ABSTRACT
Human airway basal cells are the stem (or progenitor) population of the airway epithelium, and play a central role in anchoring the epithelium to the basement membrane. The anatomic position of basal cells allows for potential paracrine signaling between them and the underlying non-epithelial stromal cells. In support of this, we have previously demonstrated that endothelial cells support growth of basal cells during co-culture through vascular endothelial growth factor A (VEGFA)-mediated signaling. Building on these findings, we found, by RNA sequencing analysis, that basal cells expressed multiple fibroblast growth factor (FGF) ligands (FGF2, FGF5, FGF11 and FGF13) and that only FGF2 and FGF5 were capable of functioning in a paracrine manner to activate classical FGF receptor (FGFR) signaling. Antibody-mediated blocking of FGFR1 during basal-cell–endothelial-cell co-culture significantly reduced the endothelial-cell-dependent basal cell growth. Stimulation of endothelial cells with basal-cell-derived growth factors induced endothelial cell expression of matrix metallopeptidase 14 (MMP14), and short hairpin RNA (shRNA)-mediated knockdown of endothelial cell MMP14 significantly reduced the endothelial-cell-dependent growth of basal cells. Overall, these data characterize a new growth-factor-mediated reciprocal ‘crosstalk’ between human airway basal cells and endothelial cells that regulates proliferation of basal cells.
KEY WORDS: Endothelial cell, Airway basal cell, MMP14, Crosstalk, Progenitor cell
Summary: Basal cell and endothelial cell crosstalk might play a substantial role in maintaining normal airway structure, with alteration of this crosstalk contributing towards smoking-dependent airway remodeling.
INTRODUCTION
The human airway epithelium consists of multiple cell types including basal, secretory and ciliated cells lining a basement membrane (Knight and Holgate, 2003; Crystal et al., 2008; Tam et al., 2011). Below the basement membrane lie multiple non-epithelial cell types, including fibroblasts, smooth muscle and capillary endothelial cells (Tam et al., 2011). Basal cells are the stem (or progenitor) population of the airway epithelium that differentiate into the other specialized epithelial cell types during turnover and repair (Evans et al., 2001; Hajj et al., 2007; Rock et al., 2009, 2010; Hackett et al., 2011; Shaykhiev et al., 2013; Staudt et al., 2014; Hogan et al., 2014). In addition, basal cells play a central role in anchoring the epithelium to the basement membrane helping to protect the underlying non-epithelial cell types from the external environment (Knight and Holgate, 2003; Tam et al., 2011). The positioning of basal cells allows for potential paracrine signaling from other non-epithelial cell types to regulate basal cell function, including proliferation and differentiation. Therefore, understanding the crosstalk between basal cells and other airway cell types is important for understanding the processes that regulate normal airway epithelial architecture.
Previous work from our laboratory has demonstrated functional crosstalk between basal cells and endothelial cells, whereby endothelial cells, in response to basal-cell-secreted vascular endothelial factor A (VEGFA), could support growth of basal cells in co-culture (Curradi et al., 2012). The present study builds on these findings and demonstrates that, in addition to VEGFA, basal cells express multiple fibroblast growth factor (FGF) ligands, a family of growth factors known to regulate activation of endothelial cells (Presta et al., 2005; Lieu et al., 2011; Belov and Mohammadi, 2013; Carter et al., 2015). These ligands function in a paracrine manner to activate endothelial cells through FGF receptor 1 (FGFR1)-dependent signaling. Furthermore, secreted basal-cell-derived growth factors induce endothelial cell expression of matrix metallopeptidase 14 (MMP14), a mediator required for endothelial-cell-dependent growth support of basal cells.
