Abstract
Surgical procedures on the trachea have only been undertaken within the past 50 years. Knowing the unique blood supply of the trachea and how to reduce tension on any anastomosis are key to a successful outcome. Tracheal conditions requiring surgery usually present with shortness of breath on exertion, and preoperative evaluation involves computed tomography and rigid bronchoscopy. Tracheal resection and reconstruction can be safely performed with excellent outcomes by following a well-described technique.
Keywords: Tracheal surgery, Trachea reconstruction
INTRODUCTION
Surgery on the trachea is a relatively new phenomenon. Prior to the pioneering work of Dr. Hermes Grillo, conventional wisdom stated that the cartilage of the trachea would not heal properly, so resection of the trachea with reconstruction was not possible [1]. During the early 1950s, with the development of respiratory intensive care units, patients were managed with mechanical ventilators with endotracheal tubes in place. These endotracheal tubes had a low volume-high pressure cuff and led to ischemic injury to the tracheal wall from pressure-induced ischemia. When these is-chemic injuries healed, they led to circumferential scarring and narrowing of the affected trachea. Simple dilation of this stricture did not solve the problem, since the injury affected the entire thickness. Only removal of the affected portion would fix the problem.
Dr. Grillo began to do experiments in his dog lab to work out the surgical treatment of tracheal stenosis (Fig. 1). He was able to show that it was possible to resect a portion of the trachea and perform a primary reanastomosis [2]. After working out the technique in the lab, he successfully applied his tracheal resection and reconstruction techniques to patients who developed tracheal stenosis. Later, this technique was generalized so that it could be applied to any type of tracheal disease that required resection, including tumors.
Fig. 1.

Dr. Hermes C. Grillo.
ANATOMY
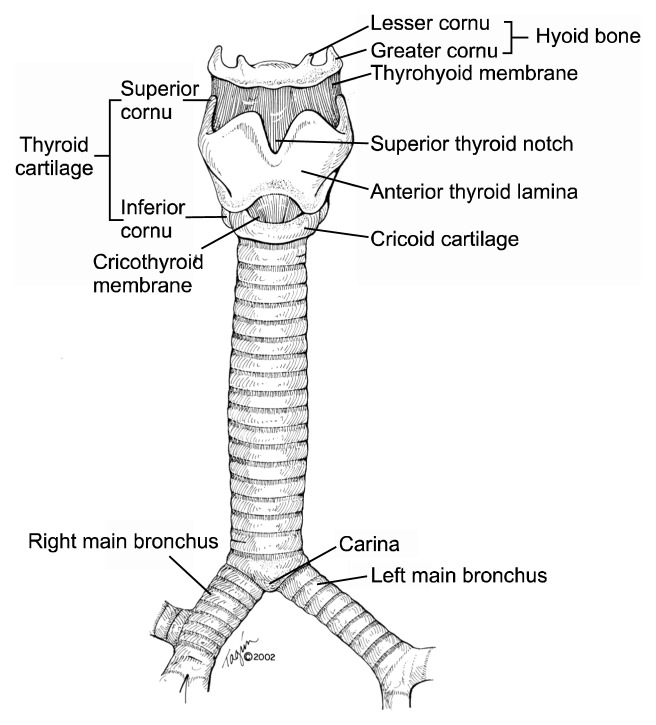
The upper airway is made up of the hyoid bone, larynx, cricoid, and trachea (Fig. 2). The larynx houses the true vocal cords. Superior to the true cords is the supraglottic airway, and the inferior area is referred to as the subglottic airway. Between the larynx and the trachea is the cricoid, which is important since the laryngeal nerves enter the airway in its posterior portion. Therefore, resection of the entire cricoid is impossible without damaging both recurrent nerves and thereby destroying the protective function of the laryngeal apparatus.
Fig. 2.
Anatomy of the trachea and larynx.
