Abstract
Our capacity to interface with the nervous system remains overwhelmingly reliant on electrical stimulation devices, such as electrode arrays and cuff electrodes that can stimulate both central and peripheral nervous systems. However, electrical stimulation has to deal with multiple challenges, including selectivity, spatial resolution, mechanical stability, implant-induced injury and the subsequent inflammatory response. Optical stimulation techniques may avoid some of these challenges by providing more selective stimulation, higher spatial resolution and reduced invasiveness of the device, while also avoiding the electrical artefacts that complicate recordings of electrically stimulated neuronal activity. This review explores the current status of optical stimulation techniques, including optogenetic methods, photoactive molecule approaches and infrared neural stimulation, together with emerging techniques such as hybrid optical-electrical stimulation, nanoparticle enhanced stimulation and optoelectric methods. Infrared neural stimulation is particularly emphasised, due to the potential for direct activation of neural tissue by infrared light, as opposed to techniques that rely on the introduction of exogenous light responsive materials. However, infrared neural stimulation remains imperfectly understood, and techniques for accurately delivering light are still under development. While the various techniques reviewed here confirm the overall feasibility of optical stimulation, a number of challenges remain to be overcome before they can deliver their full potential.
Keywords: Infrared neural stimulation, neural engineering, neural stimulation, optical stimulation, optogenetics.
1. INTRODUCTION
Despite the success of electrical stimulation for triggering neurons, there has been interest in improving the efficacy of electrical stimulation or developing alternative techniques without the disadvantages and limitation of electrical stimulation [1, 2]. The usefulness of many bionic implants has been limited by the spread of current from the electrodes [3] and the lack of spatial specificity [2]. Similarly, neuroscience would benefit from the ability to turn individual neurons on or off [1]. There has long been interest in using light to influence the behaviour of neurons [4-6] partially as it is less invasive than other techniques.
Light has been known to influence the behaviour of neurons since the work of d’Aarsonval in 1891 [4]. While there are some neurons, such as photoreceptors in the eye, that have become specialised in order to respond to light, most neurons are not normally activated by exposure to light [7]. Fork [5] showed that abdominal ganglion neurons in Aplysia californica respond to 488 nm laser light, through a reversible mechanism, despite the cells not being photosensitive. Building on the work of [5] and others, Balaban et al. [8] found that He-Ne laser irradiation (λ= 632.8 nm) of subesophageal ganglia of Helix pomatiapromoted membrane depolarisation and action potentials in spontaneously active neurons. Hirase et al. [6] demonstrated that neurons could be triggered through exposure to pulses of light from a femtosecond laser via a two-photon mechanism. More thorough reviews of these early developments can be found in the references [2, 9] and readers are referred to these.
Over the past decade, a number of new techniques have been developed to use light to trigger neurons. Early techniques were able to increase the sensitivity of neurons and generate action potential spikes with laser exposure timescales of seconds [10, 11]. These techniques include genetically modifying the target neurons to introduce photosensitive receptors in the neurons [10, 12], or the introduction of photoswitchable molecules that bind to specific ion channels and modify their behaviour when exposed to light [11, 13, 14]. In contrast, advancements in laser diode technology for infrared neural stimulation has allowed the use of wavelengths that are directly absorbed by water [2, 15]. This paper reviews the various optical techniques of nerve stimulation currently under development, with a particular focus on infrared neural stimulation due to its minimally invasive characteristics.
2. OPTOGENETICS AND PHOTOACTIVE MOLECULES
2.1. Optogenetics
Optogenetics is a technique to introduce light-sensitive ion channels to neurons, allowing them to be switched on or off optically with tight spatial and temporal confinement [16, 17]. Although early attempts at introducing light-sensitive channels had allowed tight spatial localisation of stimulation, temporal responses were on the order of seconds [10, 18]. Despite the temporal limitation, these strategies were able to induce behavioural changes in a Drosophila model [19].
The expression of Channelrhodopsin-2 (ChR2), a rapidly-gated blue (470 nm) light-sensitive cation channel, in oocytes of Xenopus laevis and mammalian cells introduced a technique to depolarise cells in timescales on the order of milliseconds [20]. Application of this to neurons was developed by Boyden et al. [12]. In that work, neurons were transfected with ChR2, introducing light sensitivity and allowing driving of single spikes with light. Introduction of photosensitivity to neurons in vivo was soon demonstrated [21], showing the potential of optogenetics for both in vitro and in vivo studies. These advances significantly boosted the field by allowing precise temporal control of neuronal firing exposed to blue light.
Since the development of ChR2 as a light-activated channel, a range of new opsins have been developed to allow inhibition of neurons, faster stimulation rates and response at longer wavelengths [22-24]. Halorhodopsins respond to light near 580 nm and act as a chlorine pump, inhibiting excitation of neurons by hyperpolarisation [23, 24]. Other work has found that high stimulation rates with ChR2 can have an inhibitory effect [25], potentially allowing the one opsin to both excite and inhibit neural activity. Genes to express opsins are typically delivered with viral transfection [12], but electroporation transfection [26] and optical transfection [27] have also been demonstrated as a feasible techniques. While ChR2 gave a large improvement in response times compared to previously used opsins, it has a typical maximum response rate of 40 Hz [22] and there has been interest in developing faster responses. As there is interest in applying optogenetic techniques to neuroprothestic implants such as the cochlear implant [28-31], higher stimulation rates may be needed to match the electrical stimulation rates that are commonly used. Recent developments have allowed spike trains of up to 200 Hz with novel opsins [22], although further develop-ment will be needed to reach the 900 Hz stimulation rate used in the cochlear implant [32]. Another limitation of ChR2 is the high scattering of 470 nm light used for excitation [33]. Much research has been focused on developing opsins with longer maximum excitation wavelengths where scattering and absorption in tissue is lower [9]. Advances include a cation channelrhodopsin (VChR1) responding at 589 nm [34] and ReaChR an opsin responding at 610 nm [35]. While promising, these opsins do not offer as fast switching as blue excitatory opsins [9].
Optogenetics have been utilised to further our unders-tanding of neural disorders [36], understand neural systems and encoding [37] and there is interest in using optogenetics to treat blindness and Parkinson’s disease [17, 38, 39]. Despite the power of these tools and their importance in the field of neuroscience, the potential for use in bionic devices or treatment of conditions in humans is currently limited. Optogenetic techniques require transfection of genes into neuronal cells, which, besides technical challenges, will face significant regulatory hurdles for human use [40, 41].
2.2. Photoactive Molecules
Another technique to optically activate neurons relies on neurotransmitters that are held in a photosensitive cage but can be liberated upon exposure to light [17]. Caged glutamate is one of the most commonly used molecules for stimulation [42], but a number of neurotransmitters have been used [43]. The short wavelengths that were initially used to photoactivate the cages (λ ~ 380 nm) tended to limit the spatial resolution of this technique, due to the high level of light scattering in tissue [44] and severly limit probing depth due to strong absorption. Furthermore, the high intensity of light and high energy of photons required for uncaging can damage tissue. The development of two-photon responsive glutamate cages allowed both finer spatial resolution and deeper penetration into tissue [45], as uncaging can occur with exposure to 800 nm light. Further refinement has reduced the optical radiation required by improving efficiency of the two-photon uncaging process [44] or developing cages which release upon exposure to visible light [46, 47]. Caged molecules are also able to inhibit neural activity, through the release of GABA [46, 44]. However, this technique requires further development as many of the compounds used also interact with the receptor before photoactivation [44].
Another approach to control neurons with photosensitive molecules is the use of bistable molecules to create photoswitches. These molecules have multiple configurations, which can change upon exposure to light of different wavelengths [17, 18, 48]. This property has given rise to photoswitchable ligands, which can regulate K+ channels and glutamate receptors [17, 49]. There is considerable potential to extend this approach by engineering new molecules for modulating a wider range of specific targets.
In addition to control of neurons, techniques are emerging to read neural activity with light [50]. These techniques include sensing of cell membrane potential, calcium and neurotransmitter release [50]. Complete optical control and monitoring of neural activity could allow the study of larger and more complex neural systems than is allowed by current electrophysiology techniques.
Caged molecules have emerged as a key technique for determining functional connectivity of neurons [44, 51]. It has been used to map the functional synaptic connections in the visual cortex [51], the cerebral cortex [52], and many other areas [53]. Despite the success of caged molecules as a tool for fundamental neuroscience studies, they have limited potential for in vivo studies or for neuroprothestic implants due to the need to replenish the caged neurotransmitter and potential toxicity of the cage [17].
3. INFRARED NEURAL STIMULATION
Infrared light has been demonstrated as an alternative technique for optical stimulation of nerves without the need for genetic manipulation or other interventions [2, 15]. The technique of using infrared light to stimulate neurons has been coined infrared neural stimulation (INS). The use of infrared light has a number of potential advantages over electrical stimulation: finer spatial resolution can in principle be achieved, no direct contact between the stimulation source and target neurons is required, there is no electrochemical junction between the source and target tissue, and there is no stimulation artefact on the recording electrodes. Disadvantages of INS include: heating of the tissue to level that could cause damage [54, 55] and a restriction on the maximum depth of stimulation due to absorption of light in the intervening tissue [55]. Compared to optogenetic and caged molecule techniques, INS requires no modification of the target tissue as it only relies upon the absorption of infrared light by water in the tissue [2, 56].
Pulses of mid-infrared light were first observed to elicit responses in mammalian nerves by Wells et al. [15]. They exposed the sciatic nerve of rats to irradiation from a free electron laser (FEL), with wavelengths between 2000 nm and 10,000 nm, observing compound nerve action potentials (CNAP) and compound muscle action potentials (CMAP) with a strong spatial specificity. Additionally a Ho:YAG laser (λ = 2120 nm) produced a response in the sciatic nerve. Histological analysis of the nerves after stimulation showed no evidence of tissue damage, confirming that the energy deposited is below tissue damage thresholds. This work was expanded upon in [57], where the dependence of the damage and stimulation thresholds on wavelength, and therefore absorption coefficient, were further investigated. Wavelengths with lower water absorption (µa ~ 3 mm–1), were found to have a greater safety ratio between the energy required for stimulation and the threshold for damage.
Since the initial demonstration of INS in the rat sciatic nerve by Wells et al. [15], the technique has been extended and demonstrated in a number of other models. A summary of these other targets is presented in Table 1, along with the laser wavelength, pulse length, fibre diameter and resultant threshold found. It is not intended as a comprehensive summary of all INS studies, but rather to provide an overview of the main targets that have been investigated.
Table 1.