RESULTS AND DISCUSSION
Expression of FGF ligands in human airway basal cells
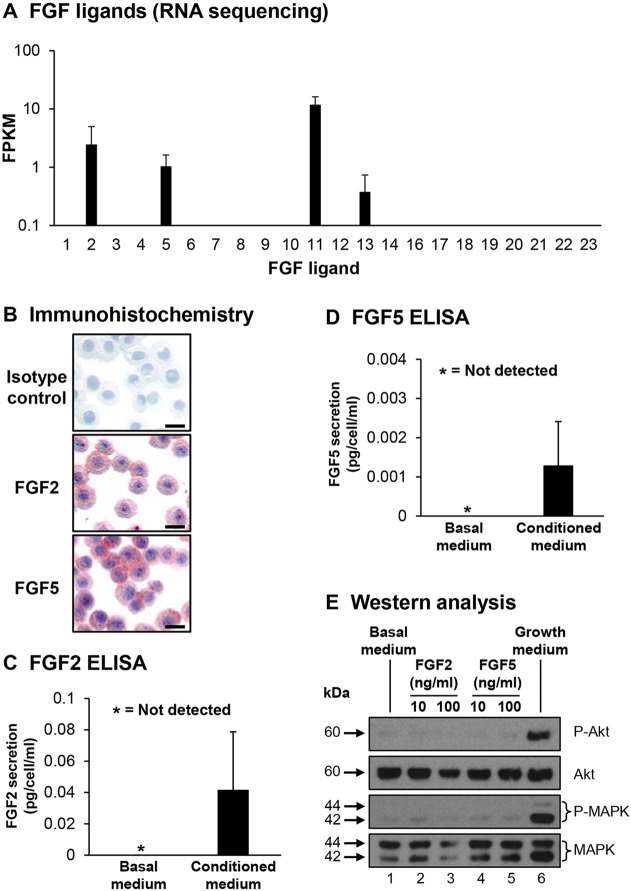
FGF ligands play an essential role in regulating diverse processes in multiple cell types during embryonic development and in differentiated tissues (Presta et al., 2005; Lieu et al., 2011; Belov and Mohammadi, 2013; Carter et al., 2015). The majority of FGF ligands function by binding to four tyrosine kinase FGF receptors (FGFR1–FGFR4) to activate downstream kinase responses including Akt and mitogen-activated protein kinase (MAPK) signaling. However, some ligands (FGF11–FGF14) do not bind FGFRs and have distinct modes of action (Olsen et al., 2003). Based on our prior work demonstrating crosstalk between endothelial cells and basal cells (Curradi et al., 2012), and the knowledge that FGF ligands play a role in regulating endothelial cell function, we assessed expression of FGF ligands in basal cells. RNA sequencing demonstrated that basal cells expressed only FGF2, FGF5, FGF11 and FGF13 (Fig. 1A). Of the ligands expressed, only FGF2 and FGF5 mediate signaling through binding to FGFRs and are capable of functioning in both an autocrine and paracrine manner, whereas FGF11 and FGF13 function independently of FGFRs (Olsen et al., 2003; Presta et al., 2005; Lieu et al., 2011; Belov and Mohammadi, 2013; Carter et al., 2015). Basal cell expression of FGF2 and FGF5 at the protein level was confirmed by immunohistochemistry (Fig. 1B). In addition, secretion of both FGF2 and FGF5 was confirmed by ELISA analysis of supernatants from basal cell cultures (Fig. 1C,D). To investigate whether FGF2 and FGF5 function in an autocrine manner to activate signaling cascades in basal cells, growth-factor-starved basal cells were stimulated with basal medium, basal medium supplemented with recombinant FGF2 or FGF5 or growth medium as a positive control. Western blot analysis for activation of Akt and the MAPKs ERK1 and ERK2 (ERK1/2, also known as MAPK3 and MAPK1, respectively) demonstrated that there was a substantial phosphorylation of both proteins in cells stimulated with growth medium (Fig. 1E, lane 6). However, in cells stimulated with basal medium or basal medium supplemented with FGF2 or FGF5, there were low levels of basal phosphorylation of both proteins with no increased activation in response to each stimuli (Fig. 1E, lanes 1–5). Equal loading of protein in each lane was confirmed by staining for total Akt and ERK1/2 (Fig. 1E, lanes 1–5). Overall, these data suggest that basal cells express FGF2 and FGF5, which function in a paracrine manner.
Fig. 1.
Basal cell expression of FGF family ligands. (A) RNA sequencing analysis of FGF ligands in basal cells. Data shown represents the mean±s.d FPKM expression from n=10 independent samples. (B) Immunohistochemical staining of cytopreparations of basal cells for FGF2, FGF5 and an isotype control. Scale bar: 20 µm. (C,D) FGF2 and FGF5 levels assessed by ELISA in medium from basal cells. Secreted FGF2 and FGF5 were normalized to the cell number and calculated as pg per cell/ml. Data shown is the mean±s.d of three independent samples, each performed in triplicate. (E) Airway basal cells were starved of growth factors for 6 h and then stimulated for 15 min with basal medium (lane 1), basal medium containing FGF2 (10 and 100 ng/ml) (lanes 2 and 3) or FGF5 (10 and 100 ng/ml) (lanes 4 and 5), or growth-factor-containing medium (lane 6). Activation of Akt and MAPK signaling was evaluated by western blot analysis and staining for phosphorylated Akt (P-Akt) and phosphorylated ERK1/2 (P-MAPK). The levels of total Akt and ERK1/2 (MAPK) were evaluated as a loading control.
