Abstract
We report the case of a 37-year-old man who suffered from biventricular failure due to left isomerism, inferior vena cava interruption with azygos vein continuation, bilateral superior vena cava, double outlet of right ventricle, complete atrioventricular septal defect, pulmonary stenosis, and isolated dextrocardia. Heart transplantation in patients with systemic venous anomalies often requires the correction and reconstruction of the upper & lower venous drainage. We present a case of heart transplantation in a patient with left isomerism, highlighting technical modifications to the procedure, including the unifocalization of the caval veins and reconstruction with patch augmentation.
Keywords: Heart transplantation, Congenital heart disease, Heterotaxy
CASE REPORT
A 37-year-old man with complex heart disease and no history of prior procedures presented to Asan Medical Center with palpitations, dyspnea (New York Heart Association [NYHA] class IV), chest discomfort, and generalized edema. He was diagnosed at the age of 18 years as having left isomerism with azygos continuation and interruption of the inferior vena cava (IVC), direct drainage of the hepatic veins to the common atrium, complete atrioventricular septal defect, double outlet of right ventricle, pulmonary stenosis, bilateral superior vena cava, right aortic arch, and isolated dextrocardia (Fig. 1A, B). At the time of his diagnosis at another institution, he was told that corrective surgery could be impossible because of Eisenmenger syndrome. He was subsequently hospitalized many times with worsening heart failure (NYHA functional class III–IV), repeated atrial fibrillations, and cyanosis (saturated arterial oxygen of 78%–80%).
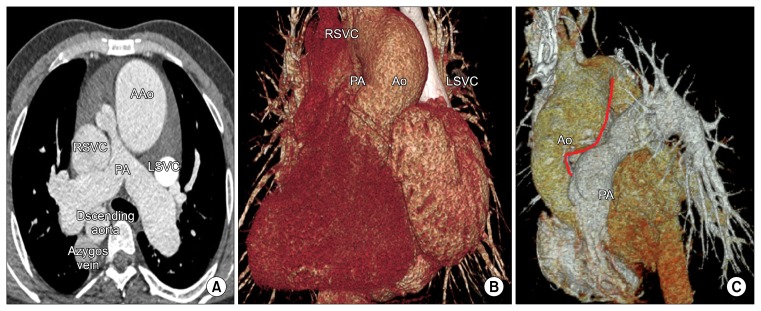
Fig. 1.
(A, B) Preoperative computed tomography. This image shows left isomerism, a dilated right superior vena cava, a dilated azygos vein, and pulmonary stenosis. The main PA is located to the right and posterior to the AAo. (C) Postoperative computed tomography. Despite the ascending aortic reduction plasty, the recipient distal AAo still push out into the main PA (Solid red line). Little space was available to insert the LSVC into the retro-aortic area. AAo, ascending aorta; RSVC, right superior vena cava; PA, pulmonary artery; LSVC, left superior vena cava; Ao, aorta.
Echocardiography showed severe atrioventricular valvular regurgitation and a dilated atrioventricular valvular annulus of 55 mm with fair systemic ventricular function. Cardiac catheterization demonstrated a mean pulmonary artery pressure of 40 mmHg, a mean pulmonary wedge pressure of 34 mmHg, and an elevated left ventricular end diastolic pressure of 35 mmHg, but an otherwise normal pulmonary vascular resistance of 1.35 Wood units · m2.
Cardiac transplantation and atrioventricular valvular replacement were considered as treatment options. At first, we thought that he was a potential candidate for valvular replacement because of his fair ventricular function. However, this therapeutic option could not treat the patient’s ongoing heart failure due to his elevated left ventricular end diastolic pressure and the problematically large size of his atrioventricular valvular annulus (55 mm). Therefore, valvular replacement was not performed, and a decision was made to offer heart transplantation.
A suitable donor heart became available from a 37-year-old male who died of suicide. The donor heart included the entire aortic arch, the main pulmonary artery and its branches, the superior vena cava (SVC) and the innominate vein, the IVC, and the pulmonary veins.
Through a median sternotomy, cardiopulmonary bypass (CPB) was instituted using an arterial cannula in the distal ascending aorta, and three venous cannulas were inserted into the bilateral SVCs and the hepatic vein. The recipient heart was excised from the thorax using the usual bicaval technique. The bilateral SVCs were dissected at the level of the SVC-atrium junction and a cuff was created from an isolated hepatic vein. The recipient main pulmonary artery was excised at the bifurcation level. An extended incision was then made toward the left pulmonary artery, because the recipient pulmonary artery was situated to the right and posterior to the ascending aorta (Fig. 2A). The recipient aorta was dilated, so we performed V-shaped reduction plasty to decrease the size discrepancy between the donor and recipient aorta (Fig. 2B).
