Abstract
Peritoneal dialysis is the renal replacement modality used by ∼20% of patients with end-stage kidney disease (S. McDonald, P. Clayton, and K. Hurst, p. 6.2–6.27, in ANZDATA 2012 Annual Report, 35th ed., 2012). A major complication of peritoneal dialysis is the development of peritonitis. We describe a case of Humicola sp. causing peritoneal dialysis (PD)-associated peritonitis, successfully treated with a prolonged course of antifungal therapy.
CASE REPORT
A 41-year-old female with end-stage renal failure secondary to systemic lupus erythematosus on peritoneal dialysis (PD) presented to the emergency department with generalized abdominal pain and cloudy PD bags. White cell count (WCC) in the peritoneal fluid was 1,080 × 106/liter, and empirical treatment was commenced with intraperitoneal (IP) vancomycin and gentamicin, as per current protocols. As she was clinically stable, she was discharged home. Three days later, she presented again with increasing abdominal pain and PD bags that remained cloudy. Cultures from her original samples remained negative, and oral ciprofloxacin was commenced. Due to increasing abdominal symptoms, she agreed to inpatient care and was transferred to our hospital. On examination, when she arrived there was generalized abdominal tenderness on deep palpation, and minimal bowel sounds were audible. The PD catheter exit site was clean with no signs of erythema. The patient was afebrile (37°C) and hemodynamically stable. Blood tests showed a hemoglobin (Hb) of 99 g/dl, WCC of 7.0 × 109/liter, platelet count of 135 × 109/liter, and a C-reactive protein (CRP) level of 160 mg/liter.
In addition to lupus nephritis, her medical history included avascular necrosis secondary to steroids requiring bilateral hip replacements, a nontraumatic left below-knee amputation, a right ankle arthrodesis, and hypertension. Her regular medications were calcitriol, darbepoetin, and gabapentin. Of note, she reported that she had been snorkeling and scuba diving in the ocean 3 to 4 weeks prior to this presentation.
On the third day after her admission, due to persisting abdominal pain and cloudy dialysate, the PD catheter was removed and hemodialysis was commenced using an existing left arteriovenous (AV) fistula. Over the next 6 days, repeated imaging showed increasing ascites and peritoneal enhancement consistent with ongoing peritonitis. No discrete abscesses or collections were visualized, and standard bacteriology cultures of PD fluid remained negative. Transthoracic and transesophageal echocardiograms did not show infective endocarditis. As she remained febrile and unwell, with nonresolving intra-abdominal collections, a further laparotomy was performed. Visual inspection of the peritoneum revealed multiple white patches with cloudy ascitic fluid, and a fluid WCC was 50 × 106/liter. Further samples for culture were taken, and a washout was performed. Empirical antimicrobial therapy with intravenous (i.v.) piperacillin-tazobactam and amphotericin B was commenced. Seventeen days later, due to ongoing fever and abdominal pain, oral voriconazole was added.
Although cultures of the original peritoneal dialysis fluid samples did not demonstrate any growth on standard bacterial culture media, a subsequent peritoneal fluid sample collected when she represented demonstrated growth of a filamentous fungus from an aerobic blood culture bottle (BD Bactec Plus Aerobic/F medium; Becton Dickinson and Company, Sparks, MD, USA) incubated according to the manufacturer's instructions on a 5-day protocol. This isolate was referred to the mycology laboratory at PathWest for identification.
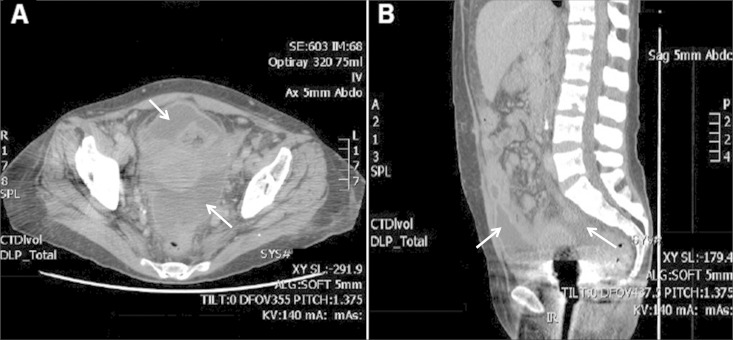
Despite ongoing treatment with amphotericin B and voriconazole, she remained febrile, and the intra-abdominal collections persisted (Fig. 1). She returned to the theater on day 49 for a further washout, which included the administration of intraperitoneal amphotericin B. Intraoperative findings showed pus in the anterior peritoneum, a frozen abdomen with mottled bowel, and infarcted parietal peritoneum at the previous wound edge, which was debrided.