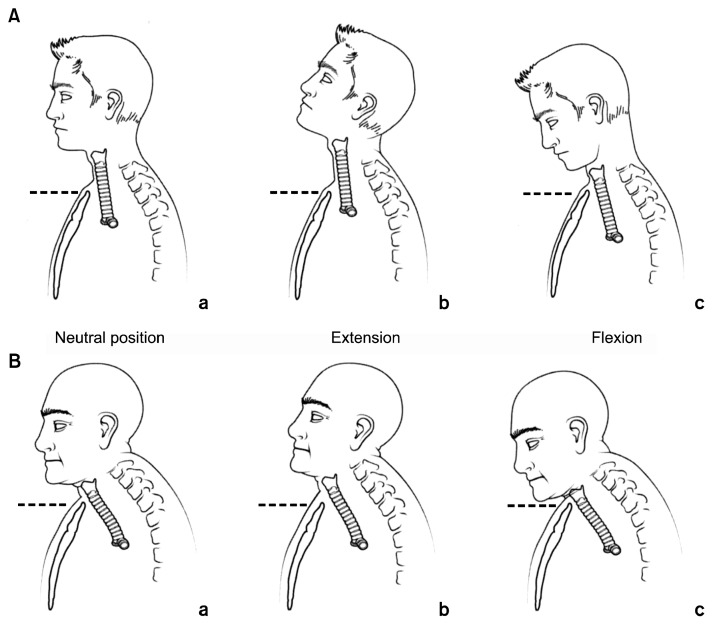
The trachea proper starts just inferior to the cricoid and extends to the main carina, some 10–12 cm distal. The shape of the normal trachea is an extended half circle with the posterior portion nearly flat and made of soft tissue, which comprises the membranous portion. The anterior portion of the trachea is made up of a series of cartilaginous rings with a membrane between each ring. The trachea is partially intra-thoracic and partially extrathoracic, as its position in the chest moves superiorly and inferiorly with extension and flexion of the neck, respectively (Fig. 3). This mobility decreases with age, which is an important consideration in tracheal resection and reconstruction, since this mobility is used to reduce tension on all tracheal anastomoses after resection.
Fig. 3.
Mobility of the trachea with flexion and extension of the neck.
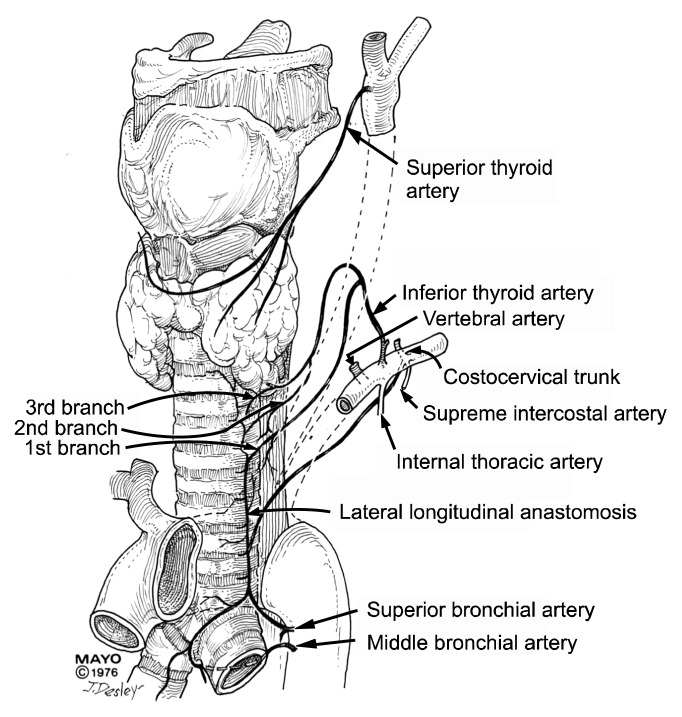
Salassa et al. [3] described the blood supply of the trachea in 1977 (Fig. 4). Salassa was a Mayo medical student at the time. The inferior thyroid artery, a branch of the subclavian artery, supplies the upper trachea. Distally, various bronchial arteries that come directly off the aorta or intercostal arteries supply the trachea. The vessels come into the trachea laterally and form a rich segmental network. Since there are no blood vessels on the anterior or posterior surface of the trachea, the trachea can be mobilized extensively, based on the lateral attachments. Once the blood vessels enter the lateral aspects of the trachea, they send smaller vessels to the anterior and posterior surfaces, which then feed the mucosal tissue [4].
