidentified fungal growth, were detected on blood agar. Cytological examination revealed numerous PMNs, erythrocytes, streptococcal chains, and fungal hyphae. In a biopsy specimen, acute endometritis, almost uniform glandular dilation, and some fibrosis were present.
FIG 1.
(Upper left) Black hairy granules in the uterine lavage fluid on a petri dish. (Upper right) Ten percent KOH mount of uterine lavage fluid showing dark brown, septate branching hyphae with single-celled oval conidia in chains (magnification, ×400). (Lower left) Lactophenol cotton blue preparation of the colony showing single-celled oval conidia in chains (magnification, ×400). (Lower right) Colonies of C. bantiana on Sabouraud dextrose agar (LabM Ltd., United Kingdom) after 2 weeks of incubation at 37°C.
Intrauterine treatment was started with a mucolytic agent, acetylcysteine, which was administered to the uterus and flushed out after 6 h. This was followed by daily flushings with isotonic saline and subsequent intrauterine administration of antifungal and antibacterial agents during the estrus cycle. During the first 2 days, 100 mg fluconazole and 5 million units of penicillin G were administered to the uterus after a flushing. On the third day, 250 ml of 2% acetic acid was placed in the uterus and maintained for 5 min before being washed out and rinsed with saline. Then, 5 million units of penicillin and 2 g gentamicin (buffered in bicarbonate solution) were administered to the uterus and continued for the rest of the estrus period. At the same time, oral fluconazole treatment was initiated (loading dose of 14 mg/kg of body weight, after which 5 mg/kg once a day was used) for 3 weeks. Oxytocin was also given to facilitate uterine emptying. On 24 and 29 May, the mare was given prostaglandin intramuscularly to induce luteolysis and subsequent estrus. By that time, the uterus was empty. At the beginning of the subsequent estrus, uterine lavage was repeated. The mare was inseminated on 5 June, but at the time of the insemination, ultrasonography of the ovaries suggested that a preovulatory follicle looked like a hemorrhagic anovulatory follicle.
The ultrasound examination for a pregnancy on 20 June was negative. Four days later, a small amount of intrauterine fluid was observed. Again, uterine lavage fluid contained numerous black hairy granules, and an unidentified fungal species was cultured at the clinic's laboratory. After this, the specimen was sent to the Mycology Unit of the Helsinki University Hospital Laboratory (HUSLAB). Lavages with saline and a mucolytic agent, as well as 2-day intrauterine administration of fluconazole together with penicillin G, were repeated. This was followed by oral fluconazole for 2 weeks. In the next estrus, only a small amount of fluid was present in the uterus. Daily saline flushing and 3 days of intrauterine treatment with fluconazole were repeated before insemination on 19 July. On 27 July, a substantial amount of uterine fluid with black hairy spheres was discovered. The uterus was edematous, and vulvar discharge was observed. An attempt to recover an embryo from the fluid was unsuccessful.
At the Mycology Unit of HUSLAB, microscopic examination of potassium hydroxide (KOH) preparations of uterine fluid revealed numerous septate and darkly pigmented fungal hyphae and conidia in chains (Fig. 1). The specimen was cultured on Sabouraud dextrose agar plates (LabM Ltd., United Kingdom) supplemented with penicillin (6 mg/liter) and dihydrostreptomycin (26 mg/liter). The plates were incubated at +37°C and +28°C. After 3 days, the plates showed tiny colonies with an olive-gray velvety appearance with a black reverse (Fig. 1). A lactophenol cotton blue preparation of the growth showed dark-walled, septate hyphae with conidia in long, poorly branched chains on undifferentiated hyphae. The conidia were one celled, pale brown, smooth walled, and ellipsoid (Fig. 1). Growth features and microscopic morphology of the fungus raised a concern that the species was probably a Cladophialophora sp. and potentially Cladophialophora bantiana, a neurotropic fungus. The laboratory alerted clinicians about the finding because of the potential occupational health hazard. The fungal isolate was sent to the CBS Fungal Biodiversity Centre (Utrecht, The Netherlands) for species confirmation.