FIG 1.
Computed tomography scans demonstrating persistent intra-abdominal collections (arrows). (A) Axial section; (B) sagittal section.
Repeat imaging on day 63 demonstrated residual pelvic collections, and following gynecological review, transvaginal drainage of the larger anterior collection was performed, after which her fever resolved. She was subsequently discharged 69 days following her initial presentation on oral voriconazole and daily i.v. amphotericin B infusions, which were continued for 4 months.
She is currently 18 months postdischarge and has remained well off all antimicrobial therapy, with normal inflammatory markers and bowel function. Following a period of home hemodialysis, she recently had a successful renal transplant with 4 weeks of voriconazole therapy (200 mg twice a day) prescribed in the immediate postoperative period, although peritoneal biopsy specimens taken at the time of transplantation did not demonstrate any fungus.
Mycological assessment.
Specific mycological examination of dialysate and biopsy samples subsequent to the initial isolate included wet microscopy of the clinical material using Parker Quink stain and culture utilizing brain heart infusion agar supplemented with chloramphenicol, Sabouraud dextrose agar supplemented with chloramphenicol, and malt extract agar. Multiple samples demonstrated filamentous fungal elements and yielded cultures of the fungus after 4 days of incubation. All isolates were examined morphologically and a selection sent for internal transcribed spacer (ITS) region of the ribosomal DNA (rDNA) sequence identification, which gave a presumptive identification of Humicola sp. At this time, it was determined that the maximum temperature of growth was 41°C and that the agent could be cultured in a NaCl concentration of ≥6.5%.
For further confirmation, three isolates from different samples were obtained and sent to the Mycology Unit, Medical School Universitat Rovira i Virgili, Sant Llorenç, Spain, for more extensive examination. Reference numbers were assigned accordingly (PWQ numbers refer to the culture collection numbers at PathWest and FRM numbers to the culture collection numbers at the Mycology Unit, Reus, Spain): PWQ2622 corresponds to FRM 13394, from the peritoneal tissue; PWQ2623 corresponds to FRM 13395, from abdominal tissue; and PWQ2624 corresponds to FRM 13396, from the dialysate bag.
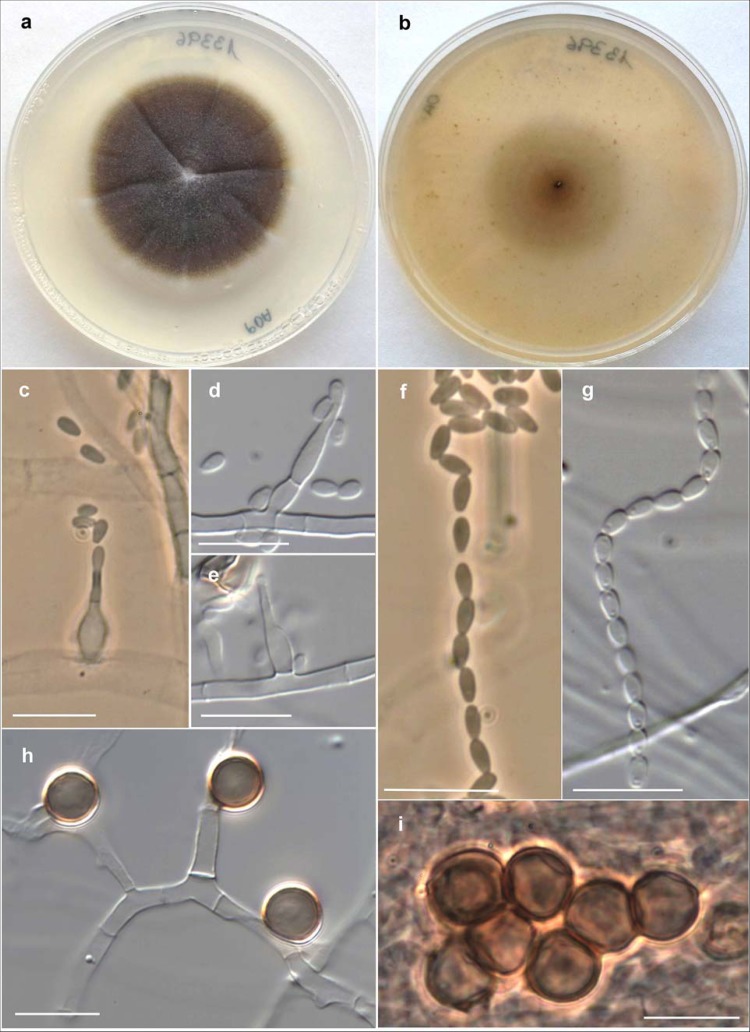
For identification purposes, the isolates were subcultured on potato dextrose agar (PDA; Pronadisa, Madrid, Spain) and oatmeal agar (OA; 30 g oat flakes, 1 g MgSO4, 1.5 g KH2PO4, 15 g agar, 1 liter tap water) and incubated at 25°C, 37°C, 40°C, and 45°C. Microscopic features were determined by making wet mounts with lactic acid and examining them under a light microscope. All three isolates showed similar morphological features and were confirmed as belonging to the genus Humicola. On PDA and OA, their colonies were glabrous to slightly cottony in the center, flat, and