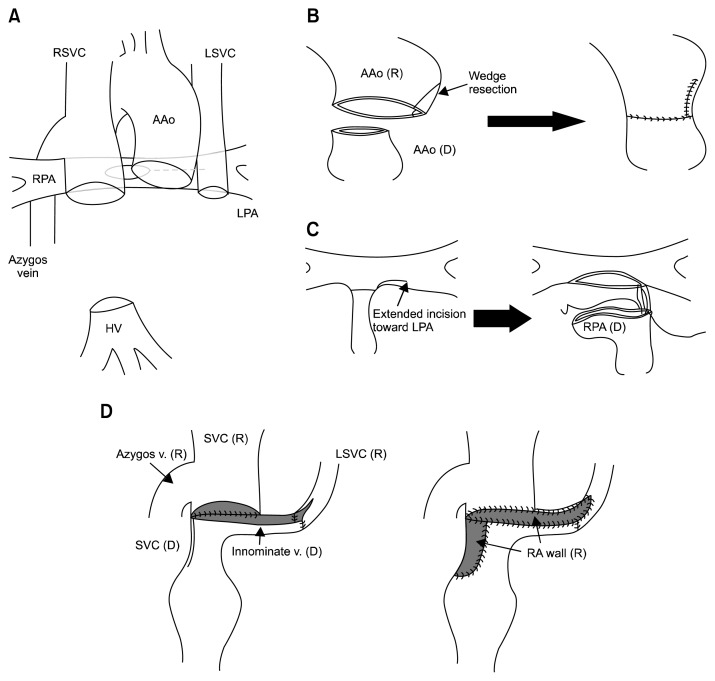
Fig. 2.
(A) Schematic drawing after the recipient’s heart excision. (B) V-shape reduction plasty of the recipient aorta. (C) The incision was extended toward the recipient’s LPA. The donor main pulmonary artery was transected at the distal part of the right pulmonary artery and then anastomosed to the recipient pulmonary artery. (D) Anastomosis between the donor SVC and innominate vein and the recipient right SVC and left SVC. SVC, superior vena cava; RSVC, right superior vena cava; AAo, ascending aorta; LSVC, left superior vena cava; RPA, right pulmonary artery; LPA, left pulmonary artery; HV, hepatic vein; R, recipient; D, donor.
The donor left atrium was anastomosed to the recipient anterior cuff of the left atrium. The donor pulmonary artery was transected at the distal part of the right pulmonary artery and was anastomosed to the recipient pulmonary artery (Fig. 2C). After the aortic anastomosis, the donor IVC was connected to the recipient hepatic vein. The aortic cross-clamp was then released. Despite the reduction aortoplasty for the dilatation of the recipient ascending aorta, the recipient distal AAo still push out into the main PA. Due to the limited space between the two vessels, the newly reconstructed SVC was positioned anterior to the ascending aorta. The donor SVC and innominate vein were connected to the recipient right SVC and left SVC, and then augmented with the recipient resected right atrial wall (Fig. 2D).
The patient was smoothly weaned from CPB without any difficulties. The CPB time was 322 minutes, and the aortic cross-clamp time was 161 minutes. The patient was extubated after 12 hours. Two weeks later, the patient complained of swelling in the left arm. Computed tomography was performed and thrombotic occlusion of the innominate vein was found. Collateral venous channels opened and the left arm swelling began to disappear gradually. Two weeks later, the patient was discharged without any complaints. Five months after the operation, chest radiography showed reduced cardiomegaly (Fig. 3). To date, the patient is in the NYHA functional class I without arrhythmic episodes, signs of transplant rejection, or left arm swelling.
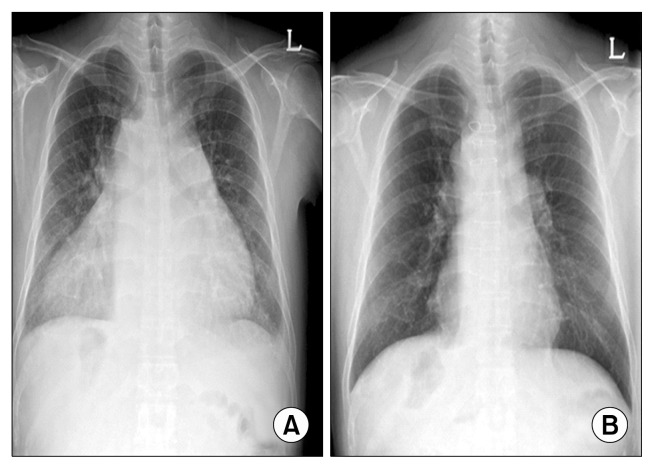
Fig. 3.