FGF2 is a pro-angiogenic factor known to regulate endothelial cell function through FGFR1-dependent signaling mechanisms (Presta et al., 2005). The role of FGF5 in regulating endothelial cell function is less well characterized, although it is overexpressed in human glioblastoma and promotes malignant progression through FGFR1-dependent signaling (Allerstorfer et al., 2008). Based on these functions, we focused on characterizing the role of FGFR1 signaling in regulating crosstalk between basal cells and endothelial cells.
Blocking of FGFR1 with a specific antibody suppresses endothelial-cell-dependent proliferation of basal cells
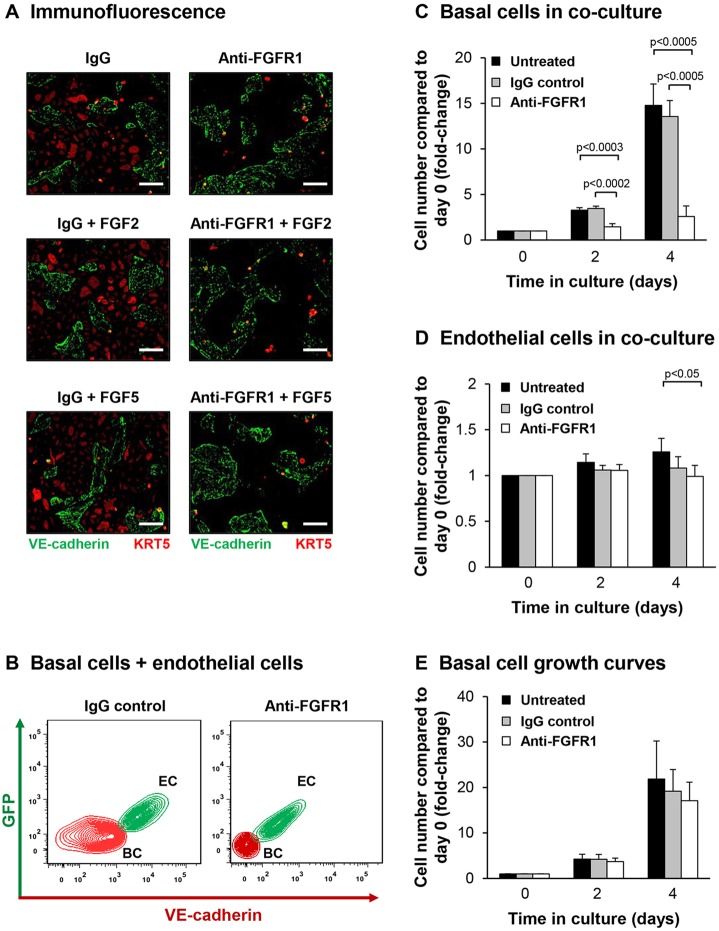
Using our previously developed cytokine- and serum-free co-culture system, which utilizes modified primary human umbilical vein endothelial cells (HUVECs) constitutively expressing active Akt (HUVEC-Akt) (Curradi et al., 2012), we assessed the role of FGFR1-mediated signaling in regulating endothelial-cell-dependent proliferation of basal cells. Co-cultures of basal cells and endothelial cells were untreated, or incubated with control IgG or an antibody against FGFR1 (which blocks FGF-ligand-dependent signaling through this receptor) in the absence and presence of recombinant FGF2 or FGF5. Immunofluorescence analysis with specific markers for endothelial cells (VE-cadherin) and basal cells (KRT5) following 4 days of co-culture in the presence of IgG or anti-FGFR1 antibody (with or without FGF2 or FGF5) demonstrated little difference in the numbers of VE-cadherin-positive endothelial cells between conditions. However, we observed a reduction in numbers of KRT5-positive basal cells in cultures treated with anti-FGFR1 even in the presence of recombinant FGF2 or FGF5 (Fig. 2A). Stimulation of co-cultures with exogenous FGF2 or FGF5 in the absence of anti-FGFR1 had little effect on KRT5-positive basal cell numbers, suggesting over-stimulation of FGF signaling has no positive effect on basal cell growth during co-culture. We next quantified the number of basal cells and endothelial cells at specific times points by flow cytometric analysis (Fig. 2B). Over 4 days, untreated basal cells proliferated, with a 14.8-fold increase in cell numbers compared to day 0 (Fig. 2C). Relative to untreated cells, incubation with IgG had no significant effect (P>0.4) on basal cell proliferation (13.6-fold; Fig. 2C). However, anti-FGFR1 antibody significantly suppressed basal cell growth (2.6-fold) compared to untreated (P<0.0005) and IgG controls (P<0.0005).
Fig. 2.