Summary of the various experimental parameters for a range of different INS studies in the literature. This table does not present data from every study discussed in this section, but provides an overview of the main results.
| Author | Neural Target | Wavelength (nm) |
Stimulation Threshold (mJ.cm.-2) |
Pulse Length (ms) |
Fibre Diameter (mm) |
|---|---|---|---|---|---|
| Wells [15] | Rat sciatic nerve | 2120, 2000 – 61001 | 320 | 0.25 | 600 |
| Wells [58] | Rat sciatic nerve | 2120 | 340 | 0.35 | 600 |
| Wells [59] | Rat sciatic nerve | 2120 | 320 | 0.35 | 600 |
| Teudt [60] | Gerbil facial nerve | 2120 | 710 | 0.25 | 600 |
| Fried [61] | Rat cavernous nerve | 1870 | 1000 | 2.5 | 300 |
| Fried [62] | Rat cavernous nerve | 1850 – 1880 | 350 | 2.5 | 400 |
| Jenkins [63] | Embryonic quail heart | 1875 | 810 | 2 | 400 |
| Jenkins [64] | Adult rabbit heart | 1851 | 7000 | 2.5 – 12 | 400 |
| Cayce [65] | Rat somatosensory cortex | 1875 | 1402 | 0.25 | 400 |
| Izzo [66] | Gerbil cochlea | 2120 | 18 | 0.25 | 100 |
| Izzo [67] | Gerbil cochlea | 1844 – 1873 | 6 | 0.035 – 1 | 200 |
| Izzo [68] | Gerbil cochlea | 1923 – 1937 | 1.6 | 0.05 – 0.3 | 200 |
| Richter [69] | Gerbil cochlea | 1844 – 1873 | 33 | 0.03 – 1.6 | 200 |
| Duke [70] | Rat sciatic nerve | 1875 | 16904 | 2 | 400 |
| Duke [71] | Aplysia buccal nerve | 1875 | 8930 | 2 – 3 | 100 |
3.1. INS in Peripheral Nerves
Work in the rat sciatic nerve has been extended to examine both the safe range of stimulation parameters [58] and a comparison between optical and electrical stimulation modalities [59]. When optical and electrical stimulation modalities were compared, Wells et al. [59] found a near linear relationship between the radiant exposure and the measured compound nerve action potential (CNAP) response, similar to that observed when using electrical stimulation. Unlike the electrical response, the minimum CNAP response from an optical stimulus was four times smaller. This lower minimum response suggests greater spatial localisation with optical stimulation. Stimulation of the rabbit sciatic nerve with 1875 nm light has also shown the strong spatial localisation of INS [72]. The authors found that not all of the nerves’ surface was sensitive to INS and that maximum INS levels typically recruited 2–9% of any muscle. The reduction in recruitment may be due to the increased size of the rabbit nerves compared to previous studies with rats.
The suitability of other wavelengths of infrared light for stimulating the rat sciatic nerve was performed by McCaughey et al. [73]. In this work wavelengths of 1540 nm, 1495 nm, 1540 nm and the more conventional 2100 nm were examined, using various diode laser sources. Stimulation was achieved with all laser sources, however it was most reliable when using the 1495 nm source. However, meaningful comparisons between the different wavelengths and laser sources is made difficult by the large variation between fibre diameters, beam divergences and pulse durations. Moreover, the long pulses (> 100 ms) used by the 1450 nm and 1540 nm sources are near the thermal diffusion time for the areas irradiated [74].
The gerbil facial nerve was stimulated using INS by Teudt et al. [60]. The authors used a Ho:YAG laser (λ = 2120 nm, ø core = 600 µm, tpuls =250 µs, f = 2 Hz). Response to the irradiation was observed when using radiant exposures from 0.71 J.cm-2 to 1.77 J.cm-2, with amplitudes similar to that observed when using electrical stimulation. Histological analysis of higher radiant exposures revealed that damage was present at levels greater than 2.00 J.cm-2. In addition to measuring the response of nerves, the authors measured the profile of the beam resulting from transmission in air, Ringer’s lactate and muscle tissue. No change in the beam profile was observed when transmitted through Ringer’s lactate when compared to air. However, transmission through tissue was found to broaden the spot when compared to the other media. These results showed that Beer’s law is a good first approximation for the spatial behaviour of light during INS, but scattering also plays a role in the propagation of light. The results suggested that INS could be beneficial clinically as a monitor of the facial nerve during surgery, as no contact to the nerve is required and it allows for greater spatial selectivity.
The rat cavernous nerve was stimulated using INS by Fried et al. [61] to establish the potential of INS for nerve mapping during nerve-sparing radical prostatectomy. A thulium fibre laser (λ = 1870 nm, øcore = 300 µm, tpuls = 2.5ms, f = 10 Hz, radiant exposure = 1.00 J.cm-2) was used. The intracavernosal pressure was monitored and showed a similar response to the laser stimulation as occurred with conventional electrical stimulation. This result demonstrated the feasibility of INS for noncontact stimulation of the cavernous nerves. The technique was further optimised in [62], where a tuneable laser (1850 nm > λ > 1880 nm,ø core = 400µm. rep rate = 10 Hz) was used. Optimal parameters of 1860 nm < λ < 1870 nm with a minimum radiant exposure of 0.35 J.cm-2 were found. Further work [75] investigated the use of continuous wave lasers (λ = 1455, 1490, 1550 nm) rather than the pulsed laser system previously used [62]. A wavelength of λ = 1490 nm with corresponding water absorption of µa = 1.9 mm-1 was found to provide the best performance, balancing absorption with penetration depth. The continuous wave system was found to give a faster intracavernosal pressure response compared to the pulsed laser system. The authors conclude that while promising, translating the technique from the rat model to human patients may be difficult as the target nerves are harder to visually identify in humans.
The heart is another target of INS that has seen interest [63, 64, 76]. Jenkins et al. [63] demonstrated that the heart of an embryonic quail could be paced using INS as a non-invasive technique. 1875 nm laser light was used and no damage was observed in the tissue. Optical pacing of the heart was further explored in [64]. Here, pulses of 1851 nm light were used to pace adult rabbit hearts at radiant exposures of 6 - 11.8 J.cm-2 and pulse duration of 2.5 ms to 12 ms. Unlike other results for INS, a pulse duration of 8 ms was found to have the lowest radiant exposure stimulation threshold. Additionally, higher stimulation frequencies resulted in a lowering of optical thresholds, similar to that observed by Duke et al. [77]. Using propidium iodine staining to determine damage, radiant exposures of 7.9 J.cm-2 or above per pulse were found to cause some disruption of cell membranes, which may limit the duration over which this technique can be used.
An attempt to stimulate nerves ex vivo was made by Cargill et al. [78]. They used a diode laser ( λ = 1850 nm, Ppeak = 5 W, τ pulse =1—5 ms øcore = 600 µm). Ten nerve samples were extracted from mice and responded to electrical stimulation. However, no activation was observed when using optical stimulation. The authors speculated that the explanation for this discrepancy between ex vivo and in vivo may be due to the nerves being at room temperature rather than body temperature, or due to other differences between the mouse and rat animal models.
3.2. INS in the Central Nervous System
Due to the improved spatial localisation of INS, it has attracted interest as a technique to stimulate the central nervous system [65, 79, 80, 81]. Exploratory work was carried out in vitro with rat thalamocortical brain slices using a free electron laser that allowed investigation of multiple wavelengths. Larger spot sizes were found to require a lower radiant exposure for stimulation, as stimulating a larger area requires fewer neurons to react to the stimulus per unit area compared to a smaller spot size [79].
The efficacy of INS to stimulate the brain in vivo has since been demonstrated in two studies [65, 80]. The somatosensory cortex of rats and primary visual cortex of macaque monkeys have shown to respond to INS and have shown strong spatial isolation.
3.3. INS in the Auditory System
Electrical stimulation of the cochlea has produced one of the world’s most successful bionic devices [32]. One current limitation of the implant is the spread of electrical current through the tissue and the perilymph, reducing the spatial selectivity that can be achieved with this stimulation modality [3]. The improved spatial selectivity of INS potentially makes it a very attractive technique for stimula-tion of nerves in the cochlea and consequently the area has seen much research.
INS of the cochlea was first performed in gerbils by Izzo et al. [66]. Optical radiation from a Ho:YAG laser (λ = 2120 nm) targeting the modiolus was delivered to the cochlea by a 100 µm core diameter fibre, with pulse durations of 250 µs at 2 Hz and a distance of 0 – 500 µm from the target. Compound action potentials (CAPs) were observed in response to the laser pulses in both normal hearing and deafened animals in which the acoustic threshold had increased by approximately 40 dB. As a result of the deafening, the authors suggest that the interaction occurs directly with the nerves and is not mediated by an optoacoustic effect involving the hair cells. No evidence of neural damage was observed during the stimulation, suggesting that stimulation at this rate was safe for the duration of the experiment (6 hrs).
While the work of Izzo et al. [66] demonstrated that the auditory system responded to the optical stimulus, it did not demonstrate the proposed advantage of improved spatial selectivity, or limiting stimulation of the auditory system to a smaller frequency range than is possible with electrical stimulation. Spatial selectivity has been investigated with a range of techniques, including: fluorescent staining with c-FOS to identify excited neurons [82]; inferior colliculus (ICC) recordings which identify the localisation of signals to different neural pathways [83]; micro-CT reconstructions of the cochlea [84] (as shown in Fig. 1) and acoustic tone masking [85]. Overall, these results showed similar localisation of neural activity for both INS and acoustic tone pips, although some results point to stimulation on the far side of the cochlea rather than neurons directly in front of the optical fibre [83].
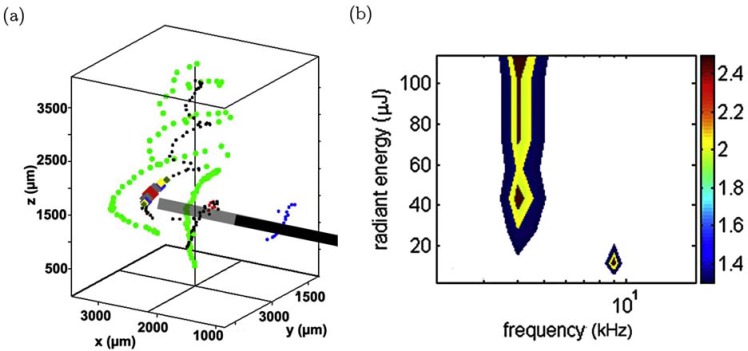
Fig. (1).
(a) 3D reconstruction of a guinea pig cochlea during stimulation. Black cylinder shows the position of the optical fibre, black dots indicates the spiral ganglion neurons, green dots are the inner pillar feet. (See online for colour) (b) The spatial tuning curve obtained from recordings of the ICC, showing stimulation at 10.8 kHz and 16.1 kHz. Fig. reproduced from [84], with permission from Elsevier.