Fig. 4.
Blood supply of the trachea.
PHYSIOLOGY
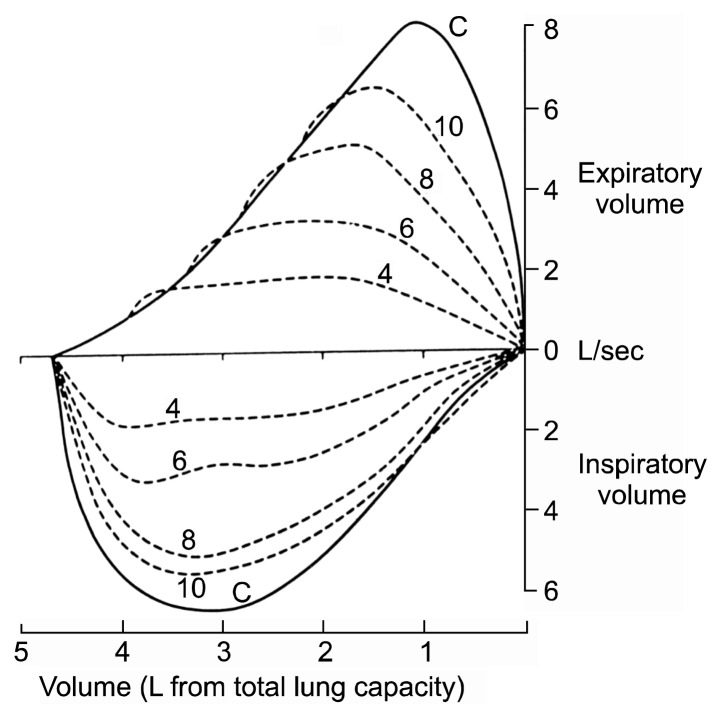
The intrathoracic and extrathoracic portions of the trachea have physiological differences with respect to airflow when the lumen is narrowed. In extrathoracic stenosis, when patients inhale, the pressure in the trachea falls, meaning that the atmospheric pressure compresses the airway more, further limiting the airflow through the trachea. This can be seen clearly on an inspiratory flow volume loop, which shows a characteristic plateau (Fig. 5). In cases of tracheal narrowing that are intrathoracic, or in the distal airway, expiration increases the pressure in the chest, thereby further collapsing the intrathoracic trachea, which leads to decreased flow through the narrowed area of the trachea. This results in a plateau on the flow volume loop on the expiratory phase of the examination.
Fig. 5.
Flow volume loops.
PRESENTATION AND EVALUATION
Patients with a tracheal tumor or tracheal stenosis usually exhibit severe symptoms of upper airway obstruction, but only after the obstruction blocks over 70% of the cross-sectional airway of the lumen [5]. Initially, patients experience wheezing or shortness of breath with exertion [6]. As the narrowing becomes more severe, the symptoms become more obvious and eventually manifest as shortness of breath with almost any activity. The presence of stridor indicates a severe obstruction that should be dealt with immediately.
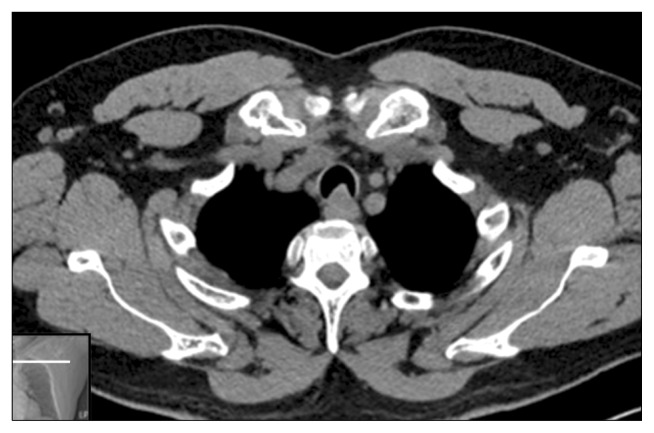
After a through history and physical examination, radiographic imaging can be performed to identify the location and extent of the tracheal pathology [7]. In the past, tomograms with a copper filter were used to delineate the pathology; however, with the advent of multiplanar computerized tomography, the older methods became obsolete. A computed tomography scan with multiple views can show the lesion and its relationships to the surrounding structures (Fig. 6).