(A) Preoperative chest radiography showing dextrocardia and cardiomegaly. (B) Chest radiography performed five months postoperatively demonstrating significantly improved cardiomegaly.
DISCUSSION
Heart transplantation is a final therapeutic option in patients with end-stage heart failure, and an increasing number of heart transplants have been performed in recent years [1]. Heart transplantation in a patient with left isomerism is considered an operative challenge to the surgeon [2]. The difficulty stems from transplanting a heart from a donor with situs solitus into a recipient with left isomerism. Patients with left isomerism are known to have an anomalous systemic venous inflow tract. Reconstructing the complex venous connections, including the left SVC and interruption of the IVC, is likely to be the key to a successful operation. Moreover, size discrepancies between the great vessels should be treated properly during anastomosis. Researchers who have reported cases of this type have proposed several solutions [3,4]. Various methods to address this difficulty have been suggested, such as forming a cavopericardial graft for the SVC and IVC [5] and using a conduit between the right and left SVC [6].
In our case, several problems remained, such as delayed occlusion of the left SVC, which we believe to have been stretched by the anteriorly positioned large ascending aorta. Joo et al. [7] performed modified cardiac transplantation in a patient with persistent left SVC and hypertrophic cardiomyopathy. In this report, they positioned the left SVC into the retroaortic area and then anastomosed it to the right SVC. It was possible for the patient to have a normally sized ascending aorta along with sufficient space between the aorta and the pulmonary artery. Unlike that case, our patient had longstanding cyanotic congenital heart disease, resulting in a dilated ascending aorta. Considering our postoperative computed tomography findings of a small space between ascending aorta and pulmonary artery, LSVC in the retroaortic area would be compressed (Fig. 1C). We therefore reconstructed the left SVC in front of the aorta, although we were reluctant to do so. In our case, however, collateral venous drainage of the left SVC contributed to the relief of swelling in the left arm. In the future, in order to prevent obstruction of the left SVC, the dilated distal aorta should undergo a more aggressive cranial reduction. Finally, this procedure should be adjusted on a case-by-case basis depending on the great arterial relationships and the systemic venous drainage.
To the best of our knowledge, heart transplantation in a patient presenting with left isomerism has not yet been described in Korea, but it appears to be a safe procedure with favorable outcomes for the treatment of end-stage heart failure in patients with complex congenital cardiac anomalies.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kim HJ, Jung SH, Kim JJ, et al. Early postoperative complications after heart transplantation in adult recipients: Asan Medical Center experience. Korean J Thorac Cardiovasc Surg. 2013;46:426–32. doi: 10.5090/kjtcs.2013.46.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horai T, Bhama JK, Fontes PA, Toyoda Y. Combined heart and liver transplantation in a patient with situs ambiguous. Ann Thorac Surg. 2011;91:600–1. doi: 10.1016/j.athoracsur.2010.07.078. [DOI] [PubMed] [Google Scholar]
- 3.Larsen RL, Eguchi JH, Mulla NF, et al. Usefulness of cardiac transplantation in children with visceral heterotaxy (asplenic and polysplenic syndromes and single right-sided spleen with levocardia) and comparison of results with cardiac transplantation in children with dilated cardiomyopathy. Am J Cardiol. 2002;89:1275–9. doi: 10.1016/S0002-9149(02)02325-1. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhajosyula P, Komlo C, Wallen TJ, Olthoff K, Pochettino A. Combined heart-liver transplant in a situs-ambiguous patient with failed Fontan physiology. J Thorac Cardiovasc Surg. 2013;145:e39–41. doi: 10.1016/j.jtcvs.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 5.Doty DB, Renlund DG, Caputo GR, Burton NA, Jones KW. Cardiac transplantation in situs inversus. J Thorac Cardiovasc Surg. 1990;99:493–9. [PubMed] [Google Scholar]
- 6.Cooper DK, Ye Y, Chaffin JS, Zuhdi N. A suggested technique for “orthotopic” heart transplantation in a patient with situs inversus. Tex Heart Inst J. 1993;20:281–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Joo S, Kim GS, Lim JY, et al. Orthotopic cardiac transplantation after inter-caval anastomosis in a patient with hypertrophic cardiomyopathy and persistent left superior vena cava. Korean J Thorac Cardiovasc Surg. 2010;43:522–4. doi: 10.5090/kjtcs.2010.43.5.522. [DOI] [Google Scholar]