A range of laser parameters have been used for INS in the cochlea and a number of studies have been performed on the effect of varying the pulse length [67-69, 74, 86] and wavelength [55, 67, 68, 74, 87] used to stimulate the neurons. The optimal wavelength depends on the fibre position, but generally a wavelength with penetration depth of greater than the distance between the neurons and the fibre is most effective. These and other results have shown that the cochlea responds to infrared pulses with roughly an order of magnitude less energy than other targets for INS (See Table 1 for an overview), although this is more likely to be attributed to a more sensitised endpoint than to an inherent difference in neural sensitivity to INS in the cochlea. Pulse lengths of 35 µs to 1600 µs have been studied, with the required energy per pulse scaling with pulse length (i.e. displaying a power dependent response). In some studies, ìs required the same total energy as the 100 ìs pulses but a higher peak power [67, 68]. However, this result has not been found in all studies [69].
As the rapid heating caused by INS laser pulses can generate an acoustic click [88], any results of INS in the cochlea require controls with deafened animals. A number of studies have demonstrated that a response can be generated when the cochlea has been acutely deafened [66, 69, 89]. Deafening techniques commonly used involve applying neomycin directly to the round window or cochleostomy and allowing it to perfuse through the cochlea. This technique typically increases acoustic thresholds by at least 40 dB [69, 89]. Generally, these results have shown a small change in response between the normal hearing and acutely deafened cases. Chronically deafened animals have shown an increase in the energy required to generate a response [69], and with a sufficiently high concentration of neomycin no response to optical or electrical stimulation has been observed in some animals [69]. The increased threshold in chronically deafened animals was attributed to a reduction in the spiral ganglion neuron count (SGNs) [69], which would imply that a larger volume of neural tissue must be stimulated to generate the same response. Recently, further research has been performed using tone masking, to test whether there is any acoustic response to the laser. Results have shown that the laser interaction with the target neurons is not primarily acoustic [89].
Currently, only limited behavioural studies have been performed on animals. Matic et al. [90] chronically implanted cats with an optical fibre targeting the spiral ganglion neurons in the cochlea. During stimulation, behavioural observations were made. Cats were individually released and observed while the laser was actively stimulating and while it was off during stimulation the cats made more turns towards the implanted ear, suggesting perception of the laser pulses. However, proof of perception requires further experimentation to explicitly show that the animals perceive a sound.
Stimulation of the cochlear nucleus was demonstrated by Lee et al. [91]. They recorded optically evoked auditory brainstem responses from a 400 µm core diameter fibre directed towards the cochlear nucleus. Unlike the results from the Richter group, this work targeted the central auditory neurons, rather than the more peripheral spiral ganglion neurons. They noted that the ABR recording was typical of that produced by acoustic stimulation and had a latency of 3 to 8 ms longer than that evoked by electrical stimulation in the same region. A follow up study found that after cutting the auditory nerve to deafen the animal, no response could be found from INS [92]. This suggests that the previous results may have been due to an acoustic artefact.
A hair cell mediated response to laser stimulation, or optoacoustic stimulation, has been demonstrated by Wenzel et al. [93]. In this work, green 532 nm laser light was delivered to the cochlea, generating an optoacoustic response. The laser pulses were 10 ns in duration with energies up to 23 µJ delivered by a 50 µm core diameter fibre. The response in most animals saturated at approximately 15 µJ and the response was similar when stimulation was performed with the fibre inserted through the round window, or with the round window intact. No damage was observed with stimulation at Epulse =13 µJ and a stimulation rate of 10 Hz for a duration of 30 mins. After deafening with kanamycin and ethacrynic acid, no response to the laser stimulation could be observed. These results suggest that the cochlea can respond to laser-induced acoustic events, although the response to optoacoustic sits in a greatly different pulse length and radiant exposure regime compared to INS in the cochlea.
Schultz et al. [94] showed that a range of wavelengths, from 400 nm to 2000 nm, can stimulate the cochlea with a nanosecond laser. In this regime, the response has a correlation between the water absorption or haemoglobin absorption of the wavelength. When the cochlea was deafened with neomycin no response to the laser was observed and no strong response has been observed in spiral ganglion neurons in vitro [95]. While this suggests that INS could be mediated through an optoacoustic mechanism, results from [66, 69] and [89] suggest that the response seen during INS is not dependent on functioning hair cells. It appears that further research is required to unravel the mechanisms behind these disparate findings for optical stimulation in the auditory system.
3.4. Safety of INS
As INS involves heating tissue, any long term use of this technique requires an understanding of the thresholds for damage and the safety margins required.
Studies on the safety margins [58] found that that the threshold to stimulate tissue and corresponding 95% confidence ranges did not overlap with the radiant exposures found to cause damage, when stimulation was performed at rates between 2 – 8 Hz. Damage was assessed by analysing histological samples for thermal lesions. Damage thresholds were found to be 0.7 J.cm-2 for a 1% probability of damage and 0.91 J.cm-2 for a 50% probability, when using histological analysis of exposed tissue. Additionally, higher pulse rates (5 – 8 Hz) were more likely to cause damage than 2 Hz. Damage from higher stimulation rates of 200 Hz have been studied in rodents and squirrel monkeys [96]. At these frequencies a damage threshold of 0.3 – 0.4 J.cm-2 was found, lower than that in the peripheral nerves. These results show that care will be needed to find the safe INS parameter space, especially at high frequencies.
Although thresholds for INS in the cochlea appear to be much lower than that required for other neural targets, maximising the usefulness of INS for implants may require high stimulation rate, on the order of several hundred Hz, to be comparable to electrical stimulation in cochlear implants [32]. To date, a number of studies focusing on the safety in both acute and chronic usage of INS in the cochlea have been performed [66, 67, 97, 98, 90]. Recent reports of acute experiments indicate that no damage occurs at stimulation rates of up to 250 Hz in a cat cochlea with a pulse energy 25 µJ over periods of up to 5 hours [98]. Cats with chronic implants have shown no evidence of damage at a stimulation rate of 200 Hz for 6 hours per day over 30 days [90]. Overall, these results are encouraging for the use of INS for long term implants.
An Arrhenius model for describing the kinetics of cellular damage from INS due to heat shocks to neurons and neuroglia was developed [99]. Experimental data from in vitro stimulation of rat astrocytes from a 1550 nm laser source provided the data on cell survival while temperatures were calculated with a model [100]. Combining these results with a finite element model of heating, such as [101, 74, 100], may allow for predictions of damage with different pulse durations and stimulation frequencies.
3.5. Mechanisms Behind INS
The mechanisms behind INS have been the topic of some discussion in the literature and a number of potential mechanisms have been identified which may contribute to the response observed from laser exposure.
Mechanisms of INS were first investigated by [54] Wells et al., using a Ho:YAG laser (λ = 2120 nm), free electron laser (λ = 2100 nm) and diode laser (λ = 1870 nm) to stimulate the rat sciatic nerve. They found that a photothermal interaction due to water absorption was the most likely mechanism behind INS, rather than photomechanical pressure waves or photochemical mechanisms. As the pulses used are well below stress confinement there is not a large pressure wave generated, reducing the possibility of a photomechanical mechanism. Photochemical effects were ruled out as direct photochemistry requires a photon energy greater than that found at the wavelength used for INS (< 0.1 eV). Additionally, transient tissue heating or a thermal gradient with respect to time was found to be required in order to achieve neural activation, as simply heating the tissue does not generate a response. The authors speculated that heat-sensitive ion channels or a change in conductance of ion channels may be behind the response to the transient heating of neurons. The importance of transient heating was also shown by Rajguru et al. [102], who investigated INS of adult oyster toadfish crista ampullaris. Exposure to 1962 nm IR radiation resulted in an increase in the firing rate, while the application of indirect heat did not.
Following the proposal of Wells et al. [54] that heat-sensitive ion channels may be a mechanism behind INS, the heat-sensitive vanilloid subfamily of TRP channels (TRPV) [103, 104] have been the subject of some research in the literature [105-107]. The TRPV channels, along with the TRPM subfamily, have the potential to detect changes in temperature from 10 to 50°C [104, 108]. Different TRPV channels activate at different temperatures, > 25°E for TRPV4 [109], >31°C for TRPV3, >43°C for TRPV1, >52°C for TRPV2 [110]. Additionally, this temperature dependence is affected by the membrane voltage [111]. Expression of TRPV channels varies in different tissue [104] and could potentially explain some variations between different neural targets. Additionally, Rhee et al. [112] exposed dissociated neurons from the vagus nerve of rats to laser stimulation and measured the change in intracellular Ca2+ concentrations. When a TRPV1 channel blocker, capsazepine was added, the laser exposure no longer generated a Ca2+ transient from an influx of Ca2+.
Further work on TRPV channels was reported by Albert et al. [107]. The response of retinal and vestibular ganglion cells to λ = 1875 nm light was examined using whole cell patch clamp recording. The influence of TRPV channels was determined by adding various channel blockers, to remove their contribution to the response. When the TRPV4 channel was blocked, a response to the laser could no longer be produced, suggesting that TRPV4 channels play a role in neural activation from INS.
Heating of neurons may also accelerate other processes in the cell membrane as the reaction rate increases with temperature. Recently, heating of hippocampal neurons with 808 nm light has been shown to accelerate Na+ current kinetics [113]. Similarly, the response of intracellular calcium due to heating from INS has been investigated by [114]. A λ = 1862 nm diode laser was used to stimulate rat ventricular cardiomyocytes and the resultant intracellular calcium wave was imaged using a confocal microscope. The authors found that TRPV channel blockers 2-APB and Ruthenium Red blocked the intracellular release in response to INS. However, CGP-37157, an inhibitor of mitochondrial Na+/Ca2+ exchange (mNCX) also blocked the response observed due to exposure to IR. The authors concluded that the mitochondria are the primary facilitator of the IR-evoked Ca2+ transients.
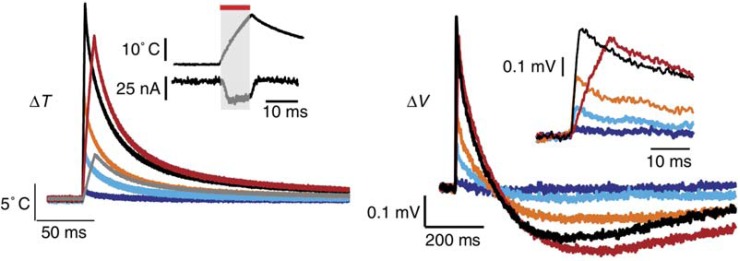
Recently Shapiro et al. [56] have shown that the membrane capacitance varies reversibly during rapid heating, such as occurs during exposure to INS. The authors propose that INS is mediated by this shift in capacitance as it causes a change in membrane voltage that can initiate an action potential. Additionally the authors used heavy water (D2O) to demonstrate the role of water as the primary chromophore absorbing laser irradiation during INS. Heavy water has approximately 20% the absorption coefficient of normal water at the wavelength investigated (1889 nm). When standard water was replaced by heavy water, the observed response reduced by 80%, demonstrating that light is primarily absorbed by water during INS. Oocytes, HEK cells and artificial lipid bilayers were exposed to 1869 nm (HEK cells) or 1889 nm laser light (ooctye and bilayers) and the response was recorded with a patch clamp.