Fig. 6.
Computed tomography of the trachea, demonstrating a tracheal tumor involving the posterior wall of the trachea.
As mentioned above, pulmonary function testing shows a characteristic plateau on the inspiratory or expiratory portion of the flow volume loop, depending on the location of the obstruction. Forced expiratory volume may be reduced as well.
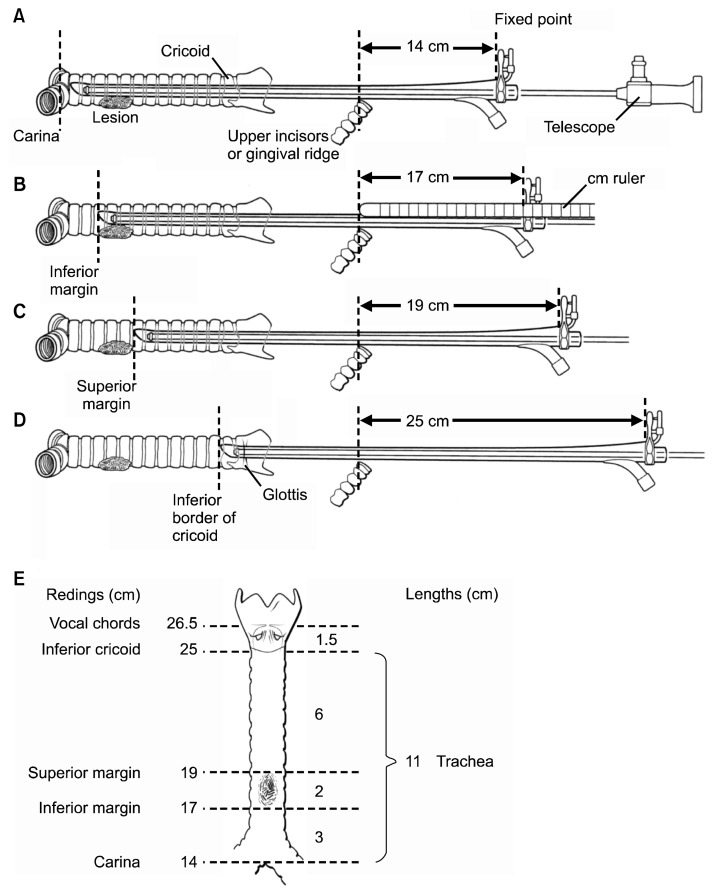
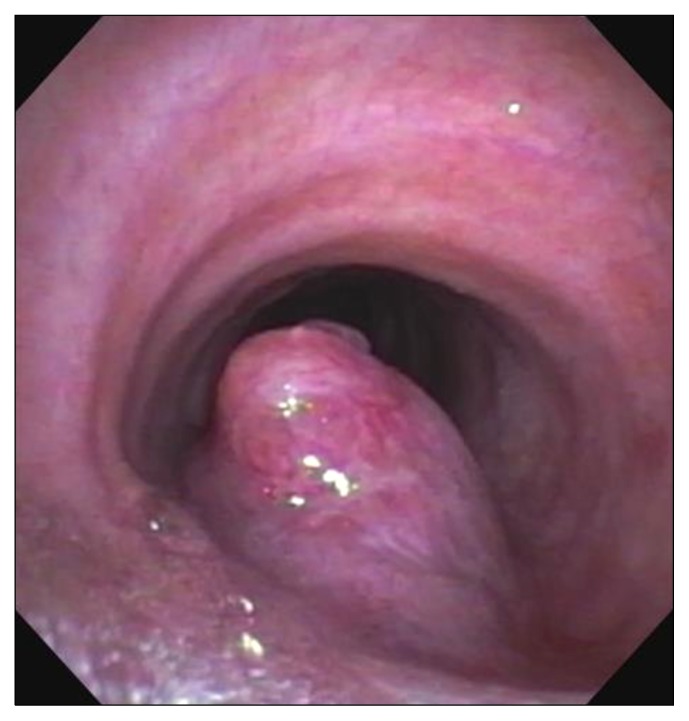
Rigid bronchoscopy is critical for examining the airway and obtaining accurate measurements of the location of the tumor. Usually done under general anesthesia, care must be taken to keep the patient spontaneously breathing in case intubation past the lesion cannot be accomplished. This is usually done using a ‘breath-down’ technique, whereby the patient’s own breathing deepens the level of anesthesia. Once the rigid bronchoscope is inserted, measurements are taken to establish the location of the carina, the distal aspect of the tumor or stenosis, the proximal aspect of the tumor or stenosis, and the location of the cricoid. These measurements allow simple calculations of the amount of the trachea that must be resected and the location of the lesion in the trachea (Fig. 7). Biopsy is not always necessary, since the lesion will need to be removed if possible regardless of the pathologic diagnosis (Fig. 8). If a tracheal tumor is present, it should not be completely removed, since locating what should be removed at a subsequent resection will be difficult. If tracheal stenosis is present, the trachea can be dilated with a rigid bronchoscope to allow for elective resection and reconstruction in the subsequent few weeks.
