Abstract
Balamuthia mandrillaris is a rare cause of human infection, but when infections do occur, they result in high rates of morbidity and mortality. A case of disseminated Balamuthia infection is presented. Early diagnosis and initiation of recommended therapy are essential for increased chances of successful outcomes.
CASE REPORT
The patient was an 82-year-old white male with a history of necrobiosis lipoidica versus granuloma annulare of the right hand who was admitted in April 2012 for acute onset of fevers, chills, nausea, vomiting, lethargy, and altered mental status (AMS). He initially presented to an outside-hospital emergency department with a temperature of 38.5°C, a heart rate of 97 beats per min, and oxygen saturation of 88% on room air. His oxygen saturation improved with administration of nasal cannula oxygen. A chest X-ray (CXR) procedure was performed and demonstrated findings concerning for granulomatous disease. Due to his condition, he was transferred to our tertiary care center for further evaluation and treatment.
Per his family, he had been healthy and without complaints prior to his current presentation. For approximately 15 months, he had been treated by a dermatologist for skin lesions located on his right thigh and hand as well as left upper extremity (LUE) and abdomen. He had multiple skin biopsy specimens from the right hand, LUE, and right thigh with negative bacterial, acid-fast bacillus (AFB), and fungal analysis results. During this period of time, he took itraconazole for 3 months without clinical response and was thus started on prednisone. All lesions resolved except those on his right hand. He was treated with a 14-day course of doxycycline and cephalexin for possible cellulitis of the right hand prior to this admission. Previous workup included a negative syphilis screen and a normal angiotensin-converting enzyme (ACE) level (drawn for possible sarcoidosis). A CXR performed at that time revealed nonspecific interstitial changes.
A review of the patient's symptoms was obtained from family members given the patient's AMS. Per their report, the patient reported a headache but no neck pain or stiffness. He had no history of mouth sores or genital ulcers and had a remote history of shingles. He had had an episode of bronchitis 1 month prior to admission (PTA) that was slow to improve but gradually resolved.
In terms of exposures, he had no history of international travel but did have seawater exposure on the Gulf Coast of Alabama 1 month prior to admission. He had no pets but had been mowing grass and spreading grass seed PTA. He had no known tick bites but spent time outdoors using a metal detector for treasure hunting and digging in soil. Previously, he had taught a bible study group in a prison but had no known tuberculosis (TB) exposure and no previous TB testing.
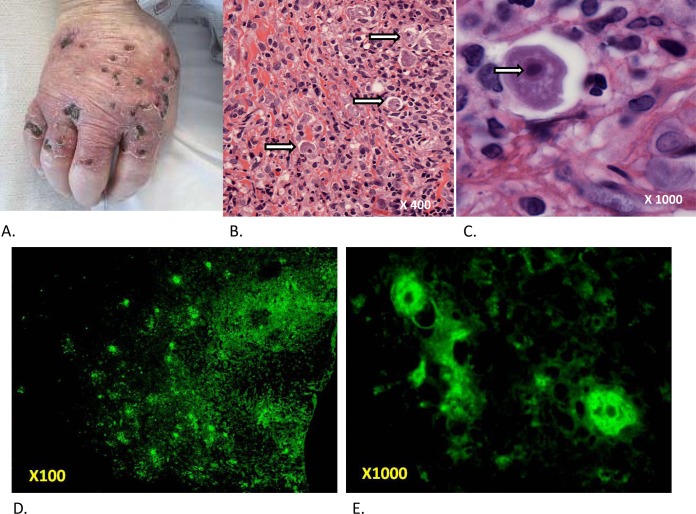
In an initial physical examination, he was febrile to 38.2°C and his oxygen saturation was 94% with 2 liters of oxygen administered using a nasal cannula. He was initially agitated and confused but progressed to somnolence over a period of 12 h. He had no cervical lymphadenopathy or nuchal rigidity. Cardiac, pulmonary, and abdominal examination results were unremarkable. On the right dorsal hand and proximal fingers, there was a collection of erythematous punched-out ulcers with overlying crusts that extended onto the palm at the 5th metacarpal-phalangeal surface (Fig. 1A). His initial laboratory values were significant for a total white blood cell (WBC) count of 8,600/μl with 88.3% neutrophils and 4.8% lymphocytes. He also had a hemoglobin level of 13.1 g/dl, hematocrit of 37.7%, sodium of 129 mmol/liter, albumin of 2.9 g/dl, creatinine of 1.3 mg/dl, and glucose of 256 g/dl.
FIG 1.