Blocking of FGFR1 suppresses endothelial cells-dependent proliferation of basal cells. (A–D) Airway basal cells were co-cultured with endothelial cells (HUVEC-Akt) and incubated with control IgG or anti-FGFR1 antibody. For A, FGF2 (10 ng/ml) or FGF5 (10 ng/ml) were also added. (A) Immunofluorescence of basal cells and endothelial cells in co-culture. Basal cells were identified by KRT5 staining (red) and endothelial cells were identified by VE-cadherin staining (green). Scale bars: 100 µm. (B) Representative flow cytometric analysis of basal cells (BC) and endothelial cells (EC) in co-culture. (C) Proliferation of basal cells co-cultured with endothelial cells. (D) Proliferation of endothelial cells in co-culture with basal cells. (E) Proliferation of basal cells cultured alone in growth medium and incubated with control IgG or anti-FGFR1 antibody. For C–E, shown are untreated (black), IgG (gray), and anti-FGFR1 antibody (white) data; results are the mean±s.d. of four independent experiments, each performed in triplicate.
We next analyzed the effect of antibody-mediated inhibition of FGFR1 on the endothelial cell population in the same experiments. Over 4 days, the untreated endothelial cells proliferated, with a 1.3-fold increase in cell numbers compared to day 0 (Fig. 2D). Treatment with IgG had no significant effect on endothelial cell numbers (1.1-fold, P>0.1) relative to untreated cells (Fig. 2D). For endothelial cells incubated with anti-FGFR1, there was a small, but significant (P<0.05), decrease in cells numbers relative to untreated cells (Fig. 2D). However, there was no significant difference (P>0.3) in cell numbers compared to IgG treatment (Fig. 2D).
To confirm the reduced basal cell growth in co-culture was due to specific inhibition of FGFR1 signaling in endothelial cells, basal cells were cultured alone in regular growth medium in the absence and presence of anti-FGFR1 antibody or control IgG (Fig. 2E). Compared to untreated cells, no significant change in basal cell growth was observed following 4 days of culture in the presence of IgG (19.2-fold versus 21.9-fold, P>0.6) and anti-FGFR1 antibody (17.1-fold versus 21.9-fold, P>0.4). Furthermore, no significant difference in cell numbers was observed between cells treated with anti-FGFR1 antibody and IgG (17.1-fold versus 19.2-fold, P>0.5). Overall, these data demonstrate that endothelial-cell-specific FGFR1 signaling is required for efficient endothelial-cell-dependent proliferation of basal cells.
Endothelial cell expression of MMP14 is required for the endothelial-cell-dependent proliferation of basal cells
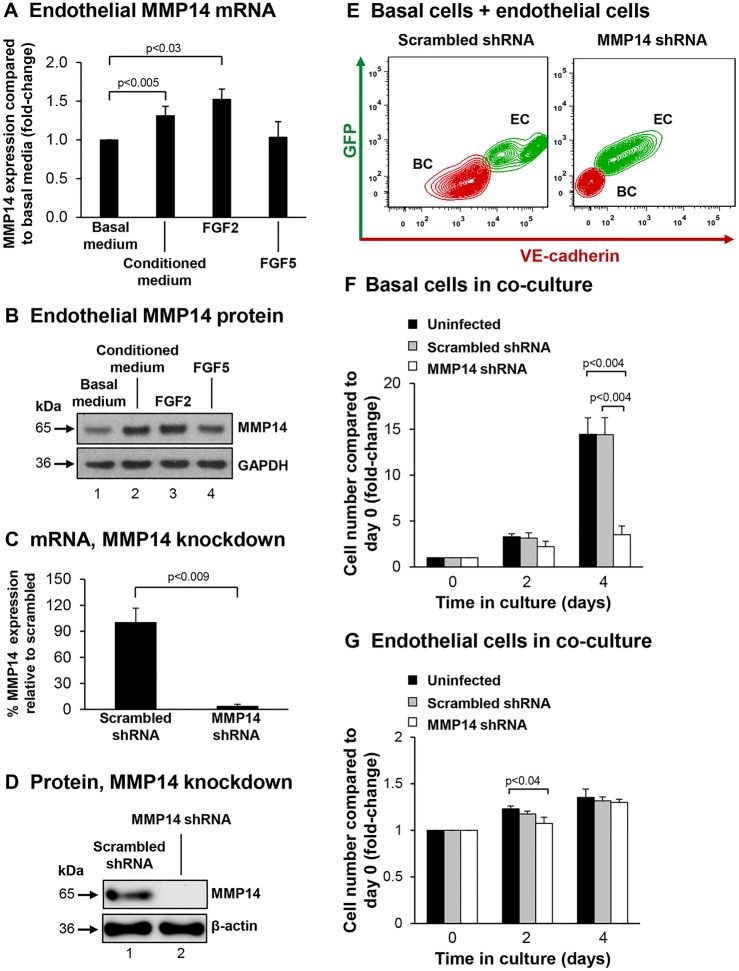
Mouse studies have shown that epithelial–endothelial crosstalk plays an important role in promoting alveologenesis following unilateral pneumonectomy (Ding et al., 2011). Pneumonectomy stimulates pulmonary capillary endothelial cells through VEGFR2- and FGFR1-signaling-dependent mechanisms to produce endothelial cell growth factors, specifically MMP14, that induce proliferation of epithelial progenitor cells to support alveologenesis. Therefore, we hypothesized that similar mechanisms might be conserved in humans and that endothelial-cell-expressed MMP14 might play a role in regulating the endothelial-cell-dependent growth support of basal cells. To assess whether MMP14 expression is upregulated in endothelial cells following stimulation with basal-cell-derived growth factors, endothelial cells were starved of growth factors, then stimulated with basal medium, conditioned medium (basal medium exposed overnight to cultured basal cells) or basal medium containing recombinant FGF2 or FGF5, before being harvested to analyze MMP14 mRNA expression. Relative to endothelial cells stimulated with basal medium, there was a significant increase in MMP14 expression in endothelial cells stimulated with basal-cell-conditioned medium (1.31-fold, P<0.005) and FGF2 (1.52-fold, P<0.03; Fig. 3A); however, FGF5 had no effect (1.03-fold, P>0.7; Fig. 3A). These data were verified at the protein level by western blot analysis for MMP14 (Fig. 3B). Overall, these data demonstrate that basal-cell-secreted growth factors (specifically FGF2) can stimulate expression of MMP14 in endothelial cells in a paracrine manner.