Recordings of the temperature shift using micropipette resistance measurements displayed a similar shape to the change in membrane voltage, as shown in the example in (Fig. 2). The observed change was consistent with a model of double layer capacitance and shows how the membrane voltage changes proportionally to the temperature. The capacitive mechanism was further investigated by Liu et al. [115], which confirmed the importance of the rate of change in temperature, rather than the absolute temperature change. However, the authors argue that the magnitude of the voltage change due to capacitance is unlikely to act as an excitatory stimulus, except for the most voltage sensitive cells [115].
Fig. (2).
Example of temperature measurement (left) and change in cell membrane potential (right) during and after exposure to INS. Adapted by permission from Macmillan Publishers Ltd: Nature Communications [56], copyright (2012).
Peterson et al. [116] applied the changes in membrane capacitance observed by Shapiro et al. [56] for the simulation of neurons to assess whether this mechanism would be adequate to explain the response observed in vivo and if myelination would change the response. They found that the capacitive mechanism was unable to activate neurons on its own and is unlikely to be the only mechanism behind INS. Additionally, they found that smaller fibres would have a lower activation threshold.
Roth et al. [117] found that cells exposed to nanosecond pulsed electric fields created nanopores in the cellular membrane, leading to an influx of Ca2+ . The authors speculate that the response seen due to exposure to INS may also be due to the formation of temporary, subtle disturbances in the cell membrane, based on the similarity in observed electrophysiological response.
Despite considerable progress towards understanding the mechanism behind INS, the details of the process have not yet been fully explained and the topic is still subject to further research. However, the current evidence points to changes in membrane capacitance underlying the primary response observed, with temperature dependent effects on transmembrance ion channels and TRPV channels playing an additional modulatory role.
4. EMERGING TECHNIQUES
4.1. Combined Optical and Electrical Stimulation
One of the potential disadvantages of INS is the heat deposited in tissue, especially at high repetition rates [118, 101]. Any reduction in the total energy delivered to the tissue would be advantageous for the development of implants both from a tissue heating consideration as well as from a laser device design point of view. Additionally, the current laser sources consume significantly more power than existing electrical implants such as the cochlear implant. Minimising total energy consumption will be important in developing portable and compact implantable stimulation systems. Duke et al. [70] proposed using sub-threshold electrical depolarisa-tion to reduce the optical energy required for stimulation. An initial investigation [70] found a three fold reduction in the optical energy when the electrical stimulus was 90% of threshold. These results showed the potential of electrical-optical hybrid stimulation. The strongest response was observed when the two pulses ended simultaneously. Additionally, the authors found that the relationship between increasing electrical current and required optical energy did not follow a linear relationship, implying that the two stimulation modalities do not function by the same mechanism.
Electrical-optical hybrid stimulation was further investigated in [71]. Here a rat sciatic nerve and Aplysia californica buccal nerve were stimulated using either a 2120 nm Ho:YAG laser or a 1875 nm diode laser and standard electrodes. For the Aplysia experiments, the light was coupled into a 100 µm or 200 µm core diameter fibre to match the nerve size; while for the rat sciatic nerve, the light was delivered by a 400 µm or 600 µm core diameter fibre as the nerve trunk is larger in this model. Both the temporal and spatial parameters of the optical and electrical stimuli were investigated. The authors found a strong spatial dependence on the location of the optical stimulus relative to the electrodes, that excitation could only be performed when stimulated near the cathode and that the excitable area was larger when the electrodes were configured transverse to the nerve than parallel. Additionally, in Aplysia, increasing the optical radiant exposure could cause inhibition of the nerve, even when the electrical pulse was set to 110% of the threshold. The authors also noted that the choice of laser source greatly affected the performance of hybrid stimulation: although both wavelengths had the same absorption coefficient in water, the Ho:YAG showed greater reproducibility than the diode.
The muscular response due to exposure of neurons to electrical-optical hybrid stimulation was investigated in [77]. Here a rat sciatic nerve was exposed to electrical and hybrid electrical-optical stimulation and the force generated in the plantarflexor muscles was measured in response to the different stimulation modalities and parameters. The optical stimulus was delivered by a 400 µm core diameter optical fibre connected to a diode laser (λ = 1875 nm), the light was delivered through a Sylgard nerve cuff which was found to have 93% transmission at the wavelength used. The nerve was stimulated at rates of 15 and 20 Hz for a period of 1 second. Unlike electrical stimulation, responses to hybrid stimulation increased during a pulse train reaching a plateau by the 20th pulse. Additionally, an isolated optical stimulus before the hybrid pulse trains showed an increase in force generated. These results suggest that an increase in the baseline temperature of the nerves is a contributing factor to the response observed during hybrid stimulation.
Hybrid electrical-optical stimulation has been used to show inhibition of electrical responses in vivo [72, 119] and spontaneous neural activity in vitro [100]. Use of a pulsed INS laser reversibly inhibited action potential creation and blocked action potential conduction in both rat sciatic nerve and Aplysia buccal nerve [119]. Infrared neural suppression is likely due to thermal block, which has previously been observed [120]. However, the technique presented allowed for suppression with a spatial resolution as small as 100 µm and temporal resolution on the order of ~500 µs. Use of this technique may allow non-invasive investigation of neuro-logical tissue and could potentially be applied to treating neurological disorders.
Overall, the work on electrical-optical hybrid stimulation suggests that the optical energy requirements could be reduced by up to 90%, without a reduction in spatial localisation compared to INS alone. However, this reduction is dependent upon electrode position relative to the target neurons and some recent research [72, 100] suggests that this process may not be applicable to all neural targets.
4.2. Nanoparticle Enhanced INS
An alternative technique to enhance INS is by adding light absorbing materials to the tissue. The use of nanoparticles to stimulate of neurons under exposure to magnetic fields has previously been demonstrated [121] and now researchers are exploring the potential of using light to stimulate neurons via heating of nanoparticles [122, 123]. If nanoparticles are selected to absorb strongly at wavelengths that are weakly absorbed by water and tissue, then the absorption can potentiallly be localised near the targeted nerves. Enhancing absorption at the neurons has the dual benefits of reducing the power required for stimulation and allowing for wavelengths with increased penetration depth. Farah et al. [122] introduced micron scale photo-absorbing particles (iron oxide) to cultured rat cortical cells. The light was holographically patterned onto the target cells, reducing the total exposure of light to the culture. Using the combination of photo-absorbers and patterned light, significant reductions in the energy required to achieve stimulation were observed. Additionally, this technique could allow for the use of wavelengths that are not strongly absorbed by water, thus allowing greater penetration depths to be obtained.
The use of gold nanorods in cells as a selective absorber of near infrared light has also been investigated for in vitro neural stimulation [123, 124]. Initial work on cells cultured with gold nanorods demonstrated intracellular Ca2+ transients in response to laser light at the plasmon resonance wavelength of the gold nanorods [124], similar to cells exposed to INS [114]. Follow-up work found that action potentials could be evoked in vitro with in spiral ganglion neurons cultured with gold NRs under exposure to 780 nm light [123]. This technique used approximately two orders of magnitude less energy than INS. While these approaches based on selective absorbers appear promising, methods for cell specific targeting of the nanoparticles may be necessary for practical applications. Obviously by introducing an exogenous substance to the target tissue this approach loses one of the main benefits of INS.
4.3. Optoelectric Neural Stimulation
Optoelectric neural stimulation is another novel technique to stimulate neurons using light [125]. This technique uses photoactive nanoparticles or surfaces which generate an electric field in response to exposure to light, resulting in localised neural stimulation. Optoelectric neural simulation may potentially combine the tight spatial localisation of light with the general applicability of electrical stimulation. A range of photoactive materials are being developed with the aim of neural stimulation, including quantum dots [126, 127], photoconductive silicon [128], organic polymers [129, 130], and carbon nanotubes [125, 131]. Many of these techniques are particularly attractive for application in to a retinal prothesis, where an optical switch at the neurons would greatly simplify the implementation of any retinal prothesis.
Quantum dots are semiconducting nanoparticles with fluorescent and other optoelectronic properties [132]. These properties allow for the generation of free electrons which can trigger action potentials in neurons [126]. Current limitations include the challenges with biocompatibility [133] of these molecules and improving efficiency to reduce the optical power required [125].
Photoconductive silicon has been demonstrated as a substrate that can generate electric charge to stimulate neurons under exposure to light [128, 134]. However, silicon is limited by its rigidity and biocompatibility, thus potentially limiting its use as a prosthetic platform [135]. Conductive polymers may overcome many of these limitations as an optoelectronic material [129, 130, 136, 137, 138, 139], although challenges in ensuring biocompatibility and stability of these polymers also remain to be resolved [125, 129].
5. LIGHT DELIVERY TECHNIQUES
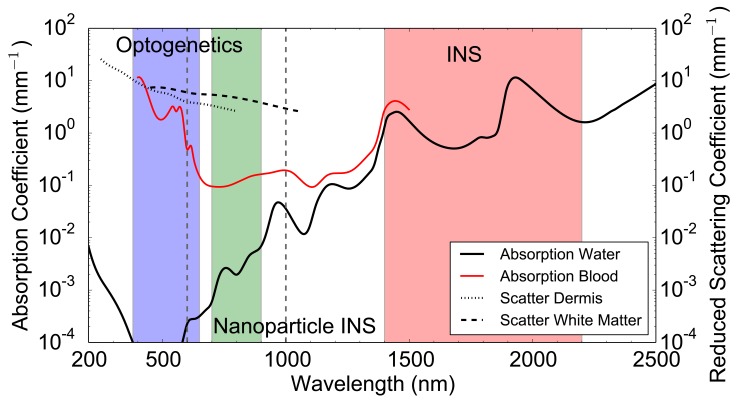
Delivery of light to the target neurons is challenge common to all optical stimulation techniques. For in vitro work, light is commonly delivered via optical fibre or microscope but for in vivo work this becomes more challenging. Different forms of optical stimulation have different challenges. Genetic and caged molecule techniques typically use lower power, but shorter wavelengths where scattering and absorption in tissue is high. Conversely, infrared techniques require delivery of higher power levels and have to contend with strong absorption in water. Some of the emerging techniques that respond to wavelength in the range of 800–1000 nm may have the least challenges with tissue or water interfering with delivery of light to the target neurons. This difference in absorption and scattering in wavelengths used for optical stimulation is shown in (Fig. 3).