Dermatologic presentation and pathology. (A) Erythematous punched-out ulcers with overlying crusts on the right dorsal hand. (B) Histopathology from the right hand showing a dense mixed acute and chronic dermal inflammatory infiltrate and scattered amebic trophozoites within lacunar spaces (arrows). (Original magnification, ×400). (C) High-power view of an amebic organism demonstrating a granular to vacuolated cytoplasm with irregular contours (pseudopods) containing a nucleus with a large central karyosome (arrow). (Original magnification, ×1,000). (D) Low-power view of immunofluorescence from skin biopsy specimen. (Original magnification, ×100). (E) High-power view of immunofluorescence from skin biopsy specimen with visible amoebic organisms. (Original magnification, ×1,000).
A computed tomography (CT) scan of the head revealed age-indeterminate infarcts in the anterior limb of the right internal capsule, left parafalcine frontal lobe, and inferior left frontal lobe thought likely to be chronic infarcts. A CXR showed diffuse, bilateral opacities. A CT scan of the chest revealed diffuse centrilobular nodules with intralobular septal thickening. Given his presentation and imaging findings, he was empirically started on intravenous (i.v.) ceftriaxone and azithromycin for coverage of community-acquired pneumonia.
On hospital day (HD) 1, staff members of the Infectious Disease department were consulted and the patient was empirically started on treatment for meningoencephalitis, including i.v. vancomycin, ceftriaxone, acyclovir, and trimethoprim-sulfamethoxazole (a reported but unknown penicillin allergy precluded the use of ampicillin for coverage of a possible Listeria infection), in addition to the antibiotics for pneumonia. A repeat examination performed at that time did reveal nuchal rigidity. Cerebrospinal fluid (CSF) analysis via lumbar puncture revealed an opening pressure of 21 cm H2O, protein at 54 mg/dl, and glucose at 86 mg/dl. A cell count was remarkable for 14 WBCs (57% lymphocytes, 3% neutrophils, and 40% monocytes). No organisms were identified on Gram stain. Dermatology analyses were also performed using skin biopsy specimens and cultures of the patient's right hand.
On HD 2, the preliminary report of the results of analyses of the patient's skin biopsy specimen revealed a dense mixed acute and chronic dermal inflammatory infiltrate. Scattered organisms, morphologically compatible with ameba, were noted within lacunar spaces (Fig. 1B; original magnification, ×400). The organisms showed granular to vacuolated cytoplasm with irregular contours and contained enlarged nuclei with large central karyosomes (Fig. 1C; original magnification, ×1,000). The patient was therefore empirically started on liposomal amphotericin and voriconazole for coverage of disseminated amebiasis and amebic encephalitis (AE). All other antibiotics except for azithromycin were discontinued. Despite treatment with broad-spectrum antibiotics, the patient continued to become increasingly obtunded, eventually requiring transfer to the intensive care unit (ICU) on HD 3 for closer monitoring and worsening hypoxia, intermittently requiring increased oxygen supplementation.
On HD 4, the Centers for Disease Control and Prevention (CDC) were contacted for further therapeutic options. CDC recommended combination therapy shown to have efficacy in previous cases, including pentamidine, sulfadiazine, flucytosine, fluconazole (or itraconazole), and azithromycin (or clarithromycin) as well as miltefosine, which at the time required approval by the Food and Drug Administration (FDA) and overseas delivery before it could be used (1–4). As there was not yet a definitive identification of the ameba species, sulfadiazine at 1.5 g every 6 h, pyrimethamine at 200 mg once and then 75 mg daily, and leucovorin at 25 mg daily were added, based on case reports of successful treatment of Acanthamoeba encephalitis (5). His azithromycin dose was increased to 1,800 mg daily as well. At this time, miltefosine was ordered.