Fig. 3.
Endothelial cell MMP14 is required for endothelial-cell-dependent induction of basal cell proliferation. (A,B) Basal-cell-derived growth factors stimulate expression of endothelial cells MMP14. (A) mRNA (TaqMan) analysis of MMP14 expression in growth-factor-starved endothelial cells (HUVECs) stimulated with either basal medium, basal medium conditioned on basal cells, or basal medium containing FGF2 (10 ng/ml) or FGF5 (10 ng/ml). Data shown is the mean±s.d. of at least three independent experiments, each performed in triplicate. (B) Protein levels of MMP14 in cells treated as in A. A representative western blot analysis is shown. (C,D) shRNA-mediated knockdown of endothelial cell (HUVEC-Akt) MMP14 expression. (C) mRNA analysis (TaqMan) of MMP14 expression in cells infected with the indicated shRNA. (D) Protein levels of MMP14 in cells treated as in C. A representative western blot analysis is shown. (E–G) Analysis of basal cell and endothelial cell co-culture. (E) Representative flow cytometric analysis of basal cells (BC) and endothelial cells (EC) infected with scrambled or MMP14 shRNA cells in co-culture. (F) Basal cell number in co-culture. (G) Endothelial cell numbers in co-culture. For F–G, shown are the endothelial cells (black), endothelial cells containing scrambled shRNA (gray) and MMP14 shRNA (white) data; results are the mean±s.d. of three independent experiments each performed in triplicate.
The functional role of endothelial-cell-expressed MMP14 in regulating endothelial-cell-dependent growth support of basal cells was assessed by knockdown of MMP14 in endothelial cells. Lentivirus infection of endothelial cells with an MMP14-specific short hairpin RNA (shRNA) resulted in a significant knockdown of MMP14 expression at the mRNA level relative to scrambled shRNA (>95% knockdown, P<0.009; Fig. 3C). These data were further validated at the protein level (Fig. 3D). Co-cultures of basal cells and uninfected endothelial cells or endothelial cells infected with scrambled or MMP14 shRNA were established, and the growth of basal cells and endothelial cells quantified (Fig. 3E). Over 4 days, basal cells cultured with uninfected endothelial cells proliferated, with a 14.4-fold increase in cell numbers compared to day 0 (Fig. 3F). Endothelial cells infected with scrambled shRNA showed no significant effect (P>0.9) on basal cell proliferation over 4 days (14.4-fold) relative to uninfected cells. However, knockdown of endothelial cell MMP14 significantly suppressed basal cell growth (3.5-fold) compared to uninfected (P<0.002) and scrambled shRNA cells (P<0.002).
We next analyzed the effect of MMP14 knockdown on the endothelial cell population in the same experiments. Over 4 days, the uninfected endothelial cells proliferated in co-culture with basal cells, with a 1.35-fold increase in cell numbers compared to day 0 (Fig. 3G). Relative to uninfected cells, treatment with scrambled or MMP14-specific shRNA had no significant effect (both P>0.4) on endothelial cell numbers at day 4 (1.3-fold increase for both; Fig. 3G). Overall these data demonstrate that endothelial cell expression of MMP14 is essential for efficient growth support of basal cells during co-culture.