Fig. (3).
Absorption coefficient in water [140] and oxygenated blood (5% haematocrit) [141]. Reduced scattering coefficient in dermis [142] and white matter [142] over the wavelength range of 200 nm to 2500 nm. Wavelength ranges commonly used for Optogenetics (380 – 650 nm), INS (1400 – 2200 nm) and Nanoparticle Enhanced INS (700 – 900 nm) are highlighted. Vertical dashed grey lines show the therapeutic window where absorption and scattering is relatively low.
5.1. Optical Waveguides
Optical fibres have provided the standard delivery mechanism for many optical stimulation experiments, as they are well-established optical components and relatively easy to use. A variety of standard silica core fibres have been used with a range of diameters, including for example, 600 µm [54, 57], 400 µm [56, 77], 200 µm [69] and 100 µm [66, 107]. Optical fibres are waveguides that have a core and cladding, most commonly constructed of silica. The refractive index of the core is slightly higher than that of the cladding, so that the light is reflected by total internal reflection if the angle of incidence exceeds the critical angle. Light is only coupled into the fibre if it falls within the acceptance cone is, which is generally often described by the numerical aperture (NA). A large numerical aperture has a wider acceptance cone. Fibres are typically protected from the environment with a number of jacket or buffer layers. For typical optical stimulation experiments, these protective layers are removed near the emitting end of the fibre. For further details on optical fibres, readers are referred to the comprehensive text on waveguides by Synder and Love [143].
Optical fibres have been implanted in vivo to allow optical control of neurons in free animals [33, 90, 144]. Aravanis et al. [33] delivered 473 nm light through an optical fibre implanted in a rat to excite neurons transfected with ChR2 in the control motor cortex of the CNS. Stimulating the neurons with blue light created an increase in whisker deflection, indicating stimulation in the motor cortex. A more integrated approach was taken by Towne et al. [144] where an optical fibre and PDMS optical nerve cuff on the sciatic nerve was implanted in rats [144]. Delivering light to the nerve demonstrated control of muscles in freely moving rats. A fully portable optical delivery system has been demonstrated by Matic et al. [90], where an optical fibre was implanted in the cochlea of cats with miniaturised laser system mounted in a backpack. This enabled the animal to move feely, while its response to stimulation could be monitored.
A laparoscopic probe designed to deliver a collimated top hat beam profile to allow uniform illumination of the cavernous nerves was developed in [145]. The probe delivered a beam with a diameter of 1 mm, when coupled to a thulium fiber laser (λ = 1870 nm). This probe showed similar results to those from previous work using a fibre [61] and may allow easier targeting of nerves for INS. This probe was adapted [146] to provide temperature monitoring and feedback with the continuous wave stimulation technique [75]. An IR sensor monitored the temperature on the surface of the nerve and controlled the laser power, allowing a fast ramp up in temperature without increasing to levels that may cause damage. This simplified the number of parameters to be optimised to achieve stimulation and allowed a faster response.
To enable an optically based implant to stimulate the cochlea, work has been performed to develop a waveguide-based optical delivery system [147]. Using optical fibres with a cladding diameter of 25 λm , bundles of eight fibres could be inserted up to 20 mm into the cochlea without breaking. Insertion forces were similar to those measured with conventional cochlear implants. These results suggest that an optically based cochlear implant may be feasible, although many challenges such as beam targeting remain. Regardless of the application, there are fundamental limitations of using fibre optics in terms of mechanical stiffness and the ability to sustain sharp bends [148], in particular when compared to coiled wire electrodes. These engineering challenges must be address before optical neural interfaces can become a reality.
Optogenetics has also generated interest in advanced light delivery techniques. Typically optogenetics requires a lower laser power level and therefore can accept reduced coupling efficiency and greater losses. Zorzos et al. [149, 150] reported custom fabricated waveguides, capable of delivering light in a 3D pattern in tissue. Holographic patterning of light onto neural tissue was demonstrated by [122, 151]. This technique uses a spatial light modulator to deliver light to individual neurons that have been photo-sensitised with optogenetic treatments or by using photo-absorbers [146].
5.2. Arrayed Light Sources
While fibres have been able to deliver light to a localised area and are able to display a significant improvement over electrical stimulation for some applications [83], there is interest in alternative delivery techniques that are able to deliver light closer to the nerves or to multiple nerves from a single implant for both INS and other optically-mediated nerve stimulation techniques [152].
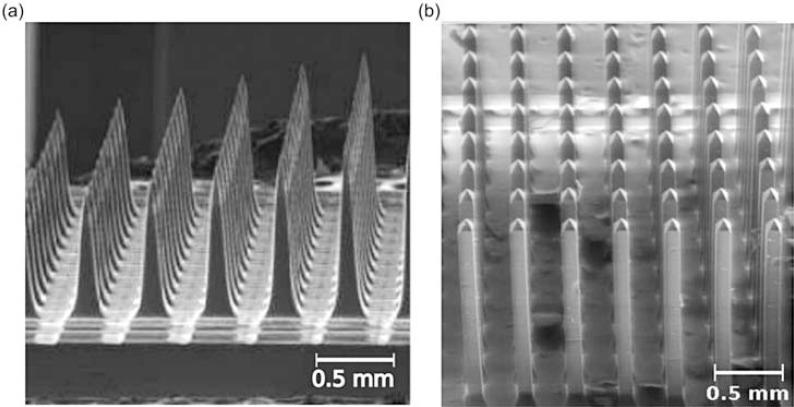
An implantable multiwaveguide device has been the topic of some research [153]. Abaya et al. [154] developed a Utah slanted optrode array (USOA), similar to the Utah slanted electrode array, which has allowed for more specific electrical stimulation of neurons in nerve trunks than was achievable with traditional electrodes [155]. The USOA is aimed at delivering light to deeper tissue and to allow an even more selective stimulation of nerve trunks. The USOA is micromachined from a silicon wafer and etched to form narrow shanks 0.5 mm to 1.5 mm in length. Transmission efficiency varied between 2 - 10% depending on the diameter of the fibre used to couple light into the array. Losses in coupling arise due to Fresnel reflection between the different interfaces, which are increased by the high refractive index of silicon compared to glass, and losses due to the tapering of the tips. The Utah slanted optrode array was improved upon in [156], which reported a 3D silica optrode array with non-tapering tips. An example of the fabricated array is shown in (Fig. 4). This resulted in a large increase in fibre-to-nerve coupling efficiency of up to 70% and significantly reduced shank transmission losses in the shank.
Fig. (4).
SEM image of (a) silicon Utah slant optrode array and (b) glass optrode array reproduced from [153], with permission from SPIE.
Another approach to deliver light to neurons is by developing microscale emitters and positioning or implanting them in close proximity to the target neurons [157-159]. Poher et al. [157] fabricated a 64x64 matrix of 20 µm micro-LEDs, which were able to photostimulate sensitised neurons. This fabrication approach has been taken up by other researchers, including fabrication of 15 LED arrays suitable for implantation in a ChR2 sensitive cochlea [158] and vertical cavity surface emitting lasers to provide high intensity infrared light for INS [159].
5.3. Modelling of Light Delivery and Heating
Recently, both analytical and numerical models have been developed to assist in understanding the flow of heat during INS, in the cochlea and in vitro models [55, 74, 101, 86, 100]. As INS depends on a thermal gradient in time [54, 56], understanding the heat distribution in tissue may help to optimise the process and assist in emitter design. Modelling may also be to provide detailed thermal information for in vitro studies aimed at uncovering the mechanisms behind INS.
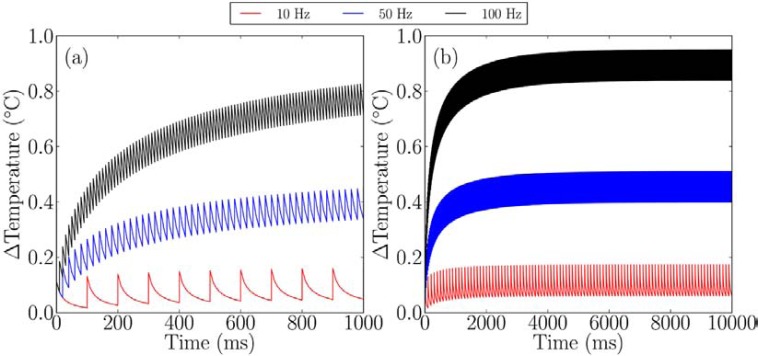
Thompson et al. presented a combined numerical Monte Carlo and finite element model that allows the prediction of temperature increases from a single pulse [55, 74] or from a pulse train and multiple emitters [101], as shown in (Fig. 5). The model compares well with experimental measurements of temperature from laser heating [55, 74] and predicts temperature increases of 0.1°C for INS in the cochlea and on the order of 1°C to 10°C for other targets. The model shows where previously used “rules of thumb” are valid and where a more detailed approach is required.
Fig. (5).
Heating from different stimulation rates with INS, when using a fibre with core diameter of 200 μm and NA = 0.22, with a wavelength of 1850 nm and pulse energy of 25 µJ. a) Over 1 second b) over 10 seconds. Reproduced from [160].
A numerical multiphysics model of heating during INS in vitro was developed by Liljemalm et al. [100]. Using an optical fibre with 200 µm core diameter and NA = 0.39, wavelength of 1550 nm, laser power of 300 mW and pulse length of 20 ms, the model predicted a temperature increase of 13.7°C at the centre of the beam, 300 µm from the fibre emitter. When using multiple pulses at 10 Hz, the peak temperature increased by a further 1.7°C, stabilising after four pulses. Results from the model compared well with local temperature measurements using changes in current in a micropipette.
Norton et al. [86] developed a Green’s function analytical model of thermal changes during INS. Rather than investigate temperature distributions, the model was applied to understanding what thermal changes are required to activate neurons, specifically the cochlea. The authors hypothesised that two thermal criteria are required for neural activation: a minimum temperature increase Tc ; and a minimum temperature rate of change dTc/dt. By optimising these criteria, a pulse length can be found that satisfies both conditions, thus reducing wasted energy. Experimental in vivo data of INS in the cat cochlea used in combination with a CAP growth function to give values of Tc = 0.8 m°C and dTc / dt = 15.1 °C.s,-1 for the thermal criteria and an optimal pulse length of 53 µs . Further use of this approach to determine optimal pulse lengths may assist in developing more efficient INS implants.