At CBS, genomic DNA was extracted from cultures grown on malt extract agar plates using a cetyltrimethylammonium bromide method, as described previously (1). Amplification and sequencing of the partial ribosomal operon were performed using primers ITS1 and ITS4 for the internal transcribed spacer (ITS) region (2). Consensus sequences were computed and edited with the SeqMan Lasergene package (DNAStar, WI, USA). The sequences were compared with deposits in GenBank and with an in-house research database on black fungi at CBS. The sequence was 100% identical to that of CBS 173.52, the type strain of Cladophialophora bantiana. The strain was deposited in the CBS reference collection with the accession number CBS 138271.
Due to the poor prognosis and zoonotic potential of the suspected pathogen, the horse was euthanized in mid-August and submitted to the Finnish Food Safety Authority Evira for a complete necropsy. Tissue samples for histological evaluation were taken from all major organs, including the reproductive tract. The samples were fixed in neutral-buffered 10% formalin, routinely processed, embedded in paraffin, sectioned at 4-μm thickness, and stained with hematoxylin and eosin (HE). Samples of the uterus were also stained with Grocott's methamine silver stain for fungal hyphae. The horse was in good body condition, and pathological changes were limited to the reproductive organs. In the ovaries, variably sized follicles with one fresh ovulation site were observed. The uterus was dilated and contained about 1 liter of yellowish-gray, fairly watery fluid. The endometrium was moderately, unevenly thickened and edematous. The cervix and both oviducts were macroscopically normal. Histologically, chronic endometritis with occasional foci of acute purulent inflammation was discovered. In the endometrium, inflammatory cells consisted of lymphocytes and plasma cells with lesser numbers of neutrophils mainly associated with the purulent foci, but neither bacteria nor fungal hyphae were observed. There were no specific changes in the cervix, but mild vaginitis characterized by mucosal infiltrations of lymphocytes and plasma cells was present. To avoid a potential health hazard, no more cultures were performed at necropsy.
To our best knowledge, this is the first report of Cladophialophora bantiana associated with equine endometritis and generally the first case of this fungus in a horse. Equine endometritis is usually accompanied by bacteria, of which group C beta-hemolytic streptococci and Escherichia coli are the most common species (3). Microbial growth is considered to be secondary due to underlying factors impairing the local immunity. Few papers have been published on the role of fungi in equine endometritis, but fungal growth is considered to be present in 1 to 7% of cases (4–6). Candida spp. and Aspergillus spp. are among the most common fungal genera in equine fungal endometritis. Clinical signs range from subclinical infection to profuse vaginal discharge. Infertility is common, and the prognosis is guarded to poor (6).
C. bantiana is a member of the order Chaetothyriales, which are often referred to as “black yeast-like fungi” due to the ability of some representatives to produce budding cells as well as dark hyphae, depending on the life cycle and environmental conditions (7). From a clinical perspective, three groups can be distinguished within the order: (i) pathogens, which have the advantage of using vertebrate vectors and which need specialized methods to be isolated from the environment; (ii) agents of chromoblastomycosis, which have a distinct pathogenic phase in mammalian tissue; and (iii) saprobes, which cause infections only by coincidence (7). The melanized fungi are unique in being capable of causing a plethora of diseases in mammals, such as phaeohyphomycosis, eumycetoma, and chromoblastomycosis (8). Among others, melanin, chitin, thermotolerance, and the assimilation of aromatic hydrocarbons are considered to play a role in pathogenesis (9).
Mammals and birds are usually well protected from infections caused by black fungi due to their skin or feather coverage (7). Ten different Cladophialophora species are known to cause infections in humans. Of these, C. bantiana is a well-known causative agent of cerebral phaeohyphomycosis, which is often a fatal condition (8, 10). Many such cases have been diagnosed in people without apparent immune deficiency (8), although there may be underlying genetic susceptibilities associated, for instance, with mutations in the CARD9 gene with subsequent decreased cytokine production by innate immune cells (7). The prognosis of cerebral phaeohyphomycosis is poor, regardless of aggressive surgical and medical treatment (8). C. bantiana has also been involved in (sub)cutaneous infections (11–13) and in a joint infection (14) in humans. The exact route of infection in systemic and/or cerebral phaeohyphomycosis is not well understood, but proposed routes include inhalation of conidia and subsequent hematogenous spread of the organism or nasal invasion (9). Penetrating trauma is a logical route for infection in localized infections.