In summary, we have identified a new function of airway basal cells in regulating activation of endothelial cells in a paracrine manner through FGFR1-mediated mechanisms. In turn, activated endothelial cells upregulate expression of endothelial-cell-specific factors, including MMP14, that support growth of basal cells in the absence of exogenous growth factors. In vivo studies of smoking-dependent airway remodeling demonstrate elevated expression of FGF2 in bronchial epithelial cells of patients with chronic obstructive pulmonary disease (COPD) (Kranenburg et al., 2005), enhanced expression of FGF and/or FGFR1 during vascular remodeling in COPD (Kranenburg et al., 2002), and altered distribution of vessels in the airway of smokers and smokers with COPD compared to healthy nonsmokers (Soltani et al., 2010). Therefore, crosstalk between basal cells and endothelial cells might play an important role in maintaining normal airway epithelial structure with alterations of this crosstalk contributing towards smoking-dependent airway remodeling.
MATERIALS AND METHODS
Culture of primary human airway basal cells
Basal cells were isolated from the large airway epithelium of healthy nonsmokers as described previously (Hackett et al., 2011). All human samples were collected with informed consent. The basal cells were maintained in bronchial epithelial growth medium (BEGM, Lonza, Walkersville, MD) and passaged by seeding at a cell density of 3000 cells/cm2. Each culture was passaged one time before study in co-culture with endothelial cells.
RNA sequencing
RNA sequencing of nonsmoker primary basal cells (n=10) was assessed as previously described (Ryan et al., 2014). The data are publically available at the Gene Expression Omnibus (GEO) site (http://www.ncbi.nlm.nih.gov/geo/), accession number 64464. FGF ligand expression was characterized as the fragments per kilobase of exon per million fragments sequenced (FPKM) being ≥0.04 in every sample.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Walters et al., 2013). The primary antibody against FGF2 was from Cell Signaling Technology (2 µg/ml; catalog number 3196), and that against FGF5 from Abcam (0.2 µg/ml; catalog number ab88118).
ELISA
The secretion of FGF2 and FGF5 by basal cells was assessed by ELISA (FGF2, catalog number ab99979, Abcam and FGF5, catalog number ELH-FGF5-1, RayBiotech, Inc., Norcross, GA) following incubation of basal cells overnight in BEBM as described previously (Walters et al., 2013).
Western blot analysis
Western blot analysis was performed as described previously (Curradi et al., 2012) using NuPAGE 4 to 12% Bis-Tris gradient gels (Invitrogen). Primary antibodies against the following proteins were used: phosphorylated Akt (1:1000, catalog number 4060), Akt (1:1000, catalog number 9272), ERK1/2 (1:1000, catalog number 9102); phosphorylated ERK1/2 (1:1000, catalog number 9101); β-actin (1:1000; catalog number 4967) (all from Cell Signaling Technology), GAPDH (1:5000, catalog number SC-32233, Santa Cruz Biotechnology) and MMP14 (1:1000; catalog number ab51074, Abcam).
Culture and maintenance of endothelial cells
Human umbilical cord vein endothelial cells (HUVECs) were isolated and cultured as previously described (Kobayashi et al., 2010). HUVEC-Akt cells were generated as previously described (Kobayashi et al., 2010) and maintained in an identical manner to HUVECs.
Co-culture proliferation assays
Co-culture assays were used to assess the ability of endothelial cells (HUVEC-Akt) to support basal cell proliferation in cytokine- and serum-free conditions as previously described (Curradi et al., 2012). To assess the role of FGFR1-mediated signaling on basal cell proliferation, human anti-FGFR1 neutralizing antibody (clone FR1-H7, ImClone, New York, NY) or IgG control was added at a final concentration of 1 µg/ml. In a subset of experiments, recombinant FGF2 (catalog number 8910LC, Cell Signaling Technology) or FGF5 (catalog number 237-F5-050, R&D Systems) was added. Fresh medium and antibody with or without growth factors was added every 2 days and at the desired time points, cells were trypsinized and cell numbers were measured with a hemocytometer and the viability assessed by counting of Trypan-Blue-excluding cells. The endothelial cells were quantified as the GFP- and VE-cadherin-positive population by flow cytometric analysis, and the GFP- and VE-cadherin-negative population was quantified as expanded basal cells. To assess the role of endothelial-cell-expressed MMP14 on basal cell proliferation in co-culture, endothelial cells were infected with lentivirus containing either pooled MMP14 specific shRNA (catalog number TRCN0000050853-56, GE Dharmacon) or scrambled control shRNA, with knockdown of MMP14 confirmed by TaqMan quantitative PCR and western blot analysis. Co-culture with basal cells was carried out as described above.