Monte Carlo models have also been applied to understand light distributions in tissue during optogenetic stimulation [9, 33, 161]. Most current opsins used for optogenetic stimulation respond at a much shorter wavelength than used in INS, where scattering in the tissue dominates over absorption. A wavelength of 473 nm, which activates commonly used opsins, has less than 20% light transmission through 500 µm of brain tissue when using a fibre core diameter of 200 µm and NA = 0.37. If light with a wavelength of 594 nm is used instead, transmission increases to 30% due to the decreased scattering at these wavelengths [9].
6. CONCLUSION
The accelerated development of optical nerve stimulation techniques over the past decade or so has provided neuroscientists with a powerful new range of tools for controlling neuronal activity. While no one tool currently offers a complete solution to all of the requirements for manipulating neuronal function, each of the techniques has some specific advantages. For example, the two-photon uncaging of glutamate allows a high degree of spatial localization and precise timing of stimulation events. However, the glutamate is gradually depleted by repeated photolysis, so this method is less effective for prolonged stimulation or high repetition rates. Likewise, genetically targeted ChR2 is attractive for controlling and mapping neural circuits in vivo, but requires suitable promoters for specific cell types and has limited depth resolution due to the use of visible light.
Infrared neural stimulation is attractive for direct activation of nerves via the transient heating of water, without any need for tissue modification. However, the technique remains relatively poorly understood, requires high light intensities and has demonstrated limited localisation and depth penetration due to the prevalence of water in all tissues. Emerging techniques based on extrinsic absorbers may overcome some of the deficiencies of infrared stimulation, but at the cost of introducing foreign materials to the nervous system. Therefore, given the undoubted attractions of optical stimulation, the remaining limitations will certainly lead to improvements in the current tools and the development of additional novel techniques. In particular, the development of a wider range of small molecule photoswitches promises to deliver great flexibility in controlling the activity of specific ion channels or receptors. This capability will provide new insights into the role of those targets in normal neuronal function.
Further progress can also be anticipated in terms of delivering light to the nervous system. Clearly those techniques that rely on near infrared light in the transparency window of tissue, such as two photon uncaging and gold nanoparticles, will have an advantage in terms of in vivo delivery. However, the need for implantable arrays of light sources or emitters will remain for many important targets, including the brain. Multiple active light sources will allow synchronous recruitment of multiple neuronal targets, while holographic illumination systems can potentially also provide simultaneous access to a large number of stimulation sites. These efforts will be facilitated by modelling of light propagation and interactions with neuronal tissue, although there is a need for more detailed data on the optical properties of biological tissues across the entire wavelength range of interest.
Indeed as novel photonic technologies are developed more and more of these emerging tools will become available to neuroscientist and clinicians. Further progress in these various domains will eventually allow optical stimulation to deliver its full potential, driving important advances in our understanding of neural disorders, unravelling neural encoding and facilitating new interventions for sensory deficiencies and neural diseases. The future of this field is bright.
CONFLICT OF INTEREST
The author confirms that this article have no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Australian Research Council (grant no: LP120100264), NIH (grant no: R01 NS052407), DOD/DARPA CIPhER Program (grant no: HR0011-10-1-0074) and Vanderbilt VIO exploratory grant.
REFERENCES
- 1.Fenno L, Yizhar O, Deisseroth K. The Development and Application of Optogenetics. Annu Rev Neurosci. 2011;34(1):389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter CP, Matic AI, Wells JD, Jansen ED, Walsh JT. Neural stimulation with optical radiation. Laser Photonics Rev. 2011;5(1):68–80. doi: 10.1002/lpor.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- 4.d'Arsonval MA. Action physiologique des courants alternatifs. CR Soc Biol. 1891;43:283–286. [Google Scholar]
- 5.Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971;171(3974):907–908. doi: 10.1126/science.171.3974.907. [DOI] [PubMed] [Google Scholar]
- 6.Hirase H, Nikolenko V, Goldberg JH, Yuste R. Multiphoton stimulation of neurons. J Neurobio. 2002;51(3):237–247. doi: 10.1002/neu.10056. [DOI] [PubMed] [Google Scholar]
- 7.Kandel ER, Schwartz JH, Jessell TM. 4th ed. New York: McGraw Hill; 2000. Principles of neural science, Health Professions Division. [Google Scholar]
- 8.Balaban P, Esenaliev R, Karu T , et al. He-Ne laser irradiation of single identified neurons. Laser Surg Med. 1992;12(3):329–337. doi: 10.1002/lsm.1900120315. [DOI] [PubMed] [Google Scholar]
- 9.Yizhar O, Fenno L, Davidson T, Mogri M, Deisseroth K. Optogenetics in Neural Systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Zemelman BV, Lee GA, Ng M, Miesenbck G. Selective Photostimulation of Genetically ChARGed Neurons. Neuron. 2002;33(1):15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 11.Zemelman BV, Nesnas N, Lee GA, Miesenbck G. Photochemical gating of heterologous ion channels Remote control over genetically designated populations of neurons. Proc Natl Acad Sci USA. 2003;100(3):1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 13.Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat Chem Biol. 2005;1(7):360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- 14.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA. 1993;90(16):7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells J, Kao C, Mariappan K , et al. Optical stimulation of neural tissue in vivo. Opt Lett. 2005;30(5):504–506. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8(8):577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 17.Kramer RH, Fortin DL, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19(5):544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 2004;7(12):1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima SQ, Miesenböck G. Remote Control of Behavior through Genetically Targeted Photostimulation of Neurons. Cell. 2005;121(1):141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Nagel G, Szellas T, Huhn W , et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. P Natl Acad Sci. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54(2):85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13(3):387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Wang LP, Brauner M , et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446(7136):633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 24.Gradinaru V, Zhang F, Ramakrishnan C , et al. Molecular and Cellular Approaches for Diversifying and Extending Optogenetics. Cell. 2010;141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liske H, Qian X, Anikeeva P, Deisseroth K, Delp S. Optical control of neuronal excitation and inhibition using a single opsin protein. ChR2. Sci Rep-UK. 2013;3 doi: 10.1038/srep03110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinyon JL, Tadros SF, Froud KE , et al. Close-Field Electroporation Gene Delivery Using the Cochlear Implant Electrode Array Enhances the Bionic Ear. Sci Transl Med. 2014;6(233):233ra54–233ra54. doi: 10.1126/scitranslmed.3008177. [DOI] [PubMed] [Google Scholar]
- 27.Antkowiak M, Torres-Mapa ML, Witts EC, Miles GB, Dholakia K, Gunn-Moore FJ. Fast targeted gene transfection and optogenetic modification of single neurons using femtosecond laser irradiation. Sci Rep-UK. 2013;3 doi: 10.1038/srep03281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darrow KN, Slama M, Kempfle J , et al. Optogenetic Control of Central Auditory Neurons. In 36th Midwinter Meeting of the ARO. Baltimore. 2013 [Google Scholar]
- 29.Schwarz UT, Goßler4 C, Schwärzle M , et al. Optogenetic stimulation of the auditory nerve for cochlear implants with increased number of frequency channels and dynamic range. In Optogenetics and Hybrid-Optical Control of Cells. San Francisco. SPIE;2013 [Google Scholar]
- 30.Shimano T, Fyk-Kolodziej B, Mirza N , et al. Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling. Brain Res. 2013;1511:138–152. doi: 10.1016/j.brainres.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez VH, Gehrt A, Reuter K , et al. Optogenetic stimulation of the auditory pathway. J Clin Invest. 2014;124(3):1114–1129. doi: 10.1172/JCI69050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark G. New York: Springer Verlag; 2003. Cochlear implants fundamentals and applications. [Google Scholar]
- 33.Aravanis AM, Wang LP, Zhang F , et al. An optical neural interface in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4(3):S143. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Prigge M, Beyrière F , et al. Red-shifted optogenetic excitation a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11(6):631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16(10):1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monesson-Olson BD, Browning-Kamins J, Aziz-Bose R, Kreines F, Trapani JG. Optical Stimulation of Zebrafish Hair Cells Expressing Channelrhodopsin-2. PLoS ONE. 2014;9(5):e96641. doi: 10.1371/journal.pone.0096641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter ME, de Lecea L. Optogenetic investigation of neural circuits in vivo. Trends mol med. 2011;17(4):197–206. doi: 10.1016/j.molmed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knpfel T, Boyden E. A comprehensive concept of optogenetics. Optogenetics Tools for Controlling and Monitoring Neuronal Activity. 2012;196:1. [Google Scholar]
- 41.Sahel JA, Roska B. Gene Therapy for Blindness. Annu Rev Neurosci. 2013;36(1):467–488. doi: 10.1146/annurev-neuro-062012-170304. [DOI] [PubMed] [Google Scholar]