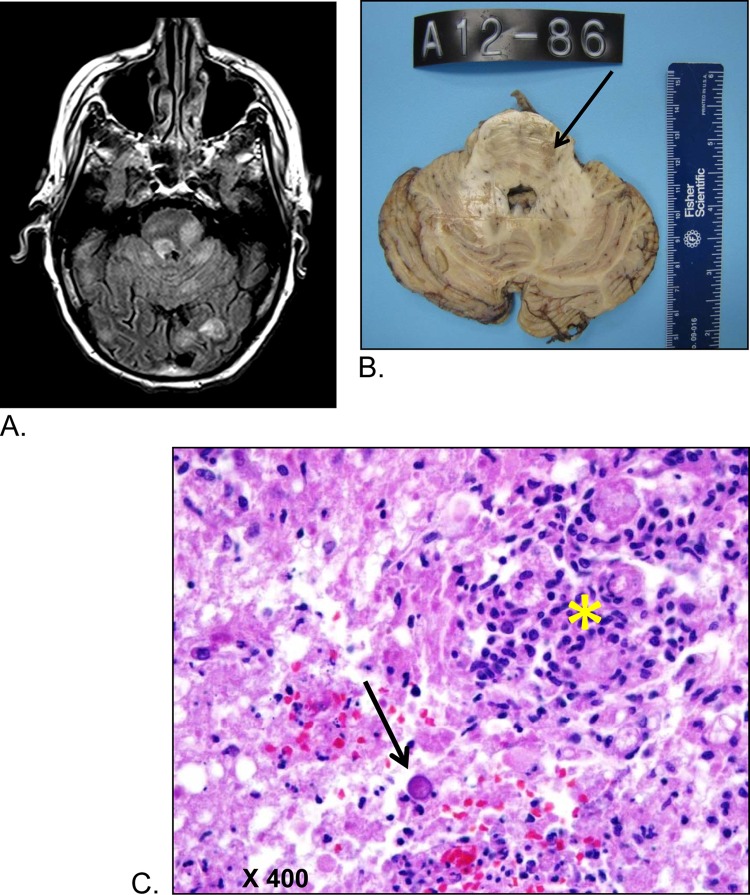
Given the persistent AMS and concern for AE, magnetic resonance imaging (MRI) of the brain was performed, which revealed multiple parenchymal supra- and infratentorial rounded lesions with surrounding white matter edema and faint enhancement. The largest lesions were in the left posterior temporal lobe. Other lesions were seen in the left dorsal pons and left frontal lobe. Radiology results indicated that these lesions were likely to be infectious in nature. Neurosurgery staff members were consulted on HD 4 for urgent biopsy of the patient's largest brain mass. At this time, the patient's sodium level continued to decrease to a nadir of 121 mmol/liter. Because of his worsening hyponatremia and the small size of the lesions, they deferred brain biopsy and recommended a repeat MRI for evaluation of progression of his lesions. Also, because of the patient's worsening respiratory symptoms, he underwent evaluation using bronchoscopy. The results of analysis of the bronchoalveolar lavage (BAL) fluid were negative for Legionella pneumophila, Pneumocystis jirovecii, acid-fast bacilli, viruses, and fungal etiologies. Gram-negative rods were visible in the BAL fluid Gram stain, so cefepime was added to his antibiotic regimen.
On HD 5, the patient's condition remained unchanged with no signs of improvement. Following CDC recommendations, flucytosine at 1,750 mg administered i.v. every 12 h (q12h) was added to his treatment regimen. Miltefosine was being held in Customs at that time, pending FDA release.
On HD 6, a repeat MRI demonstrated increases in the number and size of prior lesions. These lesions also showed perilesional edema and faint enhancement. At that time, the neurosurgery staff members planned to perform a biopsy with radiologic guidance on HD 10 as his serum sodium normalized.
On HD 7, the bacterial culture from the BAL fluid grew “a few” Pseudomonas aeruginosa bacteria which were sensitive to cefepime. The patient's inability to handle secretions became more pronounced, and his clinical status continued to deteriorate, requiring increasing oxygen and low-dose vasopressor support. A family meeting was held to discuss goals of care; the family decided to continue antibiotics but to change his code status to “Do Not Resuscitate or Intubate.”
On HD 8, CDC reported preliminary identification of ameba forms on a skin biopsy specimen which likely represented Balamuthia. The pyrimethamine and leucovorin treatments were discontinued, and the patient was started on pentamidine at 300 mg i.v. daily.”
On HD 9, the patient began to have periods of apnea and expired with family at the bedside.
The family agreed to proceed with a limited autopsy of the brain only. A postmortem gross examination revealed numerous red to brown soft lesions involving bilateral parietal, frontal, and temporal lobes, left pons and basal ganglia, and cerebellum, predominantly in subcortical white matter, with many lesions extending to the gray-white junction. Histopathologic examination revealed the granulomatous nature of the lesions, with predominantly cyst forms and occasional trophozoites, consistent with an amebic infection (Fig. 2). Additionally, purulent and granulomatous inflammation was noted in many subpial and perivascular spaces. Real-time PCR assays performed on fresh-frozen autopsy brain material determined the amebic species to be Balamuthia mandrillaris. The CDC performed immunofluorescent staining of the skin biopsy specimen, which was positive for Balamuthia mandrillaris (Fig. 1D and E). The final diagnosis was disseminated Balamuthia mandrillaris infection.