It is likely that C. bantiana has an environmental origin, but thus far, the fungus has only occasionally been isolated from environmental sources. It has been discovered from soil in Panama (15); from tree bark (16) and a brick wall of a warehouse (17) in Virginia, USA; and recently from the hot tub water of a retirement community in the United States (18). Human infections have been observed worldwide (8), but the distribution of cases indicates that C. bantiana has a preference for warmer climates with high average humidity (9). The same conclusion can be drawn from the geographical distribution of animal infections (Table 1). In this respect, it was surprising to find C. bantiana infection in an equine patient in Finland. According to HUSLAB, which also serves as a national reference laboratory for medical fungi in Finland, no C. bantiana cases in humans have been discovered. In Sweden, C. bantiana together with Mycobacterium abscessus was reported in soft tissue infections of two patients surviving the 2004 tsunami catastrophe, and these individuals were thus likely to have acquired their infections in Thailand (19). Five animal infections caused by C. bantiana have been reported from European countries: two from France (20, 21), and one each from Italy (22), the United Kingdom (23), and Switzerland (24). The horse described in our report was born in Kentucky, USA, where the climate probably favors the existence of C. bantiana. It might be possible that the horse was an asymptomatic carrier of C. bantiana until the conditions in the reproductive tract changed to support the multiplication of the fungus and subsequent development of local infection. Specimens from the vagina or clitoral fossa could have been useful to investigate whether the horse harbored the fungus in its reproductive tract, but unfortunately, they were not taken.
TABLE 1.
Summary of published Cladophialophora bantiana infections in animalsa
| Animal and disease | Age (yr) | Sex | Predisposing factor(s) | Treatment | Outcome | Location | Yr (reference) |
|---|---|---|---|---|---|---|---|
| Cat | |||||||
| Pulmonary granuloma | 12 | M | Diabetes mellitus | Surgery, oral itraconazole followed by posaconazole | Survival of 13 mo, then euthanized due to hepatocellular carcinoma | Sydney, Australia | 2011 (30) |
| Cerebral phaeohyphomycosis | 6 | M | None | No | Euthanasia | Georgia, USA | 2010 (32) |
| Fungal pyelonephritis | 11 | M | Skin scratches | Oral itraconazole | Euthanasia after 7 mo | United Kingdom | 2007 (23) |
| Systemic phaeohyphomycosis (abdomen and thorax) | 9 | M | None | Oral ketoconazole | Died after 1 wk | France | 2003 (20) |
| Cerebral phaeohyphomycosis | 0.5 | M | None | No | Euthanasia | Minnesota, USA | 2002 (29) |
| Cutaneous eumycetoma | 5 | M | None | Surgery, oral itraconazole followed by fluconazole | Cutaneous lesion relapsed, but clinically normal after 6 mo | Italy | 2002 (22) |
| Cerebral phaeohyphomycosis | 6 | M | None | No | Died | Alabama, USA | 1987 (44) |
| Cerebral phaeohyphomycosis | 1.5 | F | None | No | Died | Australia | 1985 (31) |
| Cerebral phaeohyphomycosis | 1 | M | None | No | Euthanasia | California, USA | 1977 (33) |
| Cerebral phaeohyphomycosis | 3 | M | None | No | Euthanasia | California, USA | 1977 (33) |
| Kidney granuloma | 7 | F | Leukemia | No | Euthanasia due to underlying disease | California, USA | 1974 (38) |
| Dog | |||||||
| Eumycetoma (abdominal wall) | 5 | M | None | Cryotherapy, surgery, oral itraconazole | Asymptomatic at 1-yr checkup | Taipei, Taiwan | 2013 (39) |
| Cerebral phaeohyphomycosis, pyelonephritis | 0.3 | M | Canine distemper | No | Died | Brazil | 2013 (45) |
| Eumycetoma (thoracic wall) | 3 | M | Corticosteroid treatment, dermatitis after contact with processionary caterpillars | Surgery, oral ketoconazole; surgery repeated, then oral itraconazole and flucytosine | Asymptomatic after 6 weeks | France | 2004 (21) |
| Cerebral and systemic phaeohyphomycosis (spinal cord, spleen, lymph nodes, lungs) | 2 | F | None | Oral itraconazole, intravenous amphotericin B | Euthanasia after 4 mo | South Africa | 2002 (46) |
| Cerebral and systemic phaeohyphomycosis (liver, kidney) | 8 | M | None | Oral fluconazole | Euthanasia | California, USA | 2001 (34) |