cream to pale brown, becoming brown to dark brown and reaching a 26- to 30-mm diameter in 7 days (Fig. 2a and b). The three isolates grew at 40°C, with colonies reaching a 7- to 10-mm diameter in 14 days, but did not grow at 45°C. Microscopically, at 25°C on the two agar media tested, they produced two types of conidia: (i) blastic conidia, which were large (7 to 9 μm in diameter), brown, globose to subglobose, often surrounded by melanin granules that gave a rough appearance to the conidial wall, and usually arranged singly and laterally on vegetative hyphae, sessile or on short stalks, but also forming terminal and intercalary chains or aggregates (Fig. 2h and i), and (ii) phialidic conidia, which were small (2.5 to 3 μm by 1.2 to 1.8 μm), hyaline, and obovate, with slightly truncate ends, arranged in long dry chains on the apex of the conidiogenous cells (phialides) (Fig. 2c to g). Phialides were discrete or intercalary, single and lateral, and more or less cylindrical or flask-shaped and measured 5 to 17 μm by 1.5 to 2 μm. Most of these features match the morphological description of Humicola fuscoatra, except the production of pigmented conidial chains or aggregates.
FIG 2.
Humicola sp. (FMR 13396). (a and b) Colonies on PDA and OA, respectively, after 14 days at 25°C. (c to e) Phialides and conidia. (f and g) Conidial chains. (h and i) Blastic pigmented conidia. Bars = 10 μm.
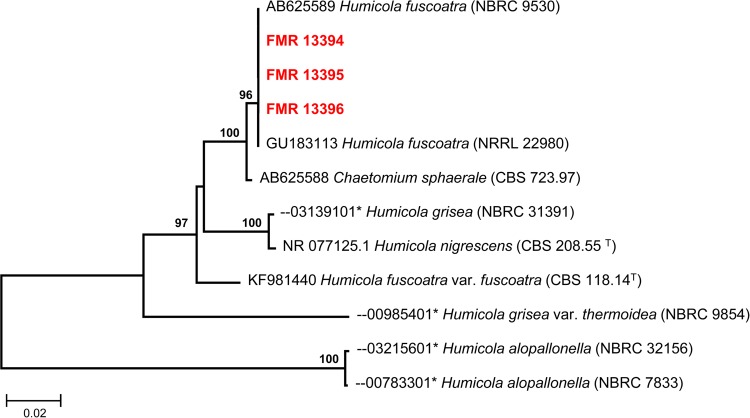
To confirm identification, the ITS region of the rDNA from the three isolates was amplified and sequenced as previously described (1). BLAST sequence homology searches were performed to compare the sequences obtained from the case isolates (approximately 560 bp) with those of other fungi deposited in GenBank and the Biological Resource Center, at the National Institute of Technology and Evaluation in Japan (NBRC) public databases (2). The BLAST query from our isolates showed a 100% similarity with several sequences of H. fuscoatra (accession no. AB625589, GU183113, KJ767116, KJ767117, and GU966514; 99% query coverage), and a similarity of 99.4% with a sequence of Chaetomium sphaerale (AB625588; 99% query coverage). However, only a 95% similarity was found with the ITS sequence of the type strain of H. fuscoatra (KF981440; 99.5% query coverage), revealing that the isolates belong to other Humicola species. Figure 3 shows the results of the analysis of ITS sequences of the available type strains of Humicola species.
FIG 3.
Maximum-likelihood (ML) tree constructed with sequences (576 bp) of the ITS region from the case isolates and from closely related fungi obtained in a BLAST search from GenBank and NBRC public databases. Bootstrap support values above 70% are indicated at the nodes. *, accession number of sequence retrieved from the NBRC. NBRC, NITE, Biological Resource Center, Chiba, Japan; CBS-KNAW, Fungal Biodiversity Centre culture collection, Utrecht, the Netherlands; NRRL, Agricultural Research Service (ARS) culture collection, Peoria, IL.