FIG 2.
Central nervous system lesions. (A) MRI axial fluid-attenuated inversion recovery (FLAIR) image demonstrating multiple focal, rounded hyperintense lesions in the pons, cerebellum, and occipital lobes. (B) Gross section of the pons and cerebellum; softened granular lesion in the pons (see arrow). (C) Microscopic examination of the pontine lesion; prominent granulomatous inflammation (yellow asterisk) in abundant necrotic background. A single amebic cyst is seen (black arrow) in the mid-lower field of the picture.
Microbiology.
Balamuthia mandrillaris is a free-living ameba that lives in soil, dust, and water (6). The species name derives from the first site from which it was isolated in 1986—the brain of a mandrill. The first human case of Balamuthia-associated granulomatous encephalitis was described in 1990. In the laboratory, Balamuthia feeds on small ameba but not bacteria, which makes its growth in the laboratory challenging. Cultivation of this organism in the laboratory requires axenic culture or growth in mammalian cell cultures (7). The exact pathogenesis of Balamuthia is incompletely defined at this time, although cell-to-cell contact appears necessary for this process, and the organism may have an affinity for binding to the extracellular matrix (2, 7–9). However, human infection appears to begin with either inhalation or percutaneous inoculation of Balamuthia cysts or trophozoites. The organisms are then believed to either migrate through tissue or via hematogenous spread to the location or locations where disease becomes manifest, which can include the brain and skin most commonly but other organs as well. A mouse model of Balamuthia encephalitis proposes the following as one possible mode of infection: the organism is inhaled through the nasal passages, adheres to the nasal epithelium, and then travels along the olfactory nerve into the central nervous system (CNS) (10).
Epidemiology.
Balamuthia has been identified in infections in both immunocompromised and immunocompetent patients. There is no clear predilection based on age or sex, but cases have been reported from individuals at the extremes of age, and the earliest reported cases were in patients with AIDS or substance abuse disorders (2, 11). While there is an overrepresentation of Hispanic ethnicity among reported cases, the cause of this association is not understood and may be related to surveillance, exposure, biology, or other factors or combinations of factors (9, 12). Transmission through organ transplantation has also occurred (13, 14).
Clinical presentation.
Balamuthia mandrillaris has two primary clinical presentations, cutaneous and granulomatous amebic encephalitis (GAE), each of which may have a prodrome of weeks to months. There are also case reports of sinus infections and pulmonary involvement, typically, in the form of abscesses (7). Patients may present with isolated cutaneous findings or typical encephalopathy/encephalitis symptoms. The latter presentation may also include meningeal signs (9). The cutaneous manifestations can vary from ecthyma-like lesions to erythematous plaques.
The pulmonary findings in our case represent a presentation different from those seen in other cases described in the literature. Although we do not have a definitive tissue diagnosis for his pulmonary process, it likely represented disseminated amebic infection given the presence of the organism in two other anatomic locations (skin and brain).
Diagnosis.
The diagnostic difficulty derives from the nonspecific presentation, which may not suggest amebic infection early in the disease course. A thorough exposure history, skin examination, and neurologic assessment may suggest inclusion of this entity in the differential diagnosis. If neurologic symptoms are present, brain MRI results may be helpful and may show space-occupying, ring-enhancing lesions. If cutaneous involvement is present, a skin biopsy specimen can be diagnostic if trophozoite forms are seen. Balamuthia species, although difficult to distinguish from Acanthamoeba species in hematoxylin and eosin (H&E) staining, may have 2 to 3 nucleoli visible. Immunofluorescence staining, PCR, and an experimental serologic test can be performed at CDC to distinguish between Balamuthia and Acanthamoeba species. A cerebrospinal fluid examination may reveal lymphocytic pleocytosis, elevated protein levels, normal or low glucose levels, and normal or mildly elevated opening pressure levels. Brain biopsy specimens may show both trophozoite and cyst forms of Balamuthia that show positive immunofluorescent staining results.
Consideration of Balamuthia infection early in the disease course is the most important element of a diagnostic workup. Once the diagnosis is included in the differential, further diagnostic workup, treatment guidance, and prompt access to miltefosine can be obtained by contacting the CDC Emergency Operations Center at (770) 488-7100. Additionally, further information about the clinical features of Balamuthia and its basic workup is available on the CDC website at http://www.cdc.gov/parasites/balamuthia/health_professionals/index.html.
Treatment/prognosis.