Immunofluorescence
Cells were fixed directly with 4% paraformaldehyde in PBS for 20 min and then permeabilized with 0.1% Triton X-100 in PBS, followed by blocking with normal serum. The samples were stained with primary antibodies against KRT5 (1 µg/ml, PA1-37974, Thermo Scientific) and VE-cadherin (1 µg/ml, catalog number AF938, R&D Systems). To visualize the antibody binding, Alexa-Fluor-594-conjugated donkey anti-rabbit-IgG (2 µg/ml, catalog number 711-585-152) and Alexa-Fluor-488-conjugated donkey anti-goat-IgG (2 µg/ml, catalog number 705-546-147) from Jackson Immunoresearch Laboratories were used for KRT5 and VE-cadherin, respectively.
Basal cell proliferation
Basal cells (2×104) were seeded into each well of a 12-well plate in BEGM. The next day (day 0) fresh growth medium was added to the cells with and without anti-FGFR1 antibody or IgG control (final concentration of 1 µg/ml). Fresh medium and antibody was added every 2 days and, at the desired time points, the cells were trypsinized and cell numbers quantified as described above.
Stimulation of endothelial MMP14 expression
HUVECs (1.5×105) were seeded into each well of a 12-well plate in the appropriate growth medium. The following day, cells were washed twice with PBS and then starved of growth factor for 12 h. Following starvation, the cells were stimulated for 6 h with either basal medium (BEBM), conditioned medium (BEBM exposed overnight to cultured basal cells) or basal medium supplemented with recombinant FGF2 or FGF5. Following stimulation, the cells were placed in TRIzol (Invitrogen) for extraction of total RNA or harvested for western blot analysis.
TaqMan quantitative PCR
The expression of MMP14 in endothelial cells was assessed using TaqMan quantitative PCR as described previously (Walters et al., 2013). Relative expression levels were determined using the ΔCt method with 18S ribosomal RNA (TaqMan® Ribosomal RNA Control, VIC, number 4308329, Applied Biosystems) as the endogenous control. A premade TaqMan Gene Expression Assay for MMP14 (Hs01037009_g1) was obtained from Applied Biosystems.
Statistical analysis
Statistical comparisons were calculated using an unpaired two-tailed Student's t-tests with unequal variance. P<0.05 was considered significant.
Acknowledgements
We thank B.-G. Harvey, R. J. Kaner, A. E. Tilley, J. Yee-Levin and M. R. Staudt for help in obtaining the clinical samples for basal cell culture, J. Heldrich, A. Rogalski and V. Arbelaez for technical assistance, and N. Mohamed for help in preparing this manuscript.
Footnotes
Competing interests
The authors declare that they have no competing interests, but notify that S.R. is the founder of and consultant to Angiocrine Bioscience New York, NY, USA.
Author contributions
B.-S.D. conceived of the study, and performed research, data analysis and manuscript writing. K.G. performed research and data analysis. S.R. conceived of the study and performed data analysis. R.G.C. conceived of the study, and performed data analysis and manuscript writing. M.S.W. conceived of the study, and performed research, data analysis and manuscript writing.
Funding
These studies are supported, in part, by the National Institutes of Health [grant numbers R01HL107882, R01HL107882-S, R01HL1189541, UL1 TR000457, UL1 RR024143]. K.G. was supported, in part, by New York State Department of Health [grant number C026878]. Deposited in PMC for release after 12 months.
