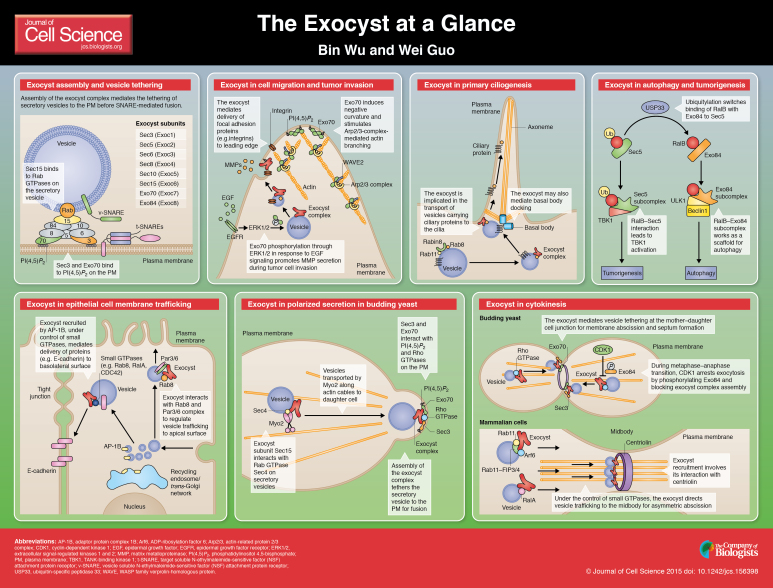
ABSTRACT
The exocyst is an octameric protein complex that is implicated in the tethering of secretory vesicles to the plasma membrane prior to SNARE-mediated fusion. Spatial and temporal control of exocytosis through the exocyst has a crucial role in a number of physiological processes, such as morphogenesis, cell cycle progression, primary ciliogenesis, cell migration and tumor invasion. In this Cell Science at a Glance poster article, we summarize recent works on the molecular organization, function and regulation of the exocyst complex, as they provide rationales to the involvement of this complex in such a diverse array of cellular processes.
KEY WORDS: Rab, Ral, Rho, Exocyst, Membrane fusion, Vesicle tether
Summary: The octameric exocyst complex is implicated in vesicle tethering during exocytosis. Recent works from different fields have revealed its role beyond membrane trafficking.
Introduction
The exocyst complex comprises eight subunits: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84. It was first identified in the budding yeast Saccharomyces cerevisiae by genetic and biochemical approaches (Novick et al., 1980; TerBush and Novick, 1995; TerBush et al., 1996). The mammalian exocyst complex was first purified from rat brain, and was found in all the tissues examined (Hsu et al., 1996, 1998). In cells, the exocyst is recruited to sites of active exocytosis and membrane expansion, where it mediates the tethering of secretory vesicles to the plasma membrane in preparation for soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE)-mediated membrane fusion.
Molecular organization of the exocyst complex
Most of the exocyst subunits interact with multiple other subunits within the complex (Guo et al., 1999a; Matern et al., 2001; Dong et al., 2005). Deep-etch electron microscopy (EM) has shown the glutaraldehyde-fixed exocyst complex to be a uniform T- or Y-shaped structure, whereas the unfixed complex displays variable shapes, which suggests conformational changes (Hsu et al., 1998). In Arabidopsis thaliana, electron tomography studies of vesicle fusion during cell plate formation showed ‘Y’- or ‘L’-shaped structures linking vesicles (Segui-Simarro et al., 2004). These structures are likely to represent the exocyst complex undergoing vesicle tethering, although further verification of their identity is needed.
The crystal structure of parts of several exocyst subunits have been resolved (Baek et al., 2010; Dong et al., 2005; Hamburger et al., 2006; Liu and Guo, 2012; Moore et al., 2007; Sivaram et al., 2006; Wu et al., 2005; Yamashita et al., 2010; Munson and Novick, 2006; Mott et al., 2003; Fukai et al., 2003; Jin et al., 2005). Many exocyst subunits contain rod-like structures that are composed of α-helical bundles, which are also found in other complex associated with tethering containing helical rods (CATCHR) family proteins (Yu and Hughson, 2010). These rod-shaped subunits are likely to pack together side by side into the ‘Y’-shaped complex, as observed by EM (Hsu et al., 1998). Structural studies have also revealed that the N-terminus of yeast Sec3 has a PH-domain-like structure that is evolutionarily conserved from yeast to mammals and plants (Baek et al., 2010; Yamashita et al., 2010), consistent with the proposed interactions of this domain with phosphatidylinositol 4,5-bisphosphate PI(4,5)P2 and the Rho family of small GTPases (Guo et al., 2001; Zhang et al., 2001, 2008). Moreover, regions of Sec5 and Exo84 have been crystalized in complex with the GTPase Ral (Mott et al., 2003; Fukai et al., 2003; Jin et al., 2005).
In addition to the holo-complex, free pools of certain subunits and sub-complexes have also been identified by biochemical fractionation experiments (Guo et al., 1999a; Jin et al., 2005; Moskalenko et al., 2003; Morgera et al., 2012; Zhao et al., 2013). The physiological significance of the different sub-complexes still needs to be investigated. In vivo studies have shown unique expression patterns for different subunits in fly and zebrafish (Mehta et al., 2005; Murthy et al., 2005; Thisse et al., 2004). Therefore, the exocyst complex is likely to be assembled and activated with spatiotemporal specificity. Understanding how the assembly of the complex is regulated is crucial to the understanding of the function of the exocyst in diverse physiological processes.
The exocyst in vesicle tethering
It has been speculated that the exocyst tethers secretory vesicles to the plasma membrane (Pfeffer, 1999; Guo et al., 2000; Whyte and Munro, 2002). However, direct evidence for the role the exocyst in tethering is missing. Live-cell imaging in HeLa cells showed that Sec8 is transported to the plasma membrane on vesicles, and remains there for seconds until fusion (Rivera-Molina and Toomre, 2013). This observation may reflect the transport and tethering of vesicles mediated by the exocyst before SNARE-mediated fusion. In yeast, it has recently been shown that ectopic targeting of Sec3 to mitochondria or peroxisomes resulted in the recruitment of secretory vesicles to these surrogate organelles, supporting the role of the exocyst in vesicle targeting and tethering (Luo et al., 2014). Future imaging studies and in vitro reconstitution experiments are needed to ultimately establish the role of the exocyst in tethering.