- 42.Wieboldt R, Gee KR, Niu L, Ramesh D, Carpenter BK, Hess GP. Photolabile precursors of glutamate synthesis, photochemical properties, and activation of glutamate receptors on a microsecond time scale. Proc Natl Acad Sci USA. 1994;91(19):8752–8756. doi: 10.1073/pnas.91.19.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis-Davies GCR. Caged compounds photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4(8):619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warther D, Gug S, Specht A , et al. Two-photon uncaging New prospects in neuroscience and cellular biology. Bioorg Med Chem. 2010;18(22):7753–7758. doi: 10.1016/j.bmc.2010.04.084. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4(11):1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Front Neural Circuit. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate Two-Photon and Visible-Light Photoactivation of Neurons and Dendritic spines. Front Neural Circuit. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beharry AA, Woolley GA. Azobenzene photoswitches for biomolecules. Chem Soc Rev. 2011;40(8):4422. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 49.Fortin DL, Banghart MR, Dunn TW , et al. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5(4):331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miesenbck G, Kevrekidis IG. Optical Imaging and Control of Genetically Designated Neurons in Functioning Circuits. Annu Rev Neurosci. 2005;28(1):533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- 51.Dalva MB, Katz LC. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science. 1994;265(5169):255–258. doi: 10.1126/science.7912852. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neural circuits. Nat Methods. 2007;4(11):943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 54.Wells J, Kao C, Konrad P , et al. Biophysical Mechanisms of Transient Optical Stimulation of Peripheral Nerve. Biophys J. 2007;93(7):2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson AC, Wade SA, Brown WGA, Stoddart PR. Modeling of light absorption in tissue during infrared neural stimulation. J Biomed Opt. 2012;17(7):075002–075002-6. doi: 10.1117/1.JBO.17.7.075002. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10(6):064003–064003-12. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 58.Wells JD, Thomsen S, Whitaker P , et al. Optically mediated nerve stimulation Identification of injury thresholds. Laser Surg Med. 2007;39(6):513–526. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 59.Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Meth. 2007;163(2):326–337. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teudt IU, Nevel AE, Izzo AD, Walsh JT, Richter CP. Optical stimulation of the facial nerve a new monitoring techniqueκ. Laryngoscope. 2007;117(9):1641–1647. doi: 10.1097/MLG.0b013e318074ec00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried NM, Lagoda GA, Scott NJ, Su LM, Burnett AL. Noncontact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser. J Endourol. 2008;22(3):409–414. doi: 10.1089/end.2008.9996. [DOI] [PubMed] [Google Scholar]
- 62.Fried NM, Lagoda GA, Scott NJ, Su LM, Burnett AL. Laser stimulation of the cavernous nerves in the rat prostate, in vivo Optimization of wavelength, pulse energy, and pulse repetition rate In Engineering in Medicine and Biology Society 2008. EMBS 2008. 30th Annual International Conference of the IEEE; 2008; pp. 2777–2780. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins MW, Duke AR, Gu S , et al. Optical pacing of the embryonic heart. Nat Photonics. 2010;4(9):623–626. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins MW, Wang YT, Doughman YQ, Watanabe M, Cheng Y, Rollins AM. Optical pacing of the adult rabbit heart. Biomed Opt Express. 2013;4(9):1626–1635. doi: 10.1364/BOE.4.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cayce JM, Friedman RM, Jansen ED, Mahavaden-Jansen A, Roe AW. Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. NeuroImage. 2011;57(1):155–166. doi: 10.1016/j.neuroimage.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Izzo AD, Richter CP, Jansen ED, Walsh Jr JT. Laser stimulation of the auditory nerve. Laser Surg Med. 2006;38(8):745–753. doi: 10.1002/lsm.20358. [DOI] [PubMed] [Google Scholar]
- 67.Izzo AD, Walsh Jr JT, Jansen ED , et al. Optical parameter variability in laser nerve stimulation A study of pulse duration, repetition rate, and wavelength. IEEE T Bio-med Eng. 2007;54(6):1108–1114. doi: 10.1109/TBME.2007.892925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Izzo AD, Walsh JT, Ralph H , et al. Laser stimulation of auditory neurons Effect of shorter pulse duration and penetration depth. Biophys J. 2008;94(8):3159–3166. doi: 10.1529/biophysj.107.117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richter CP, Bayon R, Izzo AD , et al. Optical stimulation of auditory neurons Effects of acute and chronic deafening. Hearing Res. 2008;242(1-2):42–51. doi: 10.1016/j.heares.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duke AR, Cayce JM, Malphrus JD, Konrad P, Mahadevan-Jansen A, Jansen ED. Combined optical and electrical stimulation of neural tissue in vivo. J Biomed Opt. 2009;14(6):060501–060501. doi: 10.1117/1.3257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duke AR, Lu H, Jenkins MW, Chiel HJ, Jansen ED. Spatial and temporal variability in response to hybrid electro-optical stimulation. J Neural Eng. 2012;9(3):036003. doi: 10.1088/1741-2560/9/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson EJ, Tyler DJ. Motor neuron activation in peripheral nerves using infrared neural stimulation. J Neural Eng. 2014;11(1):016001. doi: 10.1088/1741-2560/11/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCaughey RG, Chlebicki C, Wong BJF. Novel wavelengths for laser nerve stimulation. Laser Surg Med. 2010;42(1):69–75. doi: 10.1002/lsm.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson AC, Wade SA, Cadusch PJ, Brown WGA, Stoddart PR. Modeling of the temporal effects of heating during infrared neural stimulation. J Biomed Opt. 2013;18(3):035004–035004. doi: 10.1117/1.JBO.18.3.035004. [DOI] [PubMed] [Google Scholar]
- 75.Tozburun S, Stahl CD, Hutchens TC, Lagoda GA, Burnett AL, Fried NM. Continuous-wave Infrared Subsurface Optical Stimulation of the Rat Prostate Cavernous Nerves Using a 1490-nm Diode Laser. Urology. 2013;82(4):969–973. doi: 10.1016/j.urology.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 76.Wang YT, Gu S, Ma P, Watanabe M, Rollins AM, Jenkins MW. Optical stimulation enables paced electrophysiological studies in embryonic hearts. Biomed Opt Express. 2014;5(4):1000–1013. doi: 10.1364/BOE.5.001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duke AR, Peterson E, Mackanos MA, Atkinson J, Tyler D, Jansen ED. Hybrid electro-optical stimulation of the rat sciatic nerve induces force generation in the plantarflexor muscles. J Neural Eng. 2012;9(6):066006. doi: 10.1088/1741-2560/9/6/066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cargill R, Baumann T, Jacques SL. Optical stimulation of excised murine sciatic nerve using 1 µm wavelength laser. In Progress in Biomedical Optics and Imaging - Proceedings of SPIE. 2008;6854 [Google Scholar]
- 79.Cayce JM, Kao CC, Malphrus JD, Konrad PE, Mahadevan-Jansen A, Jansen ED. Infrared Neural Stimulation of Thalamocortical Brain Slices. IEEE J Quantum Elect. 2010;16(3):565–573. [Google Scholar]
- 80.Cayce JM, Friedman RM, Chen G, Jansen ED, Mahadevan-Jansen A, Roe AW. Infrared neural stimulation of primary visual cortex in non-human primates. NeuroImage. 2014;84:181–190. doi: 10.1016/j.neuroimage.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cayce JM, Bouchard MB, Chernov MM , et al. Calcium imaging of infrared-stimulated activity in rodent brain. Cell Calcium. 2014;55(4):183–190. doi: 10.1016/j.ceca.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Izzo AD, Suh E, Pathria J, Walsh J, Whitlon DS, Richter CP. Selectivity of neural stimulation in the auditory system a comparison of optic and electric stimuli. J Biomed Opt. 2007;12(2):021008. doi: 10.1117/1.2714296. [DOI] [PubMed] [Google Scholar]
- 83.Richter CP, Rajguru SM, Matic AI , et al. Spread of cochlear excitation during stimulation with pulsed infrared radiation inferior colliculus measurements. J Neural Eng. 2011;8(5):056006. doi: 10.1088/1741-2560/8/5/056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreno LE, Rajguru SM, Matic AI , et al. Infrared neural stimulation Beam path in the guinea pig cochlea. Hearing Res. 2011;282(1-2):289–302. doi: 10.1016/j.heares.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matic AI, Walsh JT, Richter CP. Spatial extent of cochlear infrared neural stimulation determined by tone-on-light masking. J Biomed Opt. 2011;16(11):118002–118002-8. doi: 10.1117/1.3655590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norton BJ, Bowler MA, Wells JD, Keller MD. Analytical approaches for determining heat distributions and thermal criteria for infrared neural stimulation. J Biomed Opt. 2013;18(9):098001–098001. doi: 10.1117/1.JBO.18.9.098001. [DOI] [PubMed] [Google Scholar]
- 87.Xia N, Wu XY, Wang X , et al. Pulsed 808-nm infrared laser stimulation of the auditory nerve in guinea pig cochlea. Laser Med Sci. 2013:1–7. doi: 10.1007/s10103-013-1348-8. [DOI] [PubMed] [Google Scholar]
- 88.Teudt IU, Maier H, Richter CP, Kral A. Acoustic Events and "Optophonic" Cochlear Responses Induced by Pulsed Near-Infrared LASER. IEEE T Bio-med Eng. 2011;58(6):1648–1655. doi: 10.1109/TBME.2011.2108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadakia S, Young H, Richter CP. Masking of infrared neural stimulation (INS) in hearing and deaf guinea pigs. In Photonic Therapeutics and Diagnostics IX. 2013;8565. SPIE:85655V. doi: 10.1117/12.2013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matic AI, Robinson AM, Young HK , et al. Behavioral and Electrophysiological Responses Evoked by Chronic Infrared Neural Stimulation of the Cochlea. PLoS ONE. 2013;8(3):e58189. doi: 10.1371/journal.pone.0058189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee DJ, Hancock KE, Mukerji S, Brown MC. Optical Stimulation of the Central Auditory System In 32nd Midwinter Meeting of the ARO. Baltimore MD. 2009 [Google Scholar]
- 92.Verma RU, Guex AA, Hancock KE , et al. Auditory responses to electric and infrared neural stimulation of the rat cochlear nucleus. Hearing Res. 2014;310:69–75. doi: 10.1016/j.heares.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wenzel GI, Balster S, Zhang K , et al. Green laser light activates the inner ear. J Biomed Opt. 2009;14(4):044007–044007. doi: 10.1117/1.3174389. [DOI] [PubMed] [Google Scholar]
- 94.Schultz M, Baumhoff P, Maier H , et al. Nanosecond laser pulse stimulation of the inner ear a wavelength study. Biomed Opt Express. 2012;3(12):3332–3345. doi: 10.1364/BOE.3.003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rettenmaier A, Lenarz T, Reuter G. Nanosecond laser pulse stimulation of spiral ganglion neurons and model cells. Biomed Opt Express. 2014;5(4):1014. doi: 10.1364/BOE.5.001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chernov MM, Chen G, Roe AW. Histological Assessment of Thermal Damage in the Brain Following Infrared Neural Stimulation. Brain Stimul. 2014;7(3):476–482. doi: 10.1016/j.brs.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rajguru SM, Matic AI, Robinson AM , et al. Optical Cochlear Implants Evaluation Of Surgical Approach And Laser Parameters In Cats. Hearing Res. 2010;269(1-2):102–111. doi: 10.1016/j.heares.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goyal V, Rajguru S, Matic AI, Stock SR, Richter CP. Acute Damage Threshold for Infrared Neural Stimulation of the Cochlea Functional and Histological Evaluation. Anat Rec. 2012;295(11):1987–1999. doi: 10.1002/ar.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liljemalm R, Nyberg T. Quantification of a Thermal Damage Threshold for Astrocytes Using Infrared Laser Generated Heat Gradients. Ann Biomed Eng. 2014;42(4):822–832. doi: 10.1007/s10439-013-0940-1. [DOI] [PubMed] [Google Scholar]