| Systemic phaeohyphomycosis | NA | NA | Lymphopenia | NA | Fatal | South Africa | 1996 (35) |
| Systemic phaeohyphomycosis | NA | NA | Lymphopenia | NA | Fatal | South Africa | 1996 (35) |
| Cerebral and systemic phaeohyphomycosis (liver, spleen, kidney, adrenal glands) | 8 | F | Corticosteroid treatment, ehrlichiosis | Ketoconazole | Euthanasia | South Africa | 1994 (36) |
| Cerebral phaeohyphomycosis | 0.3 | F | Distemper | No | Euthanasia | Alabama, USA | 1987 (44) |
| Cerebral phaeohyphomycosis | 0.5 | M | None | No | Euthanasia | Alabama, USA | 1987 (44) |
| Cerebral and systemic phaeohyphomycosis (liver, kidney, spleen) | 9 | F | None | No | Euthanasia | South Africa | 1980 (47) |
| Huacaya alpaca | |||||||
| Cerebral phaeohyphomycosis | 8 | M | None | No | Died | Indiana, USA | 2011 (37) |
| Snow leopard | |||||||
| Systemic phaeohyphomycosis (spinal cord) | 0.4 | F | None | No | Euthanasia | Switzerland | 2006 (24) |
Abbreviations: NA, data not available; M, male; F, female.
An increasing number of case reports suggests that C. bantiana may be an emerging animal pathogen capable of affecting several mammal species. Published animal cases are listed in Table 1. The table includes only infections in which the fungus has been confirmed to the species level. In addition to these cases, there are some reports of animal infections where species-level confirmation had not been performed but which were probably due to C. bantiana (25–28). In addition to the equine case of this report, animal species affected by this fungus include cats (20, 23, 29–33), dogs (21, 27, 34–36), a snow leopard (24), and an alpaca (37). The predilection of this fungus for the central nervous system (CNS) is also apparent in animals: 58% of previously reported cases had lesions in the brain, and the clinical signs in these were similar to those of humans. Dissemination of the disease in animals is common, since in 50% (12/24) of cases, lesions in various internal organs were observed with (n = 5) or without (n = 7) CNS involvement. Dark or black lesions in internal organs and the CNS are a characteristic feature of systemic C. bantiana infections (8, 20, 24, 36, 38). As in humans, the outcome of cerebral or systemic phaeohyphomycosis is often fatal, but local infections may have a better prognosis, provided that surgery and long-term medical treatment are combined. Three case reports have been published on the presence of local eumycetomas in animals: two in dogs (21, 39) and one in a cat (22). In dogs, typical chronic lesions with tumefaction, fistula, and draining discharge with black grains or granules were described. The granules are bundles of hyphae embedded in a cement-like substance and are considered highly indicative for an etiological agent belonging to black molds (8). These types of granules were also characteristic in our case.
Our patient was a classic example of equine fungal endometritis, although the fungus associated with the infection was exceptional. The mare did not show other clinical signs except infertility and the accumulation of uterine fluid. Cytology suggested that beta-hemolytic Streptococcus together with C. bantiana was associated with the infection at first, but it later became evident that C. bantiana was the pathogen persisting in the uterus. This was manifested by the repeated appearance of black granules in the uterine lavage fluids. The infection was limited to the uterine lumen, as fungal hyphae were not detected in any of the biopsy specimens taken during the treatment or at necropsy. It is a typical feature of equine fungal endometritis that the infection is superficial and that fungal elements are usually free in the uterine lumen or adjacent to the luminal surface (6). As a result, fungal structures may be rinsed off from a slide during the processing of uterine biopsy specimens, which may delay the diagnosis (6). Therefore, direct microscopic examination of uterine fluid or uterine swab cytology is an important diagnostic tool for fungal endometritis. This was also observed in our case, where cytology indicated the presence of fungal hyphae together with inflammatory cells suggesting an infection, although C. bantiana was not at this stage suspected.