At the molecular level, how is the exocyst associated with the secretory vesicles and the plasma membrane? It has been shown that two subunits, Sec3 and Exo70, directly bind to PI(4,5)P2, which is located at the inner leaflet of the plasma membrane (Liu et al., 2007; He et al., 2007b; Zhang et al., 2008). Sec3 interacts with PI(4,5)P2 through an evolutionarily conserved PH-domain-like region at its N-terminus (Baek et al., 2010; Yamashita et al., 2010), whereas Exo70 interacts with PI(4,5)P2 through a patch of basic residues at its C-terminus (He et al., 2007,b; Liu et al., 2007; Dong et al., 2005; Hamburger et al., 2006; Moore et al., 2007). In yeast, simultaneous disruption of Sec3 and Exo70 binding to PI(4,5)P2 dissociates the exocyst from the plasma membrane, and results in a block in secretion (He et al., 2007,b; Zhang et al., 2008). On the secretory vesicle, Sec15 directly interacts with the Rab protein Sec4 in its GTP-bound form (Guo et al., 1999a). Sec6 binds to the v-SNARE protein Snc2 (Shen et al., 2013). Moreover, Exo84 binds to Ral (see below) and might interact with phospholipids (Moskalenko et al., 2003; Jin et al., 2005). Overall, these combinatorial interactions might mediate exocyst–vesicle associations. Results from these molecular interaction studies are consistent with the localization analyses of individual exocyst subunits in yeast. Immuno-EM studies, coupled with fluorescence imaging experiments, have demonstrated that Sec3 and Exo70 associate with the plasma membrane, whereas the other subunits – including some Exo70 proteins – associate with secretory vesicles (Boyd et al., 2004). Furthermore, Sec3 and some of the Exo70 proteins are localized to the bud tip even in the absence of actin cables, which mediate the polarized delivery of secretory vesicles (Finger et al., 1998; Boyd et al., 2004; Zhang et al., 2008). In fission yeast and mammals, however, Sec3 and Exo70 were found to associate with vesicles as well as the plasma membrane (Yeaman et al., 2004; Liu et al., 2007; Andersen and Yeaman, 2010; Bendezu et al., 2012; Zhao et al., 2013). It is possible that, in these cells, all of the exocyst subunits can be transported on vesicles; once they arrive at the destination, Sec3 and Exo70 might then ‘anchor’ the exocyst and vesicles to the plasma membrane through their physical association with PI(4,5)P2.
Membrane fusion immediately follows vesicle tethering. Genetic and biochemical studies in yeast indicate that the exocyst functions upstream of SNAREs (Brennwald et al., 1994; Wiederkehr et al., 2004, Grote et al., 2000). The yeast exocyst subunit Sec6 was reported to interact – as a dimer – with the t-SNARE protein Sec9 (the SNAP-25 homolog) (Sivaram et al., 2005). However, it is perplexing that Sec6 has an inhibitory effect on SNARE assembly in an in vitro experiment (Sivaram et al., 2005). Sec6 also binds to the v-SNARE protein Snc2 through part of its SNARE assembly motif; a mutation of SNC2 that disrupts this interaction led to mis-localization of the exocyst and a block in exocytosis (Shen et al., 2013). Finally, Sec6 also interacts with the Sec1/Munc18 family protein Sec1 (Morgera et al., 2012). In addition to Sec6, Exo84 interacts with Sro7 and Sro77, the homologs of mammalian Lethal giant larvae (Lgl), which bind to the SNARE proteins and regulate exocytosis (Lehman et al., 1999; Zhang et al., 2005). Despite the interactions of exocyst subunits with multiple SNARE components or SNARE regulators, no data have so far provided clear support for the role of the exocyst in promoting SNARE assembly and membrane fusion. Future reconstitution experiments are called for, in order to establish the role of the exocyst in SNARE-mediated fusion.
Regulation of the exocyst
As vesicle tethering precedes fusion, spatial and temporal control of exocytosis in cells can be executed through the regulation of the exocyst. Indeed, subunits of the exocyst have been found to be direct targets of a number of small GTPases and kinases.
Regulation of the exocyst by small GTPases
Rab
The first reported interaction between the exocyst and small GTPases was that of yeast Sec15 and the exocytic Rab protein Sec4 (Guo et al., 1999a). This interaction is specific for Sec4 because other Rab proteins, such as those functioning at the endoplasmic reticulum (Ypt1) or endosomes (Ypt5p), do not interact with Sec15. Activated Sec4 might mediate the recruitment of the exocyst to secretory vesicles as well as the assembly of the complex (Guo et al., 1999a; Luo et al., 2014). In Drosophila melanogaster, Sec15 was shown to interact with a subset of Rab proteins (Rab3, Rab8, Rab11 and Rab27) that are involved in exocytic trafficking (Wu et al., 2005). In mammalian cells, Sec15 was shown to interact with Rab11, which is involved in the generation of vesicles from the trans-Golgi network (TGN) or recycling endosomes for subsequent delivery to the plasma membrane (Zhang et al., 2004). The interaction of Sec15 with Rab proteins has been implicated in E-cadherin trafficking, Notch signaling and primary ciliogenesis (Classen et al., 2005; Jafar-Nejad et al., 2005; Langevin et al., 2005; Feng et al., 2012). In yeast, Sec15 was also reported to interact with the type-V myosin Myo2p, which binds to both Sec4 and Ypt32 (the yeast homolog of Rab11), suggesting that Rab proteins orchestrate the exocyst function for vesicle transport along the cytoskeleton (Lipatova et al., 2008; Jin et al., 2011). In addition to the interaction between Rab and Sec15, another exocyst subunit, Sec10, was shown to bind to GTP–Arf6, and this interaction is important for the recycling of proteins to the plasma membrane (Prigent et al., 2003). The study by Prigent et al. further highlights the potential role of the exocyst in the trafficking from recycling endosomes to the plasma membrane.
Rho
The Rho family of small GTPases control many cellular processes, including morphogenesis and proliferation. In budding yeast, the Rho proteins are master regulators of cell polarity through their interactions with actin regulators (for a review, see Park and Bi, 2007). Rho proteins have also been implicated in exocytosis, as certain mutant alleles of Cdc42 and Rho3 display defects in secretion (Adamo et al., 1999, 2001). Cdc42, in its GTP-bound form, directly interacts with Sec3 (Zhang et al., 2001). The dual interaction of the Sec3 N-terminal PH-domain-like region with Cdc42 and PI(4,5)P2 controls the polarized localization of Sec3, and might also kinetically regulate exocytosis (Zhang et al., 2001, 2008; Adamo et al., 2001; Baek et al., 2010). In mammalian cells, Cdc42 functions together with the exocyst to regulate phagocytosis (Mohammadi and Isberg, 2013). In addition to Cdc42, another Rho family protein, Rho1, interacts with Sec3 through the same N-terminal region (Guo et al., 2001; Yamashita et al., 2010). Cdc42 and Rho1 compete with each other for their binding to Sec3, suggesting that they control Sec3 function at different stages of cell growth (Zhang et al., 2001). In addition to Sec3, Exo70 interacts with Cdc42 and Rho3 in yeast (Robinson et al., 1999; Wu et al., 2010). The interaction with Rho3 was mapped to the C-terminus of Exo70 (Dong et al., 2005), however, exactly which part of Exo70 binds to Cdc42 in yeast is unclear. It has been reported that prenylation of Cdc42 is needed for this interaction (Wu et al., 2010). In mammalian cells, Exo70 interacts, through its N-terminus, with Rho-related GTP-binding protein RhoQ (also known as TC10), a homolog of Cdc42, and this interaction is implicated in the incorporation of Glut4 to the plasma membrane in adipocytes in response to insulin stimulation (Inoue et al., 2003). Although the data for the above-mentioned interactions are quite clear, how these Rho GTPases coordinate with each other in their interaction with different exocyst subunits remains unclear.