Fig. 7.
Bronchoscopic measurements to determine the location of the tracheal tumor.
Fig. 8.
Bronchoscopic view of a tracheal tumor.
SURGICAL MANAGEMENT
Tumors in the proximal two thirds of the trachea can be approached via a collar incision, and those in the distal third can be approached by right thoracotomy [8]. Anesthetic considerations are always important, since during the resection, the anesthesiological team will cede control of the airway to the surgical team, which always causes a bit of anxiety. Almost all patients can have a small endotracheal tube passed distal to the lesion, allowing surgery to be safely initiated. If there is concern about a severe obstruction, a rigid bronchoscope can either core out a tumor or dilate a stenosis, allowing the endotracheal tube to pass. After the trachea is opened, an endotracheal tube can be passed into the distal end of the trachea and cross-table ventilation is performed. The use of cardiopulmonary bypass or jet ventilation is rarely necessary.
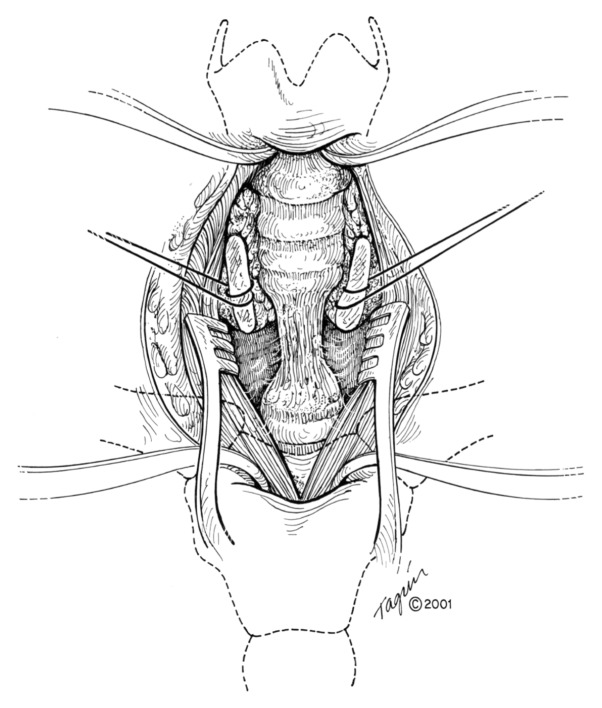
For tumors in the proximal third of the trachea, a collar incision is performed with the patient supine and the neck maximally extended [9]. The thyroid is divided in the midline and the anterior trachea is freed from the surrounding attachments. Once the tumor or stenosis is localized, dissection around the trachea is performed only at the level of the area to be removed (Fig. 9). Staying directly on the trachea avoids injury to the laryngeal nerves. Once the dissection is completed, a distal incision into the trachea is made. An endotracheal tube is placed distally for ventilation, and the initial endotracheal tube is withdrawn into the glottis area. Attaching a catheter to the end of the original endotracheal tube facilitates its replacement after the anastomosis is completed. The portion of the trachea that will be removed is resected, and the proximal and distal margins are checked with frozen sections as appropriate. Traction sutures of 3-0 vicryl are placed at the 3-o’clock and 6-o’clock positions on both the proximal and distal ends of the remaining trachea.