Our patient had several factors that may have predisposed the horse to a fungal infection: uterine fluid accumulation and the persistence of infection suggested impaired uterine emptying and/or local defense mechanisms. A cervical trauma due to fetotomy probably caused inadequate closure of the cervix. This, together with frequent manipulations (due to artificial inseminations and treatments) and repeated antimicrobial administration, may have caused disturbances of the normal vaginal microbiota. All these are considered factors associated with fungal endometritis in horses, although the mechanisms of pathogenesis of the condition are not well known (6). In addition to uterine lavages and local antimicrobials, the treatment protocol of our patient included local and systemic administration of fluconazole, a triazole. The drug has not been registered for use in horses in the European Union (EU) but can be used if the horse is removed from the food chain. The treatment response in our case was poor, but this may have been the case regardless of the fungal species, because the prognosis of fungal endometritis is guarded (6). The drug therapy was also ineffective, because fluconazole has poor or no activity against filamentous fungi, including C. bantiana (40). Nevertheless, the drug probably suppressed the growth of the fungus to some extent, as periods of clinical improvement were observed between the estrus cycles. Regrowth of the fungus in the uterus may have been due to the poor effect of fluconazole or reintroduction of the same organism from the caudal part of the reproductive tract along with inseminations and/or local treatments. The susceptibility of our strain was not tested, but reported fluconazole MICs for this species range from 16 to 64 μg/ml (40). In horses, after repeated oral administration, fluconazole concentrations in tissues vary between 11 and 57 μg/ml, depending on the type of tissue (41). Local administration of the drug is expected to raise the concentration far beyond the reported MICs, although the concentration is merely high enough to eradicate the organism. Concerning yeast infections, the primary targets for fluconazole therapy, the EUCAST susceptibility breakpoint indicating a good clinical response in humans is ≤2 μg/ml. Moreover, due to the fungistatic nature of the drug, an area under the concentration-time curve (AUC)/MIC ratio of ≥100 should be achieved in vivo to confirm a clinical cure. It is highly unlikely that this target would have been achieved for long enough, even with the local administration of fluconazole. Possible drugs that could have been used for treating infections caused by C. bantiana include itraconazole, voriconazole, posaconazole, and amphotericin B (40), but the costs often limit their use in horses. Toxicity may also limit the systemic use of the drugs, especially for amphotericin B. The treatment of our patient was stopped when the suspicion of C. bantiana species was aroused. The decision was justified by several unsuccessful inseminations, high treatment costs, the poor prognosis, and the potential health hazard to our staff.
Timely recognition of a causative organism is important in setting the prognosis and making treatment decisions. The differentiation between yeasts and filamentous fungi is important because it affects the empirical selection of an antifungal agent. Moreover, endometritis caused by filamentous fungi has a poorer prognosis than do infections caused by yeasts (6). In some circumstances, it may also be equally important to forgo treatment in animals. For example, neurotropic properties and the lack of efficient treatment regimens make C. bantiana a potential health hazard for veterinarians handling the animals affected by this fungus. In the majority of the reports concerning C. bantiana in animals, health hazard aspects have not been discussed. Coldrick et al. (23) advised that handling an affected animal or specimens containing hyphal fragments posed no health risk to humans. A similar consideration was made in our case. To our best knowledge, the transmission of C. bantiana between animals and humans has not been described. The handling of cultures is potentially more hazardous, because the fungus can produce infectious conidia in vitro. Dimorphic fungi have been reported to cause laboratory-acquired infections, but there are no such reports regarding C. bantiana (42). Nevertheless, C. bantiana is classified in the EU as a hazard category 3 agent requiring biosafety level (BSL) 3 containment facilities for manipulating cultures of this species (43). In the United States, BSL 2 containment is required (8). According to the directive 2000/54/EC, employers are obliged to keep a record of persons exposed to hazard group 3 and 4 organisms, and the records are stored for 10 years (43). In our case, the persons handling the fungal cultures at the clinic's laboratory were considered exposed to C. bantiana, because cultures were not handled in a biosafety cabinet. The risk of infection, however, was considered by an infectious disease specialist to be low. Specimens with suspected fungal growth are now required to be sent to an experienced mycological laboratory. Clinicians were advised to consider C. bantiana as one possibility among differential diagnoses if discharge with black granules is observed. It was further advised that proper precautions, such as the use of gloves and protective clothing (i.e., masks in procedures creating aerosols), as well as the cautious use of sharp objects, are necessary when suspect animals are handled, although this should be routine in any case.