Ral
In metazoans, Ral GTPases (RalA and RalB) interact with both Sec5 and Exo84, and these interactions have been implicated in many processes, including cell migration, autophagy, neurogenesis and cancer (Brymora et al., 2001; Sugihara et al., 2002; Moskalenko et al., 2002, 2003; Polzin et al., 2002; Moskalenko et al. ; Balakireva et al., 2006; Bodemann et al., 2011; Chen et al., 2006, 2007; Chien et al., 2006; Hazelett et al., 2011; Lalli, 2009; Rosse et al., 2006). Cell fractionation studies have shown that Sec5 and Exo84 are in separate sub-complexes (Moskalenko et al., 2003), and structure analysis has demonstrated that Sec5 and Exo84 competitively bind to GTP–Ral (Jin et al., 2005). Recent studies have shown different subcellular localizations of these two sub-complexes, and suggested their involvement in different cellular functions, such as autophagy and innate immunity (Bodemann et al., 2011). Furthermore, ubiquitylation of RalB promotes the switch from an interaction between RalB, Exo84 and beclin1 to that beteen RalB, Sec5 and TBK1, which further differentiates the roles of Ral and the exocyst in different processes (Simicek et al., 2013).
Regulation of the exocyst by kinases
Components of the exocyst are targets for a number of kinases. In mammalian cells, Exo70 is phosphorylated by ERK1/2 in response to epidermal growth factor (EGF) signaling (Ren and Guo, 2012). Phosphorylation of Exo70 promotes its association with the rest of the exocyst complex and stimulates exocytosis. Because ERK1 and ERK2 are pivotal kinases in the classic Ras–MEK–ERK signaling cascade, ERK1/2 phosphorylation might coordinate exocytosis with other cellular processes – such as cell migration – in response to growth factor signaling. In budding yeast, Exo84 is phosphorylated by the cyclin-dependent kinase Cdk1 during mitosis (Luo et al., 2013). Phosphorylation of Exo84 disrupts its association with the rest of the exocyst subunits, resulting in a block of exocytosis before the transition from metaphase to anaphase. The study by Luo et al. might provide a molecular mechanism for the mitotic cell-growth arrest observed in most eukaryotic cells. In the human fungal pathogen Candida albicans, Exo84 is phosphorylated by Cdk1, which is bound to the hypha-specific cyclin Hgc1, at residues that are specific to this pathogen, which is important for filamentous growth (Caballero-Lima and Sudbery, 2014). Phosphorylation of Exo84 by Cdk1–Hgc1 alters its affinity for phosphatidylserine, although how this change affects filamentous growth remains unclear. Another subunit, Sec5, can be phosphorylated by PKC at its Ral-binding domain, which allosterically leads to dissociation of Sec5 from RalA in mammalian cells (Chen et al., 2011). This cycle of engagement and disengagement with RalA is likely to allow continuous rounds of exocytic trafficking to the plasma membrane. Overall, these studies demonstrate that subunits of the exocyst can be phosphorylated in different modes that serve a diverse array of cellular processes.
Cellular functions of the exocyst complex
The basic function of the exocyst complex is exocytosis. However, recent studies implicate the exocyst in many other functions. A rationale is that many cellular processes involve, or need to coordinate with, exocytosis through the exocyst complex. In other cases, studies link the exocyst to cellular functions through binding partners that have no obvious connection to exocytosis (see supplementary material Table S1 for a list of proteins and molecules that interact with the exocyst complex). Next, we summarize several major cellular processes involving the exocyst.
The exocyst in polarized exocytosis
In budding yeast, mutations in the exocyst subunits lead to accumulation of post-Golgi secretory vesicles in the cytoplasm (Novick, et al., 1980; Finger and Novick, 1997; Guo et al., 1999b; He et al., 2007a,b). The exocyst is localized to the emerging bud tip, where it mediates exocytosis for the asymmetric expansion of daughter cell surfaces during polarized cell growth. During cytokinesis, the exocyst is localized to the mother–daughter cell junction to mediate abscission (TerBush and Novick, 1995; Finger et al., 1998; Guo et al., 1999b; Zhang et al., 2008). The polarized localization of the exocyst stands in contrast to the localization of t-SNARE proteins, which are distributed along the entire plasma membrane (Brennwald et al., 1994). In Arabidopsis thaliana, the exocyst is localized to growing pollen tubes and root tips, which are regions of active membrane expansion (Hala et al., 2008; Fendrych et al., 2013). In epithelial cells, the exocyst was shown to mediate vesicle trafficking to the basolateral domain, and is important for the establishment of epithelial polarity (Grindstaff et al., 1998; Lipschutz et al., 2000; Langevin et al., 2005; Yeaman et al., 2004; Andersen and Yeaman, 2010; Xiong et al., 2012). The recruitment of the exocyst complex to the vesicle is facilitated by the clathrin adaptor complex AP-1B (Folsch et al., 2003). Genetic analyses in worms suggest that the exocyst functions together with Rab10 in basolateral recycling; ablation of the exocyst subunits disrupts endosomal tubules (Chen et al., 2014). Studies have also shown that the exocyst functions together with Rab8 and the Par3/6 complex in membrane trafficking to the apical domain (Blankenship et al., 2007; Oztan et al., 2007; Bryant et al., 2010). In neurons, the exocyst is localized to the growth cones, tips of neurites or points of branching, where exocytosis is needed for membrane addition and surface expansion (Hazuka et al., 1999; Vega and Hsu, 2001; Lalli and Hall, 2005; Das et al., 2014). Inhibition of the exocyst does not affect neural transmitter release at mature synapses (Andrews et al., 2002; EauClaire and Guo, 2003; Murthy et al., 2003). However, it has been shown that delivery of the NMDA and AMPA receptors to the post-synaptic membrane is regulated by the exocyst complex (Sans et al., 2003; Riefler et al., 2003; Gerges et al., 2006).
The exocyst in cell migration and tumor invasion
Directional cell migration requires the coordination of actin cytoskeleton remodeling, plasma membrane reorganization and polarized exocytosis. The exocyst, together with Rab and Ral, mediates the trafficking of signaling proteins and adhesion molecules to the leading edge (Rosse et al., 2006; Rosse et al., 2009; Spiczka and Yeaman, 2008; Assaker et al., 2010; Thapa et al., 2012). In addition, the secretion of matrix metalloproteinases (MMPs) is important for the formation of invadopodia during tumor cell invasion (Sakurai-Yageta et al., 2008; Liu et al., 2009; Yamamoto et al., 2013). ERK1/2 phosphorylation of Exo70 promotes MMP secretion by promoting the assembly of the exocyst complex (Ren and Guo, 2012). The exocyst was also reported to interact with the endosomal Wiskott–Aldrich syndrome protein and Scar homolog (WASH) complex, thus coupling generation of membrane Type-1 MMP-containing late endosomes to their exocytosis at the plasma membrane (Monteiro et al., 2013). In neurons, the exocyst interacts with the Par3/6 complex under the control of Ral to regulate polarized neuronal cell migration (Lalli, 2009; Das et al., 2014).