Fig. 9.
Trachea exposed after initial dissection.
An end-to-end anastomosis is performed using 4-0 vicryl (Fig. 10). The trachea can be divided into four quadrants to make organization of the anastomosis easier. Once the sutures are placed, the distal airway is suctioned of any blood or debris, and the original endotracheal tube is passed through the anastomosis and into the distal airway. The patient’s neck is flexed and the ends of the trachea are brought together. The anastomosis should be free of tension. If too much tension is present, a laryngeal release maneuver can be performed, but this will interfere with the swallowing mechanism for about three weeks, so it should not be done unless necessary. Once the tracheal ends are together, the sutures are tied down. Bronchoscopy should be performed to examine the anastomotic area, and if found to be deficient, the anastomosis should be redone. A small drain is placed in the neck area, and the soft tissue and skin are closed. In order to keep the neck in flexion, a stout stich is placed from the mentum to the sternum (Fig. 11). This ‘reminder’ helps keep the neck flexed in the postoperative period. Patients are extubated in the operating room and should be breathing freely without stridor after extubation.
Fig. 10.
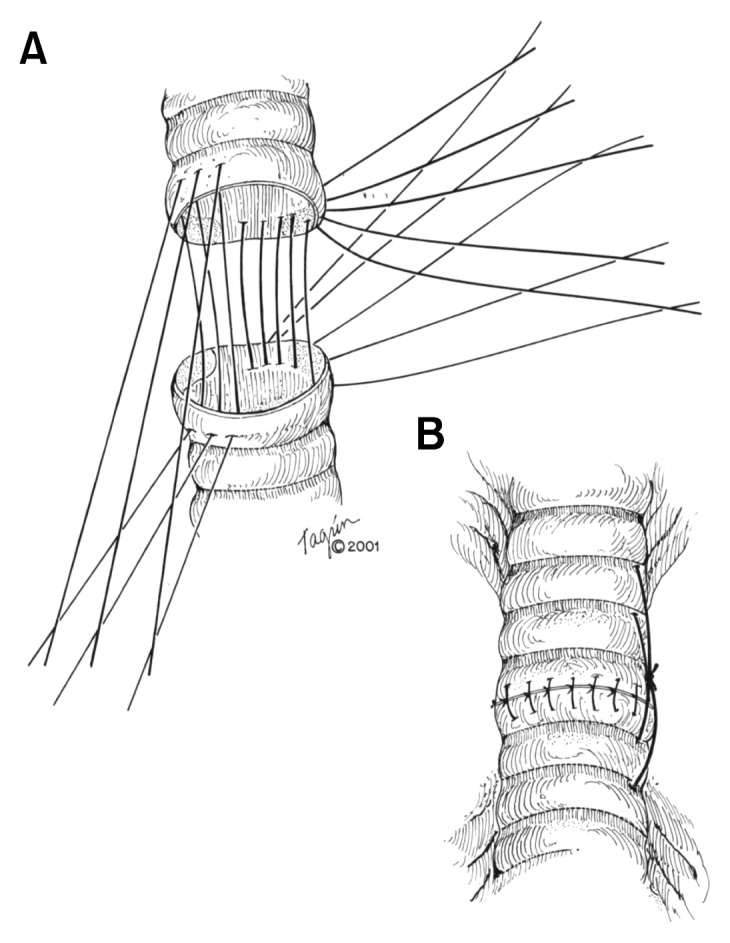
Tracheal anastomosis.