ACKNOWLEDGMENTS
We thank Roy Siddall for reviewing the English of our manuscript and Thomas Grönthal for providing invaluable comments on the content of the manuscript.
REFERENCES
- 1.Moller EM, Bahnweg G, Sandermann H, Geiger HH. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116. doi: 10.1093/nar/20.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc, New York, NY. [Google Scholar]
- 3.LeBlanc MM, Causey RC. 2009. Clinical and subclinical endometritis in the mare: both threats to fertility. Reprod Domest Anim 44(Suppl 3):10–22. doi: 10.1111/j.1439-0531.2009.01485.x. [DOI] [PubMed] [Google Scholar]
- 4.Dascanio JJ, Schweizer C, Ley WB. 2001. Equine fungal endometritis. Equine Vet Educ 13:324–329. [Google Scholar]
- 5.Albihn A, Baverud V, Magnusson U. 2003. Uterine microbiology and antimicrobial susceptibility in isolated bacteria from mares with fertility problems. Acta Vet Scand 44:121–129. doi: 10.1186/1751-0147-44-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutinho da Silva MA, Alvarenga MA. 2011. Fungal endometritis, p 2643–2651. In McKinnon AO, Squires EL, Vaala WE, Varner DD (ed), Equine reproduction, 2nd ed, vol 2 John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 7.Seyedmousavi S, Netea MG, Mouton JW, Melchers WJ, Verweij PE, de Hoog GS. 2014. Black yeasts and their filamentous relatives: principles of pathogenesis and host defense. Clin Microbiol Rev 27:527–542. doi: 10.1128/CMR.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin Microbiol Rev 23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A, Perfect J, de Hoog GS. 2014. Black molds and melanized yeasts pathogenic to humans. Cold Spring Harb Perspect Med doi: 10.1101/cshperspect.a019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzoni C, Markham L, Bijlenga P, Garbino J. 2008. Cladophialophora bantiana: a rare cause of fungal brain abscess. Clinical aspects and new therapeutic options. Med Mycol 46:481–486. doi: 10.1080/13693780801914906. [DOI] [PubMed] [Google Scholar]
- 11.Hussey SM, Gander R, Southern P, Hoang MP. 2005. Subcutaneous phaeohyphomycosis caused by Cladophialophora bantiana. Arch Pathol Lab Med 129:794–797. [DOI] [PubMed] [Google Scholar]
- 12.Jacyk WK, Du Bruyn JH, Holm N, Gryffenberg H, Karusseit VO. 1997. Cutaneous infection due to Cladophialophora bantiana in a patient receiving immunosuppressive therapy. Br J Dermatol 136:428–430. doi: 10.1046/j.1365-2133.1997.d01-1216.x. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, Agrawal SC, Jain PC. 2003. Subcutaneous phaeohyphomycosis on face caused by Cladophialophora bantiana. Mycoses 46:237–239. [DOI] [PubMed] [Google Scholar]
- 14.Lim A, Speers D, Inderjeeth C. 2013. Cladophialophora (Xylohypha) bantiana—an unusual cause of septic arthritis. Rheumatology 52:958–959. doi: 10.1093/rheumatology/kes317. [DOI] [PubMed] [Google Scholar]
- 15.Klite PD, Kelley HB Jr, Diercks FH. 1965. A new soil sampling technique for pathogenic fungi. Am J Epidemiol 81:124–130. [DOI] [PubMed] [Google Scholar]
- 16.Dixon DM, Shadomy HJ, Shadomy S. 1977. Isolation of Cladosporium trichoides from nature. Mycopathologia 62:125–127. doi: 10.1007/BF01259404. [DOI] [PubMed] [Google Scholar]