Actin remodeling is required for membrane protrusion at the leading edge. The Arp2/3 complex is the core machinery for the generation of a branched actin network that pushes the plasma membrane (Goley and Welch, 2006; Pollard and Borisy, 2003; Suraneni et al., 2012). Exo70 directly interacts with the ARPC1 subunit of the Arp2/3 complex, and promotes actin branching (Liu et al., 2012). Interaction between Exo70 and Arp2/3 is stimulated by EGF signaling, which is known to promote cell migration and tumor invasion (Liu et al., 2009; Zuo et al., 2006). In addition to modulating actin dynamics, Exo70 interacts with PI(4,5)P2 and induces a high curvature on the plasma membrane (Zhao et al., 2013). The coordinated regulation of actin polymerization and membrane deformation through Exo70 leads to effective membrane protrusion during cell migration. The ability of Exo70 to induce high membrane curvature might also contribute to the formation of tunneling nanotubes, which mediate cell–cell communication (Hase et al., 2009; Schiller et al., 2013).
The exocyst in cytokinesis
The exocyst functions in multiple stages of cytokinesis. As discussed above, CDK1 regulates the assembly of the exocyst complex through phosphorylation of Exo84, thus arresting exocytosis before the transition from metaphase to anaphase (Luo et al., 2013). At telophase, the exocyst directs the trafficking of vesicles to the cell–cell junction for abscission (Martin-Cuadrado et al., 2005; Wang et al., 2002 and 2003). In Arabidopsis, the exocyst subunits are localized to the separation sites during initiation of the cell plate and maturation, when vesicle fusion is in high demand (Fendrych et al., 2010). In mammalian cells, the exocyst is involved in the trafficking of secretory vesicles to the cleavage furrow and midbody under the control of Rab11 and RalA (Chen et al., 2006; Fielding et al., 2005; Neto et al., 2013). Because cleavage often takes place at one side of the midbody (also known as asymmetric abscission), the vesicles appear to be recruited to the cleavage site of one of the daughter cells (Gromley et al., 2005; Schiel et al., 2013).
The exocyst in tumorigenesis and autophagy
Ral GTPases are crucial for tumorigenesis (Camonis and White, 2005; Hamad et al., 2002; Lim et al., 2005; Rangarajan et al., 2004). As a direct downstream effector of Ral, the exocyst has been shown to participate in tumorigenic signaling processes. Knockdown of Sec5 and Exo84, which interact with GTP–Ral, reduces oncogenic transformation and tumor cell growth (Issaq et al., 2010). The exocyst is also proposed to function as a scaffold for signaling molecules. RalB–Sec5 recruits and activates TBK1, which in turn activates Akt to overcome apoptosis in cancer cells (Chien et al., 2006; Chien and White, 2008; Ou et al., 2011). The interaction between RalB, Sec5 and TBK1 also initiates innate immune responses following viral infection (Chien et al., 2006). In addition, the RalB–Exo84 complex was shown to interact with beclin-1 to regulate autophagosome assembly in response to starvation (Bodemann et al., 2011). The switch of RalB binding from Exo84 to Sec5 is mediated by ubiquitylation of RalB (Simicek et al., 2013). It is interesting to note that the exocyst mostly plays a role as a signaling scaffold in the above mentioned process. It is not clear whether and how this scaffold role is linked to exocytosis.
The exocyst in primary ciliogenesis
The primary cilium is a microtubule-based membrane protrusion on the cell surface that functions as a signaling organelle. Cilia malfunction has been implicated in many diseases, such as Bardet-Biedl syndrome, Joubert syndrome and polycystic kidney diseases. Exocyst subunits have been detected at the base or inside of primary cilia by immunofluorescence microscopy or EM; inhibition of exocyst function affected ciliogenesis (Rogers et al., 2004; Zuo et al., 2009; Feng et al., 2012). In addition, an interaction network between Rab11, Rabin8 (the guanine nucleotide exchange factor for Rab8), Rab8 and the exocyst complex regulates primary ciliogenesis – probably by mediating the transport of transmembrane proteins or basal bodies to the cilia membrane, although further experimental evidence is needed to support this hypothesis (Knodler, et al., 2010; Das and Guo, 2011; Feng et al., 2012). Rab10 also interacts with the exocyst and mediate ciliogenesis in renal epithelial cells, probably through a similar mechanism (Babbey et al., 2010). The interaction of exocyst with the Par3/6 complex might also contribute to primary ciliogenesis, although direct evidence for this notion is lacking (Zuo et al., 2009; Lalli, 2009; Das et al., 2014).
Functional diversification of the exocyst
As discussed above, the exocyst is involved in many biological processes from cell migration to primary ciliogenesis. Although its basic role in exocytosis is linked to many of these processes, its functional diversification likely involves many different regulatory mechanisms. In addition to small GTPases and kinases, alternative splicing of the exocyst at the mRNA level could serve as a means to diversify exocyst function. In humans, Exo70 has five splicing isoforms. During epithelialization, epithelial splicing regulatory proteins 1 and 2 (ESRP1 and ESRP2) mediate the generation of a particular isoform of Exo70 (also known as Exo70-E; Lu et al., 2013) that differs from other Exo70 isoforms by a 23-amino-acid insertion. This Exo70 isoform cannot interact with the Arp2/3 complex and fails to stimulate cell motility (Warzecha et al., 2010; Lu et al., 2013). Isoform switching of Exo70 takes place during epithelial-to-mesenchymal transition, and is implicated in breast cancer metastasis (Lu et al., 2013). Exo70 was also found to be the most diversified subunit of the exocyst complex in plants – many of them have dozens of Exo70 paralogues per genome. This might correlate with the many unique structures of endomembranes and surface domains observed in terrestrial plants and their adaptation to different environments (Cvrckova et al., 2012; Zarsky et al., 2009). For example, the Exo70 isoform Exo70E2 marks a recently discovered organelle, named exocyst-positive organelle (EXPO), that is implicated in an unconventional secretion pathway in plants (Wang et al., 2010). In addition to Exo70, many of the other exocyst subunits also have multiple isoforms or paralogues. The existence of isoforms or paralogues of house-keeping proteins, such as Exo70, might account for the many different functions observed for this evolutionarily conserved complex.