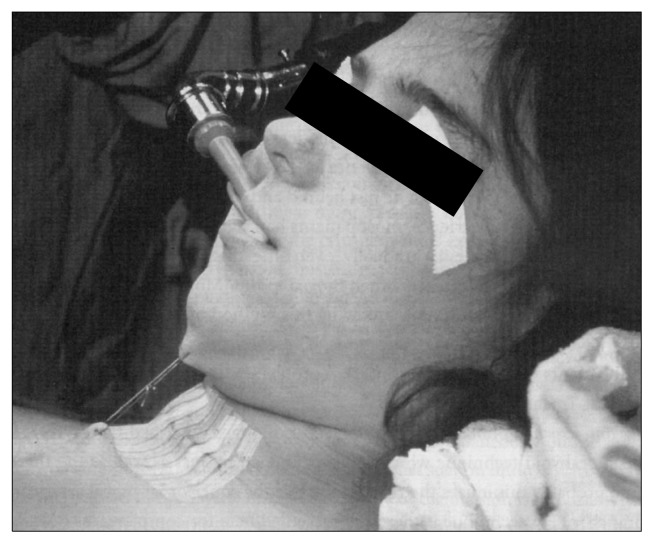
Fig. 11.
Lateral view of a patient showing the ‘chin stitch’ that provides a reminder to keep the neck flexed until the tracheal anastomosis is healed.
Postoperatively, patients are kept nil per os for 24 hours, then fed a diet as tolerated, watching carefully for any signs of aspiration. On the seventh postoperative day, a bronchoscopy is done and if all looks well, the chin stitch is removed and the patient is discharged from the hospital. Additional treatment of malignancies depends on the pathology, but in general, nothing else is required, with the exception of radiation therapy for adenoid cystic carcinomas [10].
RESULTS
Grillo et al. [2] reported the results of all of their tracheal work, including 503 patients from 1965 through 1992. They had good or satisfactory results in 93.7% of patients, failure (requiring a tracheostomy) in 3.9%, and death in 2.4%. Other researchers have reported similar results [11].
CONCLUSION
Surgery of the trachea is now a standard operation for a well-trained general thoracic surgeon. With careful attention to detail and proper patient selection, outstanding results are obtainable using the basic principles outlined by Dr. Grillo.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Grillo HC, Mathisen DJ. The trachea. Ann Thorac Surg. 1990;49:845–6. doi: 10.1016/0003-4975(90)90045-8. [DOI] [PubMed] [Google Scholar]
- 2.Grillo HC, Mathisen DJ, Wain JC. Management of tumors of the trachea. Oncology (Williston Park) 1992;6:61–7. [PubMed] [Google Scholar]
- 3.Salassa JR, Pearson BW, Payne WS. Gross and microscopical blood supply of the trachea. Ann Thorac Surg. 1977;24:100–7. doi: 10.1016/S0003-4975(10)63716-2. [DOI] [PubMed] [Google Scholar]
- 4.Minnich DJ, Mathisen DJ. Anatomy of the trachea, carina, and bronchi. Thorac Surg Clin. 2007;17:571–85. doi: 10.1016/j.thorsurg.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Honings J, Gaissert HA, Ruangchira-Urai R, et al. Pathologic characteristics of resected squamous cell carcinoma of the trachea: prognostic factors based on an analysis of 59 cases. Virchows Arch. 2009;455:423–9. doi: 10.1007/s00428-009-0843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson GA, Campbell DB. Clinical-pathologic conference in thoracic surgery: basaloid squamous carcinoma of the trachea. J Thorac Cardiovasc Surg. 2000;120:187–93. doi: 10.1067/mtc.2000.107520. [DOI] [PubMed] [Google Scholar]
- 7.Zwischenberger JB, Sankar AB. Surgery of the thoracic trachea. J Thorac Imaging. 1995;10:199–205. doi: 10.1097/00005382-199522000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mathisen DJ. The trachea. Ann Thorac Surg. 2001;71:2075–6. doi: 10.1016/S0003-4975(01)02602-9. [DOI] [PubMed] [Google Scholar]
- 9.Raman R, Jalaluddin MA. Preoperative estimation of resectable trachea by the cervical approach in surgery for tracheal stenosis. Thorac Cardiovasc Surg. 1998;46:43–4. doi: 10.1055/s-2007-1010184. [DOI] [PubMed] [Google Scholar]
- 10.Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78:1889–96. doi: 10.1016/j.athoracsur.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 11.Prommegger R, Salzer GM. Long-term results of surgery for adenoid cystic carcinoma of the trachea and bronchi. Eur J Surg Oncol. 1998;24:440–4. doi: 10.1016/S0748-7983(98)92465-9. [DOI] [PubMed] [Google Scholar]