Peritoneal dialysis (PD) is the renal replacement modality of choice for over 20% of patients with end-stage renal disease and the only option for some patients in remote locations (3). A major complication of PD therapy is the development of peritonitis, currently occurring ∼1 in every 25 patients on therapy in Australia (4). Most episodes of peritonitis are caused by Gram-positive bacteria, with Gram-negative organisms being responsible for ∼20% of cases. More rarely, peritonitis may be due to fungal species; Australian and New Zealand Registry data report a fungus, usually Candida species, as the primary cause of PD peritonitis in 3% of cases (5).
Fungal peritonitis is a serious complication of PD associated with high morbidity and mortality (6). Attempts to preserve the dialysis catheter through prolonged treatment with antifungal agents are generally unsuccessful, and the current recommendation is catheter removal when fungal peritonitis is identified (7). As empirical treatment for PD peritonitis does not cover fungal species, delays in diagnosis and commencement of appropriate antimicrobial therapy are common and may contribute to the poor outcomes. We strongly advocate that fungal culture be performed where standard bacterial cultures are negative or there is a strong clinical suspicion of atypical organisms (∼20% of cases) and that routine antifungal prophylaxis be used when patients present with peritonitis, in keeping with current guidelines (7). In the present case, peritonitis caused by Humicola sp. was diagnosed and successfully treated with prolonged administration of voriconazole and amphotericin B as well as multiple washouts and drainage of infected collections.
Where an infective agent is suspected and routine bacteriology culture is inconclusive, a request for extended incubation and mycological examination should be instituted. Collection of samples in blood culture bottles for prolonged culture in fluid phase may facilitate the growth of organisms that are present in low numbers or that are slow growing, as demonstrated in this case. The initial identification of the etiological agent as a possible Humicola sp. was obtained by ITS sequencing once the organism was isolated. Further elucidation of the species identification was sought from a reference laboratory. However, identification at the species level was not possible due to the confusing taxonomy of the genus Humicola and to the scarcity of available molecular data on these fungi in public databases.
Humicola is a genus of hyphomycetes related to the family Chaetomiaceae, which includes fungi commonly isolated from soil and plant debris (8). There are numerous diagnostic features to distinguish this genus from others that are morphologically similar, such as Leohumicola, Scytalidium, Staphylotrichum, and Thermomyces. These include the production of two types of conidia: large, dark, more or less globose blastoconidia borne singly on vegetative hyphae and small, hyaline phialoconidia, although the latter are associated with only some species (9, 10). The genus Humicola comprises more than 20 species, although H. fuscoatra and Humicola grisea are the species most frequently isolated from the environment; they are also known to be strongly cellulolytic fungi (8, 10). Disease caused by Humicola species is rare, with one previous human case of peritonitis described in an abstract, as well as a case of Humicola-associated hypersensitivity pneumonitis (11, 12). In both cases, H. fuscoatra was identified morphologically as the causative organism but not confirmed molecularly.
In our case, the three isolates investigated were all morphologically similar to H. fuscoatra, but the comparative analysis of the ITS barcode region showed that they differed considerably from the type culture (CBS 118.14), indicating that they are clearly different fungi (Fig. 3). Our isolates also differed from the typical features of H. fuscoatra in the production of pigmented blastoconidia that formed chains or aggregates and in their ability to grow at 40°C. Given the halophilic nature of the organism and the development of peritonitis after the patient had been scuba diving, we hypothesize that this organism may have been acquired from the ocean.
Despite the morphological differences, ITS sequences of our isolates had 100% identity to other sequences retrieved from GenBank and deposited as H. fuscoatra (Fig. 3). We suspect that this is due to Humicola species, including cryptic taxa, as demonstrated in other medically important fungi with poorly structured conidiogenous apparatus (1, 13, 14). Our isolates were also demonstrated to be phylogenetically associated with Chaetomium sphaerale (Fig. 3). Relationships between Humicola and the ascomycetous genus Chaetomium have been considered by several authors, but no anamorph-teleomorph connection has ever been established (10, 15). Little is known about C. sphaerale, and no asexual morph was mentioned in the original description of the species (16). Since no ex-type strain of this Chaetomium species is available in any culture collection, the taxonomy of this fungus remains uncertain.