Perspectives
Since its initial purification (TerBush and Novick, 1995), the last two decades have seen exciting progress in the understanding of the exocyst complex. Research in the field has not only led to a better understanding of the exocyst as a tether complex in exocytosis, but also revealed the role of the exocyst in a wide range of cell biological processes. The expansion of our knowledge about the exocyst also raises new questions. To elucidate the fundamental mechanism of exocyst function in exocytosis, we must understand how the exocyst complex interacts with SNARE proteins and potentially activates them for vesicle fusion with the plasma membrane. Despite the efforts from a number of laboratories, a clear model is still lacking. What is the structure of the complex and how is it assembled? With the recent revolution in cryo-EM technology, it is possible that a near-atomic-level-resolution structure of the holo-complex can be obtained. In fact, cryo-EM is probably the only approach for a high-resolution structure of this macromolecular complex. Does assembly of the exocyst complex (or sub-complexes) coordinate vesicle tethering with upstream vesicle budding from the TGN and recycling endosomes? What are the molecular consequences of the exocyst interactions with small GTPases? At the organismal level, how is the exocyst complex involved in tissue and organ development? With the advent of new technologies in cell biology, the next decade will see more exciting progress in the understanding of the exocyst, exocytosis and beyond.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
W.G.’s laboratory is supported by grants from National Institute of General Medical Sciences [grant numbers GM085146 and GM111128]. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.156398/-/DC1
Supplementary material
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.156398/-/DC2
References
- Adamo J. E., Rossi G. and Brennwald P. (1999). The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10, 4121-4133. 10.1091/mbc.10.12.4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo J. E., Moskow J. J., Gladfelter A. S., Viterbo D., Lew D. J. and Brennwald P. J. (2001). Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell. Biol. 155, 581-592. 10.1083/jcb.200106065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen N. J. and Yeaman C. (2010). Sec3-containing exocyst complex is required for desmosome assembly in mammalian epithelial cells. Mol. Biol. Cell. 21, 152-164. 10.1091/mbc.E09-06-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews H. K., Zhang Y. Q., Trotta N. and Broadie K. (2002). Drosophila sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic 3, 906-921. 10.1034/j.1600-0854.2002.31206.x [DOI] [PubMed] [Google Scholar]
- Assaker G., Ramel D., Wculek S. K., Gonzalez-Gaitan M. and Emery G. (2010). Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc. Natl. Acad. Sci. USA 107, 22558-22563. 10.1073/pnas.1010795108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbey C. M., Bacallao R. L. and Dunn K. W. (2010). Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal. Physiol. 299, F495-F506. 10.1152/ajprenal.00198.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K., Knodler A., Lee S. H., Zhang X., Orlando K., Zhang J., Foskett T. J., Guo W. and Dominguez R. (2010). Structure-function study of the N-terminal domain of exocyst subunit Sec3. J. Biol. Chem. 285, 10424-10433. 10.1074/jbc.M109.096966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva M., Rosse C., Langevin J., Chien Y. C., Gho M., Gonzy-Treboul G., Voegeling-Lemaire S., Aresta S., Lepesant J. A., Bellaiche Y. et al. (2006). The Ral/exocyst effector complex counters c-Jun N-terminal kinase-dependent apoptosis in Drosophila melanogaster. Mol. Cell. Biol. 26, 8953-8963. 10.1128/MCB.00506-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú F. O., Vincenzetti V. and Martin S. G. (2012). Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS ONE 7, e40248 10.1371/journal.pone.0040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. T., Fuller M. T. and Zallen J. A. (2007). The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120, 3099-3110. 10.1242/jcs.004770 [DOI] [PubMed] [Google Scholar]
- Bodemann B. O., Orvedahl A., Cheng T., Ram R. R., Ou Y.-H., Formstecher E., Maiti M., Hazelett C. C., Wauson E. M., Balakireva M. et al. (2011). RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144, 253-267. 10.1016/j.cell.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C., Hughes T., Pypaert M. and Novick P. (2004). Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167, 889-901. 10.1083/jcb.200408124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns B., Champion K., Keränen S., Bankaitis V. and Novick P. (1994). Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79, 245-258. 10.1016/0092-8674(94)90194-5 [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodríguez-Fraticelli A. E., Peränen J., Martín-Belmonte F. and Mostov K. E. (2010). A molecular network for de novo generation of the apical surface and lumen. Nat. Cell. Biol. 12, 1035-1045. 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brymora A., Valova V. A, Larsen M. R., Roufogalis B. D. and Robinson P. J. (2001). The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276, 29792-29797. 10.1074/jbc.C100320200 [DOI] [PubMed] [Google Scholar]
- Caballero-Lima D. and Sudbery P. E. (2014). In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol. Biol. Cell. 25, 1097-1110. 10.1091/mbc.E13-11-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camonis J. H. and White M. A. (2005). Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 15, 327-332. 10.1016/j.tcb.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Chen X. W., Inoue M., Hsu S. C. and Saltiel A. R. (2006). RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 281, 38609-38616. 10.1074/jbc.M512847200 [DOI] [PubMed] [Google Scholar]
- Chen X.-W., Leto D., Chiang S.-H., Wang Q. and Saltiel A. R. (2007). Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391-404. 10.1016/j.devcel.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Chen X.-W., Leto D., Xiao J., Goss J., Wang Q., Shavit J. A., Xiong T., Yu G., Ginsburg D., Toomre D. et al. (2011). Exocyst function is regulated by effector phosphorylation. Nat. Cell Biol. 13, 580-588. 10.1038/ncb2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Li L., Li J., Liu B., Zhu X., Zheng L., Zhang R. and Xu T. (2014). SEC-10 and RAB-10 coordinate basolateral recycling of clathrin-independent cargo through endosomal tubules in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 111, 15432-15437. 10.1073/pnas.1408327111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. and White M. A. (2008). Characterization of RalB-Sec5-TBK1 function in human oncogenesis. Methods Enzymol. 438, 321-329. 10.1016/S0076-6879(07)38022-1 [DOI] [PubMed] [Google Scholar]
- Chien Y., Kim S., Bumeister R., Loo Y.-M., Kwon S. W., Johnson C. L., Balakireva M. G., Romeo Y., Kopelovich L., Gale M. Jr. et al. (2006). RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127, 157-170. 10.1016/j.cell.2006.08.034 [DOI] [PubMed] [Google Scholar]
- Classen A. K., Anderson K. I., Marois E. and Eaton S. (2005). Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell 9, 805-817. 10.1016/j.devcel.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Cvrčková F., Grunt M., Bezvoda R., Hála M., Kulich I., Rawat A. and Žárský V. (2012). Evolution of the land plant exocyst complexes. Front. Plant Sci. 3, 159 10.3389/fpls.2012.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. and Guo W. (2011). Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol. 21, 383-386. 10.1016/j.tcb.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Gajendra S., Falenta K., Oudin M. J., Peschard P., Feng S., Wu B., Marshall C. J., Doherty P., Guo W. et al. (2014). RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J. Cell Sci. 127, 686-699. 10.1242/jcs.145037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Hutagalung A. H., Fu C., Novick P. and Reinisch K. M. (2005). The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat. Struct. Mol. Biol. 12, 1094-1100. 10.1038/nsmb1017 [DOI] [PubMed] [Google Scholar]