References
- Allerstorfer S., Sonvilla G., Fischer H., Spiegl-Kreinecker S., Gauglhofer C., Setinek U., Czech T., Marosi C., Buchroithner J., Pichler J. et al. (2008). FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene 27, 4180-4190. 10.1038/onc.2008.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov A. A. and Mohammadi M. (2013). Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb. Perspect. Biol. 5, a015958 10.1101/cshperspect.a015958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E. P., Fearon A. E. and Grose R. P. (2015). Careless talk costs lives: fibroblast growth factor receptor signalling and the consequences of pathway malfunction. Trends Cell Biol. 25, 221-233. 10.1016/j.tcb.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Randell S. H., Engelhardt J. F., Voynow J. and Sunday M. E. (2008). Airway epithelial cells: current concepts and challenges. Proc. Am. Thorac. Soc. 5, 772-777. 10.1513/pats.200805-041HR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curradi G., Walters M. S., Ding B.-S., Rafii S., Hackett N. R. and Crystal R. G. (2012). Airway basal cell vascular endothelial growth factor-mediated cross-talk regulates endothelial cell-dependent growth support of human airway basal cells. Cell. Mol. Life Sci. 69, 2217-2231. 10.1007/s00018-012-0922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B.-S., Nolan D. J., Guo P., Babazadeh A. O., Cao Z., Rosenwaks Z., Crystal R. G., Simons M., Sato T. N., Worgall S. et al. (2011). Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539-553. 10.1016/j.cell.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Van Winkle L. S., Fanucchi M. V. and Plopper C. G. (2001). Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 27, 401-415. 10.1080/019021401300317125 [DOI] [PubMed] [Google Scholar]
- Hackett N. R., Shaykhiev R., Walters M. S., Wang R., Zwick R. K., Ferris B., Witover B., Salit J. and Crystal R. G. (2011). The human airway epithelial basal cell transcriptome. PLoS ONE 6, e18378 10.1371/journal.pone.0018378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R., Baranek T., Le Naour R., Lesimple P., Puchelle E. and Coraux C. (2007). Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 25, 139-148. 10.1634/stemcells.2006-0288 [DOI] [PubMed] [Google Scholar]
- Hogan B. L. M., Barkauskas C. E., Chapman H. A., Epstein J. A., Jain R., Hsia C. C. W., Niklason L., Calle E., Le A., Randell S. H. et al. (2014). Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123-138. 10.1016/j.stem.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. A. and Holgate S. T. (2003). The airway epithelium: structural and functional properties in health and disease. Respirology 8, 432-446. 10.1046/j.1440-1843.2003.00493.x [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Butler J. M., O'Donnell R., Kobayashi M., Ding B.-S., Bonner B., Chiu V. K., Nolan D. J., Shido K., Benjamin L. et al. (2010). Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046-1056. 10.1038/ncb2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg A. R., de Boer W. I., Van Krieken J. H., Mooi W. J., Walters J. E., Saxena P. R., Sterk P. J. and Sharma H. S. (2002). Enhanced expression of fibroblast growth factors and receptor FGFR-1 during vascular remodeling in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 27, 517-525. 10.1165/rcmb.4474 [DOI] [PubMed] [Google Scholar]
- Kranenburg A. R., Willems-Widyastuti A., Mooi W. J., Saxena P. R., Sterk P. J., de Boer W. I. and Sharma H. S. (2005). Chronic obstructive pulmonary disease is associated with enhanced bronchial expression of FGF-1, FGF-2, and FGFR-1. J. Pathol. 206, 28-38. 10.1002/path.1748 [DOI] [PubMed] [Google Scholar]
- Lieu C., Heymach J., Overman M., Tran H. and Kopetz S. (2011). Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin. Cancer Res. 17, 6130-6139. 10.1158/1078-0432.CCR-11-0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S. K., Garbi M., Zampieri N., Eliseenkova A. V., Ornitz D. M., Goldfarb M. and Mohammadi M. (2003). Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 278, 34226-34236. 10.1074/jbc.M303183200 [DOI] [PubMed] [Google Scholar]
- Presta M., Dell'Era P., Mitola S., Moroni E., Ronca R. and Rusnati M. (2005). Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16, 159-178. 10.1016/j.cytogfr.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Rock J. R., Onaitis M. W., Rawlins E. L., Lu Y., Clark C. P., Xue Y., Randell S. H. and Hogan B. L. M. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 106, 12771-12775. 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. R., Randell S. H. and Hogan B. L. M. (2010). Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 3, 545-556. 10.1242/dmm.006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. M., Vincent T. L., Salit J., Walters M. S., Agosto-Perez F., Shaykhiev R., Strulovici-Barel Y., Downey R. J., Buro-Auriemma L. J., Staudt M. R. et al. (2014). Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS ONE 9, e88051 10.1371/journal.pone.0088051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R., Zuo W.-L., Chao I., Fukui T., Witover B., Brekman A. and Crystal R. G. (2013). EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc. Natl. Acad. Sci. USA 110, 12102-12107. 10.1073/pnas.1303058110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A., Reid D. W., Sohal S. S., Wood-Baker R., Weston S., Muller H. K. and Walters E. H. (2010). Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: a cross-sectional study. Respir. Res. 11, 105 10.1186/1465-9921-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M. R., Buro-Auriemma L. J., Walters M. S., Salit J., Vincent T., Shaykhiev R., Mezey J. G., Tilley A. E., Kaner R. J., Ho M. W. Y. et al. (2014). Airway Basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 190, 955-958. 10.1164/rccm.201406-1167LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A., Wadsworth S., Dorscheid D., Man S. F. P. and Sin D. D. (2011). The airway epithelium: more than just a structural barrier. Ther. Adv. Respir. Dis. 5, 255-273. 10.1177/1753465810396539 [DOI] [PubMed] [Google Scholar]
- Walters M. S., Gomi K., Ashbridge B., Moore M. A. S., Arbelaez V., Heldrich J., Ding B.-S., Rafii S., Staudt M. R. and Crystal R. G. (2013). Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity. Respir. Res. 14, 135 10.1186/1465-9921-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]