In conclusion, we report a case of fungal peritoneal dialysis-associated peritonitis caused by a novel filamentous fungus related to Humicola sp. successfully treated with multiple operative interventions and prolonged antifungal therapy. Given the halophilic nature of the fungus isolated, we speculate that it may have been acquired from a marine environment. The taxonomy of the genus Humicola is unclear, and a re-evaluation of its species by molecular techniques would be welcome to elucidate the phylogeny of these fungi. Only a correct delineation of the species will allow us to advance our understanding of the pathogenic role of these fungi, as has recently been shown with Madurella and Acrophialophora, two clinically relevant hyphomycetous genera of the family Chaetomiaceae (17–19).
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers KR259874, KR259875, and KR259876.
ACKNOWLEDGMENTS
We are indebted to Gracy Cherian from the Mycology Laboratory and Adam Merrit and staff of the Molecular Diagnostics Laboratory, Division of Microbiology and Infectious Diseases, PathWest, QEII Medical Centre.
REFERENCES
- 1.Gilgado F, Cano J, Gene J, Guarro J. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol 43:4930–4942. doi: 10.1128/JCM.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.McDonald S, Clayton P, Hurst K. 2012. Peritoneal dialysis, p 6.2–6.27. In ANZDATA 2012 annual report, 35th ed Australia and New Zealand Dialysis and Transplant Registry, Adelaide, South Australia, Australia. [Google Scholar]
- 4.Brown F, Gulyani A, McDonald S, Hurst K. 2012. Peritoneal dialysis. Australian and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. [Google Scholar]
- 5.Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, McDonald SP. 2011. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 31:651–662. doi: 10.3747/pdi.2010.00131. [DOI] [PubMed] [Google Scholar]
- 6.Indhumathi E, Chandrasekaran V, Jagadeswaran D, Varadarajan M, Abraham G, Soundararajan P. 2009. The risk factors and outcome of fungal peritonitis in continuous ambulatory peritoneal dialysis patients. Indian J Med Microbiol 27:59–61. [PubMed] [Google Scholar]
- 7.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG. 2010. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30:393–423. doi: 10.3747/pdi.2010.00049. [DOI] [PubMed] [Google Scholar]
- 8.Seifert K, Morgan-Jones G, Gams W, Kendrick B. 2011. The genera of hyphomycetes. CBS biodiversity series, vol 9 Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- 9.Ellis MB. 1971. Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, England. [Google Scholar]
- 10.Domsch KH, Gams W, Anderson TH. 2007. Compendium of soil fungi, 2nd ed IHW-Verlag, Eching, Germany. [Google Scholar]
- 11.Kita T, Nishi K, Fujimura M, Abo M, Ohka T, Yasui M, Ogawa H, Minato H, Kurumaya H, Nakao S. 2003. A case of hypersensitivity pneumonitis caused by Humicola fuscoatra. Respirology 8:95–98. doi: 10.1046/j.1440-1843.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Xie X-L, Wang H, Dou H-T, Sun H-L, Wang H, Xu Y-C. 2011. A case of fungal peritonitis caused by Humicola fuscoatra. Chin J Mycol 1:015. [Google Scholar]
- 13.Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. 2007. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 45:3198–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdomo H, Sutton DA, Garcia D, Fothergill AW, Cano J, Gene J, Summerbell RC, Rinaldi MG, Guarro J. 2011. Spectrum of clinically relevant Acremonium species in the United States. J Clin Microbiol 49:243–256. doi: 10.1128/JCM.00793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarro J, Gené J, Stchigel AM, Figueras MJ. 2012. Atlas of soil ascomycetes. CBS biodiversity series, vol 10 Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- 16.Chivers AH. 1912. Preliminary diagnoses of new species of Chaetomium. Proc Am Acad Arts Sci 48:83–88. doi: 10.2307/20022812. [DOI] [Google Scholar]
- 17.Sandoval-Denis M, Gene J, Sutton DA, Wiederhold NP, Guarro J. 2015. Acrophialophora, a poorly known fungus with clinical significance. J Clin Microbiol 53:1549–1555. doi: 10.1128/JCM.00279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Hoog GS, van Diepeningen AD, Mahgoub E-S, van de Sande WW. 2012. New species of Madurella, causative agents of black-grain mycetoma. J Clin Microbiol 50:988–994. doi: 10.1128/JCM.05477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Hoog GS, Ahmed SA, Najafzadeh MJ, Sutton DA, Keisari MS, Fahal AH, Eberhardt U, Verkleij GJ, Xin L, Stielow B, van de Sande WW. 2013. Phylogenetic findings suggest possible new habitat and routes of infection of human eumyctoma. PLoS Negl Trop Dis 7:e2229. doi: 10.1371/journal.pntd.0002229. [DOI] [PMC free article] [PubMed] [Google Scholar]