- EauClaire S. and Guo W. (2003). Conservation and specialization. The role of the exocyst in neuronal exocytosis. Neuron 37, 369-370. 10.1016/S0896-6273(03)00059-X [DOI] [PubMed] [Google Scholar]
- Fendrych M., Synek L., Pecenkova T., Toupalova H., Cole R., Drdova E., Nebesarova J., Sedinova M., Hala M., Fowler J. E. et al. (2010). The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22, 3053-3065. 10.1105/tpc.110.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M., Synek L., Pecenkova T., Drdova E. J., Sekeres J., de Rycke R., Nowack M. K. and Zarsky V. (2013). Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol. Biol. Cell 24, 510-520. 10.1091/mbc.E12-06-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Knodler A., Ren J., Zhang J., Zhang X., Hong Y., Huang S., Peranen J. and Guo W. (2012). A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J. Biol. Chem. 287, 15602-15609. 10.1074/jbc.M111.333245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding A. B., Schonteich E., Matheson J., Wilson G., Yu X., Hickson G. R., Srivastava S., Baldwin S. A., Prekeris R. and Gould G. W. (2005). Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 24, 3389-3399. 10.1038/sj.emboj.7600803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger F. P. and Novick P. (1997). Sec3p is involved in secretion and morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 647-662. 10.1091/mbc.8.4.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger F. P., Hughes T. E. and Novick P. (1998). Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92, 559-571. 10.1016/S0092-8674(00)80948-4 [DOI] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Maday S., Pelletier L. and Mellman I. (2003). The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163, 351-362. 10.1083/jcb.200309020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai S., Matern H. T., Jagath J. R., Scheller R. H. and Brunger A. T. (2003). Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. EMBO J. 22, 3267-3278. 10.1093/emboj/cdg329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges N. Z., Backos D. S., Rupasinghe C. N., Spaller M. R. and Esteban J. A. (2006). Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 25, 1623-1634. 10.1038/sj.emboj.7601065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D. and Welch M. D. (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol. Cell Biol. 7, 713-726. 10.1038/nrm2026 [DOI] [PubMed] [Google Scholar]
- Grindstaff K. K., Yeaman C., Anandasabapathy N., Hsu S.-C., Rodriguez-Boulan E., Scheller R. H. and Nelson W. J. (1998). Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93, 731-740. 10.1016/S0092-8674(00)81435-X [DOI] [PubMed] [Google Scholar]
- Gromley A., Yeaman C., Rosa J., Redick S., Chen C.-T., Mirabelle S., Guha M., Sillibourne J. and Doxsey S. J. (2005). Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123, 75-87. 10.1016/j.cell.2005.07.027 [DOI] [PubMed] [Google Scholar]
- Grote E., Carr C. M. and Novick P. J. (2000). Ordering the final events in yeast exocytosis. J. Cell Biol. 151, 439-452. 10.1083/jcb.151.2.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C. and Novick P. (1999a). The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071-1080. 10.1093/emboj/18.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Grant A. and Novick P. (1999b). Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 274, 23558-23564. 10.1074/jbc.274.33.23558 [DOI] [PubMed] [Google Scholar]
- Guo W., Sacher M., Barrowman J., Ferro-Novick S. and Novick P. (2000). Protein complexes in transport vesicle targeting. Trends Cell Biol. 10, 251-255. 10.1016/S0962-8924(00)01754-2 [DOI] [PubMed] [Google Scholar]
- Guo W., Tamanoi F. and Novick P. (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3, 353-360. 10.1038/35070029 [DOI] [PubMed] [Google Scholar]
- Hala M., Cole R., Synek L., Drdova E., Pecenkova T., Nordheim A., Lamkemeyer T., Madlung J., Hochholdinger F., Fowler J. E. et al. (2008). An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20, 1330-1345. 10.1105/tpc.108.059105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad N. M., Elconin J. H., Karnoub A. E., Bai W., Rich J. N., Abraham R. T., Der C. J. and Counter C. M. (2002). Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 16, 2045-2057. 10.1101/gad.993902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger Z. A., Hamburger A. E., West A. P. Jr and Weis W. I. (2006). Crystal structure of the S.cerevisiae exocyst component Exo70p. J. Mol. Biol. 356, 9-21. 10.1016/j.jmb.2005.09.099 [DOI] [PubMed] [Google Scholar]
- Hase K., Kimura S., Takatsu H., Ohmae M., Kawano S., Kitamura H., Ito M., Watarai H., Hazelett C. C., Yeaman C. et al. (2009). M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427-1432. 10.1038/ncb1990 [DOI] [PubMed] [Google Scholar]
- Hazelett C. C., Sheff D. and Yeaman C. (2011). RalA and RalB differentially regulate development of epithelial tight junctions. Mol. Biol. Cell 22, 4787-4800. 10.1091/mbc.E11-07-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuka C. D., Foletti D. L., Hsu S. C., Kee Y., Hopf F. W. and Scheller R. H. (1999). The sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J. Neurosci. 19, 1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Xi F., Zhang J., TerBush D., Zhang X. and Guo W. (2007a). Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J. Cell Biol. 176, 771-777. 10.1083/jcb.200606134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Xi F., Zhang X., Zhang J. and Guo W. (2007b). Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 26, 4053-4065. 10.1038/sj.emboj.7601834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.-C., Ting A. E., Hazuka C. D., Davanger S., Kenny J. W., Kee Y. and Scheller R. H. (1996). The mammalian brain rsec6/8 complex. Neuron 17, 1209-1219. 10.1016/S0896-6273(00)80251-2 [DOI] [PubMed] [Google Scholar]
- Hsu S.-C., Hazuka C. D., Roth R., Foletti D. L., Heuser J. and Scheller R. H. (1998). Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 20, 1111-1122. 10.1016/S0896-6273(00)80493-6 [DOI] [PubMed] [Google Scholar]
- Inoue M., Chang L., Hwang J., Chiang S.-H. and Saltiel A. R. (2003). The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629-633. 10.1038/nature01533 [DOI] [PubMed] [Google Scholar]
- Issaq S. H., Lim K. H. and Counter C. M. (2010). Sec5 and Exo84 foster oncogenic ras-mediated tumorigenesis. Mol. Cancer Res. 8, 223-231. 10.1158/1541-7786.MCR-09-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A. and Bellen H. J. (2005). Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351-363. 10.1016/j.devcel.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Jin R., Junutula J. R., Matern H. T., Ervin K. E., Scheller R. H. and Brunger A. T. (2005). Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 24, 2064-2074. 10.1038/sj.emboj.7600699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Sultana A., Gandhi P., Franklin E., Hamamoto S., Khan A. R., Munson M., Schekman R. and Weisman L. S. (2011). Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev. Cell 21, 1156-1170. 10.1016/j.devcel.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler A., Feng S., Zhang J., Zhang X., Das A., Peranen J. and Guo W. (2010). Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA 107, 6346-6351. 10.1073/pnas.1002401107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G. (2009). RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J. Cell Sci. 122, 1499-1506. 10.1242/jcs.044339 [DOI] [PubMed] [Google Scholar]
- Lalli G. and Hall A. (2005). Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J. Cell Biol. 171, 857-869. 10.1083/jcb.200507061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Rossé C, Racine V, Sibarita J.-B., Aresta S., Murthy M., Schwarz T., Camonis J. and Bellaïche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365-376. 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Lehman K., Rossi G., Adamo J. E. and Brennwald P. (1999). Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 146, 125-140. 10.1083/jcb.146.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.-H., Baines A. T., Fiordalisi J. J., Shipitsin M., Feig L. A., Cox A. D., Der C. J. and Counter C. M. (2005). Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7, 533-545. 10.1016/j.ccr.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Lipatova Z., Tokarev A. A., Jin Y., Mulholland J., Weisman L. S. and Segev N. (2008). Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol. Biol. Cell 19, 4177-4187. 10.1091/mbc.E08-02-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschutz J. H., Guo W., O'Brien L. E., Nguyen Y. H., Novick P. and Mostov K. E. (2000). Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol. Biol. Cell 11, 4259-4275. 10.1091/mbc.11.12.4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. and Guo W. (2012). The exocyst complex in exocytosis and cell migration. Protoplasma 249, 587-597. 10.1007/s00709-011-0330-1 [DOI] [PubMed] [Google Scholar]