Prognosis is poor, in part because the diagnosis is often not considered until late in the disease course. There is no clear standard therapy for infections with B. mandrillaris. Pentamidine, flucytosine, fluconazole, and sulfadiazine plus either azithromycin or clarithromycin (combined with surgical resection if CNS lesions are present) have been used successfully in a few cases (2). Additionally, miltefosine, an agent used to treat leishmaniasis, has demonstrated some in vitro effect on Balamuthia infections and has been used successfully in combination therapy of Balamuthia GAE and skin infections (4, 15). Miltefosine is not readily available in the United States but can now be obtained directly from the CDC under an FDA-approved investigational new drug protocol for treatment of free-living ameba infections (16). Amphotericin B, trimethoprim-sulfamethoxazole (TMP-SMX), and polymyxin B have shown little to no in vitro effects on Balamuthia (7). Other work suggests that cyst formation in brain tissue may be hindered by ketoconazole, cytochalasin D, and cycloheximide (17).
In summary, Balamuthia mandrillaris infection is a rare, but important, cause of encephalitis and cutaneous infection. It is difficult to diagnose, and early tissue biopsy is extremely important. This disease manifests with a very high mortality rate. Treatment is poorly defined, but early diagnosis, prompt ameba-specific treatment, and increased availability of miltefosine through the CDC may have a positive effect on outcomes in the future.
ACKNOWLEDGMENTS
The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C II, Robertson W Jr. 2010. Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics 125:e699–e703. doi: 10.1542/peds.2009-1797. [DOI] [PubMed] [Google Scholar]
- 2.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. 2003. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis 37:1304–1312. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 3.Jung S, Schelper RL, Visvesvara GS, Chang HT. 2004. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med 128:466–468. [DOI] [PubMed] [Google Scholar]
- 4.Martínez DY, Seas C, Bravo F, Legua P, Ramos C, Cabello AM, Gotuzzo E. 2010. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 51:e7–e11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 5.Seijo Martinez M, Gonzalez-Mediero G, Santiago P, Rodriguez De Lope A, Diz J, Conde C, Visvesvara GS. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J Clin Microbiol 38:3892–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John D, Howard M. 1995. Seasonal distribution of pathogenic free-living amebae in Oklahoma waters. Parasitol Res 81:193–201. [DOI] [PubMed] [Google Scholar]
- 7.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 8.Kiderlen AF, Tata PS, Ozel M, Laube U, Radam E, Schäfer H. 2006. Cytopathogenicity of Balamuthia mandrillaris, an opportunistic causative agent of granulomatous amebic encephalitis. J Eukaryot Microbiol 53:456–463. doi: 10.1111/j.1550-7408.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 9.Mandell GL, Bennett JE, Dolin R. 2009. Mandell, Douglas, and Bennett's principles and practice of infectious diseases, vol 7 Churchill Livingstone, London, United Kingdom. [Google Scholar]
- 10.Kiderlen AF, Laube U. 2004. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res 94:49–52. [DOI] [PubMed] [Google Scholar]
- 11.Visvesvara GS. 2010. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr Opin Infect Dis 23:590–594. doi: 10.1097/QCO.0b013e32833ed78b. [DOI] [PubMed] [Google Scholar]
- 12.Botterill E, Yip G. 2011. A rare survivor of Balamuthia granulomatous encephalitis. Clin Neurol Neurosurg 113:499–502. doi: 10.1016/j.clineuro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). 2010. Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. MMWR Morb Mortal Wkly Rep 59:1165–1170. [PubMed] [Google Scholar]
- 14.Gupte AA, Hocevar SN, Lea AS, Kulkarni RD, Schain DC, Casey MJ, Zendejas-Ruiz IR, Chung WK, Mbaeyi C, Roy SL, Visvesvara GS, da Silva AJ, Tallaj J, Eckhoff D, Baddley JW. 2014. Transmission of Balamuthia mandrillaris through solid organ transplantation: utility of organ recipient serology to guide clinical management. Am J Transplant 14:1417–1424. doi: 10.1111/ajt.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bravo FG, Alvarez PJ, Gotuzzo E. 2011. Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis 24:112–117. doi: 10.1097/QCO.0b013e3283428d1e. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). 2013. Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep 62:666. [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui R, Matin A, Warhurst D, Stins M, Khan NA. 2007. Effect of antimicrobial compounds on Balamuthia mandrillaris encystment and human brain microvascular endothelial cell cytopathogenicity. Antimicrob Agents Chemother 51:4471–4473. doi: 10.1128/AAC.00373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]