- 17.Espinel-Ingroff A, Kerkering TM, Shadomy HJ. 1982. Isolation of dematiaceous pathogenic fungi from a feed and seed warehouse. J Clin Microbiol 15:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurjevic Z, Li C, Dobranic J. 2013. First finding of Cladophialophora bantiana from the indoor environment in the United States, abstr SR-140-02. Am Ind Hyg Conf Expos (AIHce), Montreal, Canada. [Google Scholar]
- 19.Petrini B, Farnebo F, Hedblad MA, Appelgren P. 2006. Concomitant late soft tissue infections by Cladophialophora bantiana and Mycobacterium abscessus following tsunami injuries. Med Mycol 44:189–192. doi: 10.1080/13693780500294949. [DOI] [PubMed] [Google Scholar]
- 20.Elies L, Balandraud V, Boulouha L, Crespeau F, Guillot J. 2003. Fatal systemic phaeohyphomycosis in a cat due to Cladophialophora bantiana. J Vet Med A Physiol Pathol Clin Med 50:50–53. doi: 10.1046/j.1439-0442.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 21.Guillot J, Garcia-Hermoso D, Degorce F, Deville M, Calvie C, Dickele G, Delisle F, Chermette R. 2004. Eumycetoma caused by Cladophialophora bantiana in a dog. J Clin Microbiol 42:4901–4903. doi: 10.1128/JCM.42.10.4901-4903.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramo F, Bastelli F, Nardoni S, Mancianti F. 2002. Feline cutaneous phaeohyphomycosis due to Cladophialophora bantiana. J Feline Med Surg 4:157–163. doi: 10.1053/jfms.2002.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coldrick O, Brannon CL, Kydd DM, Pierce-Roberts G, Borman AM, Torrance AG. 2007. Fungal pyelonephritis due to Cladophialophora bantiana in a cat. Vet Rec 161:724–727. doi: 10.1136/vr.161.21.724. [DOI] [PubMed] [Google Scholar]
- 24.Janovsky M, Grone A, Ciardo D, Vollm J, Burnens A, Fatzer R, Bacciarini LN. 2006. Phaeohyphomycosis in a snow leopard (Uncia uncia) due to Cladophialophora bantiana. J Comp Pathol 134:245–248. doi: 10.1016/j.jcpa.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Miller DM, Blue JL, Winston SM. 1983. Keratomycosis caused by Cladosporium sp in a cat. J Am Vet Med Assoc 182:1121–1122. [PubMed] [Google Scholar]
- 26.Migaki G, Casey HW, Bayles WB. 1987. Cerebral phaeohyphomycosis in a dog. J Am Vet Med Assoc 191:997–998. [PubMed] [Google Scholar]
- 27.Bentley RT, Faissler D, Sutherland-Smith J. 2011. Successful management of an intracranial phaeohyphomycotic fungal granuloma in a dog. J Am Vet Med Assoc 239:480–485. doi: 10.2460/javma.239.4.480. [DOI] [PubMed] [Google Scholar]
- 28.Mariani CL, Platt SR, Scase TJ, Howerth EW, Chrisman CL, Clemmons RM. 2002. Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthair cats. J Am Anim Hosp Assoc 38:225–230. doi: 10.5326/0380225. [DOI] [PubMed] [Google Scholar]
- 29.Bouljihad M, Lindeman CJ, Hayden DW. 2002. Pyogranulomatous meningoencephalitis associated with dematiaceous fungal (Cladophialophora bantiana) infection in a domestic cat. J Vet Diagn Invest 14:70–72. doi: 10.1177/104063870201400116. [DOI] [PubMed] [Google Scholar]
- 30.Evans N, Gunew M, Marshall R, Martin P, Barrs V. 2011. Focal pulmonary granuloma caused by Cladophialophora bantiana in a domestic short haired cat. Med Mycol 49:194–197. doi: 10.3109/13693786.2010.519349. [DOI] [PubMed] [Google Scholar]
- 31.Shinwari MW, Thomas AD, Orr JS. 1985. Feline cerebral phaeohyphomycosis associated with Cladosporium bantianum. Aust Vet J 62:383–384. doi: 10.1111/j.1751-0813.1985.tb14216.x. [DOI] [PubMed] [Google Scholar]