- Liu J., Zuo X., Yue P. and Guo W. (2007). Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18, 4483-4492. 10.1091/mbc.E07-05-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yue P., Artym V. V., Mueller S. C. and Guo W. (2009). The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol. Biol. Cell 20, 3763-3771. 10.1091/mbc.E08-09-0967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhao Y., Sun Y., He B., Yang C., Svitkina T., Goldman Y. E. and Guo W. (2012). Exo70 stimulates the Arp2/3 complex for lamellipodia formation and directional cell migration. Curr. Biol. 22, 1510-1515. 10.1016/j.cub.2012.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Liu J., Liu S., Zeng J., Ding D., Carstens R. P., Cong Y., Xu X. and Guo W. (2013). Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev Cell. 27, 560-573. 10.1016/j.devcel.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Zhang J., Luca F. C. and Guo W. (2013). Mitotic phosphorylation of Exo84 disrupts exocyst assembly and arrests cell growth. J. Cell Biol. 202, 97-111. 10.1083/jcb.201211093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Zhang J. and Guo W. (2014). The Role of Sec3p in Secretory Vesicle Targeting and Exocyst Complex Assembly. Mol. Biol. Cell 25, 3813-3822. 10.1091/mbc.e14-04-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cuadrado A. B., Morrell J. L., Konomi M., An H., Petit C., Osumi M., Balasubramanian M., Gould K. L., del Rey F. and de Aldana C. R. V. (2005). Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 16, 4867-4881. 10.1091/mbc.E04-12-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H. T., Yeaman C., Nelson W. J. and Scheller R. H. (2001). The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc. Natl. Acad. Sci. USA 98, 9648-9653. 10.1073/pnas.171317898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. Q., Hiesinger P. R., Beronja S., Zhai R. G., Schulze K. L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M. C. et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219-232. 10.1016/j.neuron.2005.02.029 [DOI] [PubMed] [Google Scholar]
- Mohammadi S. and Isberg R. R. (2013). Cdc42 interacts with the exocyst complex to promote phagocytosis. J. Cell Biol. 200, 81-93. 10.1083/jcb.201204090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P., Rosse C., Castro-Castro A., Irondelle M., Lagoutte E., Paul-Gilloteaux P., Desnos C., Formstecher E., Darchen F., Perrais D. et al. (2013). Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J. Cell. Biol. 10.1083/jcb.201306162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. A., Robinson H. H. and Xu Z. (2007). The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J. Mol. Biol. 371, 410-421. 10.1016/j.jmb.2007.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgera F., Sallah M. R., Dubuke M. L., Gandhi P., Brewer D. N., Carr C. M. and Munson M. (2012). Regulation of exocytosis by the exocyst subunit Sec6 and the SM protein Sec1. Mol. Biol. Cell 23, 337-346. 10.1091/mbc.E11-08-0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S., Henry D. O., Rosse C., Mirey G., Camonis J. H. and White M. A. (2002). The exocyst is a Ral effector complex. Nat. Cell Biol. 4, 66-72. 10.1038/ncb728 [DOI] [PubMed] [Google Scholar]
- Moskalenko S., Tong C., Rosse C., Mirey G., Formstecher E., Daviet L., Camonis J. and White M. A. (2003). Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278, 51743-51748. 10.1074/jbc.M308702200 [DOI] [PubMed] [Google Scholar]
- Mott H. R., Nietlispach D., Hopkins L. J., Mirey G., Camonis J. H. and Owen D. (2003). Structure of the GTPase-binding domain of Sec5 and elucidation of its Ral binding site. J. Biol. Chem. 278, 17053-17059. 10.1074/jbc.M300155200 [DOI] [PubMed] [Google Scholar]
- Munson M. and Novick P. (2006). The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 13, 577-581. 10.1038/nsmb1097 [DOI] [PubMed] [Google Scholar]
- Murthy M., Garza D., Scheller R. H. and Schwarz T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433-447. 10.1016/S0896-6273(03)00031-X [DOI] [PubMed] [Google Scholar]
- Murthy M., Ranjan R., Denef N., Higashi M. E., Schupbach T. and Schwarz T. L. (2005). Sec6 mutations and the Drosophila exocyst complex. J. Cell Sci. 118, 1139-1150. 10.1242/jcs.01644 [DOI] [PubMed] [Google Scholar]
- Neto H., Kaupisch A., Collins L. L. and Gould G. W. (2013). Syntaxin 16 is a master recruitment factor for cytokinesis. Mol. Biol. Cell 24, 3663-3674. 10.1091/mbc.E13-06-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C. and Schekman R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205-215. 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- Ou Y.-H., Torres M., Ram R., Formstecher E., Roland C., Cheng T., Brekken R., Wurz R., Tasker A., Polverino T. et al. (2011). TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol. Cell 41, 458-470. 10.1016/j.molcel.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan A., Silvis M., Weisz O. A., Bradbury N. A., Hsu S.-C., Goldenring J. R., Yeaman C. and Apodaca G. (2007). Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol. Biol. Cell 18, 3978-3992. 10.1091/mbc.E07-02-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-O. and Bi E. (2007). Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71, 48-96. 10.1128/MMBR.00028-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. (1999). Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1, E17-E22. 10.1038/8967 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. and Borisy G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Polzin A., Shipitsin M., Goi T., Feig L. A. and Turner T. J. (2002). Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol. Cell Biol. 22, 1714-1722. 10.1128/MCB.22.6.1714-1722.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent M., Dubois T., Raposo G., Derrien V., Tenza D., Rosse C., Camonis J. and Chavrier P. (2003). ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 163, 1111-1121. 10.1083/jcb.200305029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A., Hong S. J., Gifford A. and Weinberg R. A. (2004). Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6, 171-183. 10.1016/j.ccr.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Ren J. and Guo W. (2012). ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev. Cell 22, 967-978. 10.1016/j.devcel.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler G. M., Balasingam G., Lucas K. G., Wang S., Hsu S.-C. and Firestein B. L. (2003). Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor). Biochem. J. 373, 49-55. 10.1042/BJ20021838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Molina F. and Toomre D. (2013). Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J. Cell Biol. 201, 673-680. 10.1083/jcb.201212103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. G., Guo L., Imai J., Toh E. A., Matsui Y. and Tamanoi F. (1999). Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19, 3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. K., Wilson P. D., Snyder R. W., Zhang X., Guo W., Burrow C. R. and Lipschutz J. H. (2004). The exocyst localizes to the primary cilium in MDCK cells. Biochem. Biophys. Res. Commun. 319, 138-143. 10.1016/j.bbrc.2004.04.165 [DOI] [PubMed] [Google Scholar]
- Rosse C., Hatzoglou A., Parrini M. C., White M. A., Chavrier P. and Camonis J. (2006). RalB mobilizes the exocyst to drive cell migration. Mol. Cell. Biol. 26, 727-734. 10.1128/MCB.26.2.727-734.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C., Formstecher E., Boeckeler K., Zhao Y., Kremerskothen J., White M. D., Camonis J. H. and Parker P. J. (2009). An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 7, e1000235 10.1371/journal.pbio.1000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Yageta M., Recchi C., Le Dez G., Sibarita J. B., Daviet L., Camonis J., D'Souza-Schorey C. and Chavrier P. (2008). The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 181, 985-998. 10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N., Prybylowski K., Petralia R. S., Chang K., Wang Y.-X., Racca C., Vicini S. and Wenthold R. J. (2003). NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 5, 520-530. 10.1038/ncb990 [DOI] [PubMed] [Google Scholar]