- 32.Simmons JK, McManamon R, Rech RR, Phillips AE, Howerth EW. 2010. Pathology in practice. Necrotizing pyogranulomatous meningoencephalitis with intralesional fungal hyphae, consistent with Cladophialophora bantiana. J Am Vet Med Assoc 236:295–297. doi: 10.2460/javma.236.3.295. [DOI] [PubMed] [Google Scholar]
- 33.Jang SS, Biberstein EL, Rinaldi MG, Henness AM, Boorman GA, Taylor RF. 1977. Feline brain abscesses due to Cladosporium trichoides. Sabouraudia 15:115–123. doi: 10.1080/00362177785190191. [DOI] [PubMed] [Google Scholar]
- 34.Anor S, Sturges BK, Lafranco L, Jang SS, Higgins RJ, Koblik PD, LeCouteur RA. 2001. Systemic phaeohyphomycosis (Cladophialophora bantiana) in a dog—clinical diagnosis with stereotactic computed tomographic-guided brain biopsy. J Vet Intern Med 15:257–261. doi: 10.1111/j.1939-1676.2001.tb02321.x. [DOI] [PubMed] [Google Scholar]
- 35.Lobetti RG. 1996. Leukogram and serum globulin values in two dogs with systemic Xylohypha bantiana infection. J S Afr Vet Assoc 67:91–92. [PubMed] [Google Scholar]
- 36.Schroeder H, Jardine JE, Davis V. 1994. Systemic phaeohyphomycosis caused by Xylohypha bantiana in a dog. J S Afr Vet Assoc 65:175–178. [PubMed] [Google Scholar]
- 37.Frank C, Vemulapalli R, Lin T. 2011. Cerebral phaeohyphomycosis due to Cladophialophora bantiana in a Huacaya alpaca (Vicugna pacos). J Comp Pathol 145:410–413. doi: 10.1016/j.jcpa.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Reed C, Fox JG, Campbell LH. 1974. Leukaemia in a cat with concurrent Cladosporium infection. J Small Anim Pract 15:55–62. doi: 10.1111/j.1748-5827.1974.tb05644.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun PL, Peng PC, Wu PH, Chiang YL, Ju YM, Chang CC, Wang PC. 2013. Canine eumycetoma caused by Cladophialophora bantiana in a Maltese: case report and literature review. Mycoses 56:376–381. doi: 10.1111/myc.12033. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Florl C, Lortholary O, Meletiadis J, Munoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 20(Suppl 3):S47–S75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 41.Latimer FG, Colitz CM, Campbell NB, Papich MG. 2001. Pharmacokinetics of fluconazole following intravenous and oral administration and body fluid concentrations of fluconazole following repeated oral dosing in horses. Am J Vet Res 62:1606–1611. doi: 10.2460/ajvr.2001.62.1606. [DOI] [PubMed] [Google Scholar]
- 42.Singh K. 2009. Laboratory-acquired infections. Clin Infect Dis 49:142–147. doi: 10.1086/599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Economic Community. 2000. Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC). Office for Official Publication of the European Communities, Luxembourg City, Luxembourg. [Google Scholar]
- 44.Dillehay DL, Ribas JL, Newton JC Jr, Kwapien RP. 1987. Cerebral phaeohyphomycosis in two dogs and a cat. Vet Pathol 24:192–194. [DOI] [PubMed] [Google Scholar]
- 45.Docal CR, Lopes LL, de Campos CG, da Silveira MM, de Paula DAJ, Pescador CA. 2013. Feohifomicose no cérebro e nefrite em cão infectado pelo vírus da cinomose canina. Arch Vet Sci 18:619–621. [Google Scholar]
- 46.Leisewitz AL, Rademeyer C, Picard J. 2002. The use of liposomal amphotericin B in the management of Xylohypha bantiana mycosis in a dog. J S Afr Vet Assoc 73:79–82. [DOI] [PubMed] [Google Scholar]
- 47.Newsholme SJ, Tyrer MJ. 1980. Cerebral mycosis in a dog caused by Cladosporium trichoides Emmons 1952. Onderstepoort J Vet Res 47:47–49. [PubMed] [Google Scholar]