- Schiel J. A., Childs C. and Prekeris R. (2013). Endocytic transport and cytokinesis: from regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol. 23, 319-327. 10.1016/j.tcb.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller C., Diakopoulos K. N., Rohwedder I., Kremmer E., von Toerne C., Ueffing M., Weidle U. H., Ohno H. and Weiss E. H. (2013). LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J. Cell Sci. 126, 767-777. 10.1242/jcs.114033 [DOI] [PubMed] [Google Scholar]
- Segui-Simarro J. M., Austin J. R. II, White E. A. and Staehelin L. A. (2004). Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16, 836-856. 10.1105/tpc.017749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D., Yuan H., Hutagalung A., Verma A., Kummel D., Wu X., Reinisch K., McNew J. A. and Novick P. (2013). The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p. J. Cell Biol. 202, 509-526. 10.1083/jcb.201211148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simicek M., Lievens S., Laga M., Guzenko D., Aushev V. N., Kalev P., Baietti M. F., Strelkov S. V., Gevaert K., Tavernier J. et al. (2013). The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response. Nat. Cell Biol. 15, 1220-1230. 10.1038/ncb2847 [DOI] [PubMed] [Google Scholar]
- Sivaram M. V., Saporita J. A., Furgason M. L., Boettcher A. J. and Munson M. (2005). Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry 44, 6302-6311. 10.1021/bi048008z [DOI] [PubMed] [Google Scholar]
- Sivaram M. V., Furgason M. L., Brewer D. N. and Munson M. (2006). The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat. Struct. Mol. Biol. 13, 555-556. 10.1038/nsmb1096 [DOI] [PubMed] [Google Scholar]
- Spiczka K. S. and Yeaman C. (2008). Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J. Cell Sci. 121, 2880-2891. 10.1242/jcs.031641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K., Asano S., Tanaka K., Iwamatsu A., Okawa K. and Ohta Y. (2002). The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 4, 73-78. 10.1038/ncb720 [DOI] [PubMed] [Google Scholar]
- Suraneni P., Rubinstein B., Unruh J. R., Durnin M., Hanein D. and Li R. (2012). The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J. Cell Biol. 197, 239-251. 10.1083/jcb.201112113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa N., Sun Y., Schramp M., Choi S., Ling K. and Anderson R. A. (2012). Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev. Cell 22, 116-130. 10.1016/j.devcel.2011.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D. R. and Novick P. (1995). Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130, 299-312. 10.1083/jcb.130.2.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D. R., Maurice T., Roth D. and Novick P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483-6494. [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Heyer V., Lux A., Alunni V., Degrave A., Seiliez I., Kirchner J., Parkhill J.-P. and Thisse C. (2004). Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 77, 505-519. 10.1016/S0091-679X(04)77027-2 [DOI] [PubMed] [Google Scholar]
- Vega I. E. and Hsu S. C. (2001). The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 21, 3839-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Tang X., Liu J., Trautmann S., Balasundaram D., McCollum D. and Balasubramanian M. K. (2002). The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 515-529. 10.1091/mbc.01-11-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Tang X. and Balasubramanian M. K. (2003). Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 164, 1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ding Y., Wang J., Hillmer S., Miao Y., Lo S. W., Wang X., Robinson D. G. and Jiang L. (2010). EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 22, 4009-4030. 10.1105/tpc.110.080697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha C. C., Jiang P., Amirikian K., Dittmar K. A., Lu H., Shen S., Guo W., Xing Y. and Carstens R. P. (2010). An ESRP-regulated splicing programme is abrogated during the epithelial–mesenchymal transition. EMBO J. 29, 3286-3300. 10.1038/emboj.2010.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J. R. and Munro S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627-2637. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A., De Craene J. O., Ferro-Novick S. and Novick P. (2004). Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J. Cell. Biol. 167, 875-887. 10.1083/jcb.200408001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Mehta S. Q., Pichaud F., Bellen H. J. and Quiocho F. A. (2005). Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 12, 879-885. 10.1038/nsmb987 [DOI] [PubMed] [Google Scholar]
- Wu H., Turner C., Gardner J., Temple B. and Brennwald P. (2010). The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol. Biol. Cell 21, 430-442. 10.1091/mbc.E09-06-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Xu Q., Huang Y., Singh R. D., Anderson R., Leof E., Hu J. and Ling K. (2012). An association between type Igamma PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol. Biol. Cell 23, 87-98. 10.1091/mbc.E11-05-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Kasamatsu A., Ishige S., Koike K., Saito K., Kouzu Y., Koike H., Sakamoto Y., Ogawara K., Shiiba M. et al. (2013). Exocyst complex component Sec8: a presumed component in the progression of human oral squamous-cell carcinoma by secretion of matrix metalloproteinases. J. Cancer Res. Clin. Oncol. 139, 533-542. 10.1007/s00432-012-1356-2 [DOI] [PubMed] [Google Scholar]
- Yamashita M., Kurokawa K., Sato Y., Yamagata A., Mimura H., Yoshikawa A., Sato K., Nakano A. and Fukai S. (2010). Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nat. Struct. Mol. Biol. 17, 180-186. 10.1038/nsmb.1722 [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K. and Nelson W. J. (2004). Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 117, 559-570. 10.1242/jcs.00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.-M. and Hughson F. M. (2010). Tethering factors as organizers of intracellular vesicular traffic. Annu. Rev. Cell Dev. Biol. 26, 137-156. 10.1146/annurev.cellbio.042308.113327 [DOI] [PubMed] [Google Scholar]
- Žárský V., Cvrčková F., Potocký M. and Hála M. (2009). Exocytosis and cell polarity in plants - exocyst and recycling domains. New Phytol. 183, 255-272. 10.1111/j.1469-8137.2009.02880.x [DOI] [PubMed] [Google Scholar]
- Zhang X., Bi E., Novick P., Du L., Kozminski K. G., Lipschutz J. H. and Guo W. (2001). Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276, 46745-46750. 10.1074/jbc.M107464200 [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Ellis S., Sriratana A., Mitchell C. A. and Rowe T. (2004). Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027-43034. 10.1074/jbc.M402264200 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang P., Gangar A., Zhang J., Brennwald P., TerBush D. and Guo W. (2005). Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 170, 273-283. 10.1083/jcb.200502055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Orlando K., He B., Xi F., Zhang J., Zajac A. and Guo W. (2008). Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 180, 145-158. 10.1083/jcb.200704128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu J., Yang C., Capraro B. R., Baumgart T., Bradley R. P., Ramakrishnan N., Xu X., Radhakrishnan R., Svitkina T. et al. (2013). Exo70 generates membrane curvature for morphogenesis and cell migration. Dev. Cell 26, 266-278. 10.1016/j.devcel.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X., Zhang J., Zhang Y., Hsu S.-C., Zhou D. and Guo W. (2006). Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat. Cell Biol. 8, 1383-1388. 10.1038/ncb1505 [DOI] [PubMed] [Google Scholar]
- Zuo X., Guo W. and Lipschutz J. H. (2009). The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 20, 2522-2529. 10.1091/mbc.E08-07-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.