- 100.Liljemalm R, Nyberg T, von Holst H. Heating during infrared neural stimulation. Laser Surg Med. 2013;45(7):469–481. doi: 10.1002/lsm.22158. [DOI] [PubMed] [Google Scholar]
- 101.Thompson AC, Wade SA, Pawsey NC, Stoddart PR. Infrared Neural Stimulation Influence of Stimulation Site Spacing and Repetition Rates on Heating. IEEE T Bio-med Eng. 2013;60(12):3534–3541. doi: 10.1109/TBME.2013.2272796. [DOI] [PubMed] [Google Scholar]
- 102.Rajguru SM, Richter CP, Matic AI , et al. Infrared photostimulation of the crista ampullaris. J Physiol. 2011;589(6):1283–1294. doi: 10.1113/jphysiol.2010.198333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 104.Montell C. The TRP Superfamily of Cation Channels. Sci Signal. 2005;2005(272):re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 105.Suh E, Izzo Matic A, Otting M, Walsh JT , Jr, Richter CP. USA: San Jose CA.; 2009. Optical stimulation in mice lacking the TRPV1 channel In Proceedings of SPIE; pp. 71800S–71800S-5. [Google Scholar]
- 106.Katz EJ, Ilev IK, Krauthamer V, Kim DH, Weinreich D. Excitation of primary afferent neurons by near-infrared light in vitro. NeuroReport. 2010;21(9):662–666. doi: 10.1097/WNR.0b013e32833add3a. [DOI] [PubMed] [Google Scholar]
- 107.Albert ES, Bec JM, Desmadryl G , et al. TRPV4 Channels Mediate the Infrared Laser Evoked Response in Sensory Neurons. J Neurophysiol. 2012;107(12):3227–3234. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- 108.Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1(2):85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- 109.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-Evoked Activation of the Ion Channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold-and heat-sensitive TRP channels. Nature. 2004;430(7001):748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 111.Talavera K, Nilius B, Voets T. Neuronal TRP channels thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31(6):287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Rhee AY, Li G, Wells J, Kao JPY. Photostimulation of sensory neurons of the rat vagus nerve. Proceedings of SPIE. 2008;6854(1):68540E–68540E-5. [Google Scholar]
- 113.Li X, Liu J, Liang S , et al. Temporal Modulation of Sodium Current Kinetics in Neuron Cells by Near-Infrared Laser. Cell Biochem Biophys. 2013:1–11. doi: 10.1007/s12013-013-9674-9. [DOI] [PubMed] [Google Scholar]
- 114.Dittami GM, Rajguru SM, Lasher RA, Hitchcock RW, Rabbitt RD. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J Physiol. 2011;589(6):1295–1306. doi: 10.1113/jphysiol.2010.198804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Q, Frerck M, Holman H, Jorgensen E, Rabbitt R. Exciting Cell Membranes with a Blustering Heat Shock. Biophys J. 2014;106(8):1570–1577. doi: 10.1016/j.bpj.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peterson EJ, Tyler DJ. Activation using infrared light in a mammalian axon model. In 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2012; pp. 1896–9. [DOI] [PubMed] [Google Scholar]
- 117.Roth CC, Tolstykh GP, Payne JA , et al. Nanosecond pulsed electric field thresholds for nanopore formation in neural cells. J Biomed Opt. 2013;18(3):035005–035005. doi: 10.1117/1.JBO.18.3.035005. [DOI] [PubMed] [Google Scholar]
- 118.Izzo AD, Walsh Jr JT, Ralph H , et al. Laser stimulation of the auditory system at 1 94 um and microsecond pulse durations. In Proc. of SPIE. 2008;6854:68540C–1. [Google Scholar]
- 119.Duke AR, Jenkins MW, Lu H, McManus JM, Chiel HJ, Jansen ED. Transient and selective suppression of neural activity with infrared light. Sci Rep-UK. 2013;3 doi: 10.1038/srep02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis FA, Schauf CL, Reed BJ, Kesler RL. Experimental studies of the effects of extrinsic factors on conduction in normal and demyelinated nerve 1. Temperature. J Neurol Neurosur Ps. 1976;39(5):442–448. doi: 10.1136/jnnp.39.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol. 2010;5(8):602–606. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- 122.Farah N, Zoubi A, Matar S , et al. Holographically patterned activation using photo-absorber induced neural-thermal stimulation. J Neural Eng. 2013;10(5):056004. doi: 10.1088/1741-2560/10/5/056004. [DOI] [PubMed] [Google Scholar]
- 123.Yong J, Needham K, Brown WGA , et al. Gold-Nanorod-Assisted Near-Infrared Stimulation of Primary Auditory Neurons. Adv Healthc Mater. 2014;3(11):1862–1868. doi: 10.1002/adhm.201400027. [DOI] [PubMed] [Google Scholar]
- 124.Paviolo C, Haycock JW, Cadusch PJ, McArthur SL, Stoddart PR. Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophoton. 2013;7(10):761–765. doi: 10.1002/jbio.201300043. [DOI] [PubMed] [Google Scholar]
- 125.Bareket-Keren L, Hanein Y. Novel interfaces for light directed neuronal stimulation advances and challenges. Int J Nanomed. 2014;9(1):65–83. doi: 10.2147/IJN.S51193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pappas TC, Wickramanyake WMS, Jan E, Motamedi M, Brodwick M, Kotov NA. Nanoscale engineering of a cellular interface with semiconductor nanoparticle films for photoelectric stimulation of neurons. Nano lett. 2007;7(2):513–519. doi: 10.1021/nl062513v. [DOI] [PubMed] [Google Scholar]
- 127.Lugo K, Miao X, Rieke F, Lin LY. Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots. Biomed Opt Express. 2012;3(3):447–454. doi: 10.1364/BOE.3.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suzurikawa J, Takahashi H, Kanzaki R, Nakao M, Takayama Y, Jimbo Y. Light-addressable electrode with hydrogenated amorphous silicon and low-conductive passivation layer for stimulation of cultured neurons. Appl Phys Lett. 2007;90(9):093901. doi: 10.1109/IEMBS.2006.259828. [DOI] [PubMed] [Google Scholar]
- 129.Ghezzi D, Antognazza MR, Dal Maschio M, Lanzarini E, Benfenati F, Lanzani G. A hybrid bioorganic interface for neuronal photoactivation. Nat Commun. 2011;2:166. doi: 10.1038/ncomms1164. [DOI] [PubMed] [Google Scholar]
- 130.Ghezzi D, Antognazza MR, Maccarone R , et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat Photon. 2013;7(5):400–406. doi: 10.1038/nphoton.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bareket-Keren L, Hanein Y. Carbon nanotube-based multi electrode arrays for neuronal interfacing progress and prospects. Front Neural Circuit. 2013;6:122. doi: 10.3389/fncir.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Michalet X, Pinaud FF, Bentolila LA , et al. Quantum Dots for Live Cells, in vivo Imaging, and Diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gomez N, Winter JO, Shieh F, Saunders AE, Korgel BA, Schmidt CE. Challenges in quantum dot-neuron active interfacing. Talanta. 2005;67(3):462–471. doi: 10.1016/j.talanta.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 134.Wang L, Mathieson K, Kamins TI , et al. Photovoltaic retinal prosthesis implant fabrication and performance. J Neural Eng. 2012;9(4):046014. doi: 10.1088/1741-2560/9/4/046014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Poole-Warren L, Lovell N, Baek S, Green R. Development of bioactive conducting polymers for neural interfaces. Expert Rev Med Devic. 2010;7(1):35–49. doi: 10.1586/erd.09.58. [DOI] [PubMed] [Google Scholar]
- 136.Gautam V, Bag M, Narayan KS. Single-Pixel, Single-Layer Polymer Device as a Tricolor Sensor with Signals Mimicking Natural Photoreceptors. J Am Chem Soc. 2011;133(44):17942–17949. doi: 10.1021/ja207853e. [DOI] [PubMed] [Google Scholar]
- 137.Gautam V, Rand D, Hanein Y, Narayan KS. A Polymer Optoelectronic Interface Provides Visual Cues to a Blind Retina. Adv Mater. 2014;26(11):1751–1756. doi: 10.1002/adma.201304368. [DOI] [PubMed] [Google Scholar]
- 138.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces Challenges in developing an effective long-term implant. Biomaterials. 2008;29(24-25):3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 139.Green RA, Matteucci PB, Hassarati RT , et al. Performance of conducting polymer electrodes for stimulating neuroprosthetics. J Neural Eng. 2013;10(1):016009. doi: 10.1088/1741-2560/10/1/016009. [DOI] [PubMed] [Google Scholar]
- 140.Hale GM, Querry MR. Optical Constants of Water in the 200-nm to 200-um Wavelength Region. Appl Optics. 1973;12(3):555–563. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 141.Roggan A, Friebel M, Dörschel K, Hahn A, Müller G. Optical properties of circulating human blood in the wavelength range 400-2500 nm. J Biomed Opt. 1999;4(1):36. doi: 10.1117/1.429919. [DOI] [PubMed] [Google Scholar]
- 142.Vo-Dinh T. handbook CRC Press: 2003. Biomedical photonics . [Google Scholar]
- 143.Snyder AW, Love JD. Optical waveguide theory. Springer. 1983;190 [Google Scholar]
- 144.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic Control of Targeted Peripheral Axons in Freely Moving Animals. PLoS ONE. 2013;8(8):e72691. doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tozburun S, Mayeh M, Lagoda GA, Farahi FA, Burnett AL, Fried NM. A Compact Laparoscopic Probe for Optical Stimulation of the Prostate Nerves. IEEE J Sel Top Quant. 2010;16(4):941–945. [Google Scholar]
- 146.Tozburun S, Hutchens TC, McClain MA, Lagoda GA, Burnett AL, Fried NM. Temperature-controlled optical stimulation of the rat prostate cavernous nerves. J Biomed Opt. 2013;18(6):067001–067001. doi: 10.1117/1.JBO.18.6.067001. [DOI] [PubMed] [Google Scholar]
- 147.Balster S, Wenzel GI, Warnecke A , et al. Optical cochlear implant evaluation of insertion forces of optical fibres in a cochlear model and of traumata in human temporal bones. Biomed Tech. 2014;59(1):19–28. doi: 10.1515/bmt-2013-0038. [DOI] [PubMed] [Google Scholar]
- 148.Thompson AC, Cadusch PJ, Robertson DF, Stoddart PR, Wade SA. Origins of Spectral Changes in Fiber Bragg Gratings Due to Macrobending. J Lightwave Technol. 2012;30(22):3500 –3511. [Google Scholar]
- 149.Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett. 2010;35(24):4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt Lett. 2012;37(23):4841–4843. doi: 10.1364/OL.37.004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reutsky-Gefen I, Golan L, Farah N , et al. Holographic optogenetic stimulation of patterned neuronal activity for vision restoration. Nat Commun. 2013;4:1509. doi: 10.1038/ncomms2500. [DOI] [PubMed] [Google Scholar]
- 152.Chernov MM, Duke AR, Cayce JM, Crowder SW, Sung HJ, Jansen ED. Material considerations for optical interfacing to the nervous system. MRS Bull. 2012;37(06):599–605. [Google Scholar]
- 153.Abaya TVF, Diwekar M, Blair S, Tathireddy P, Rieth L, Solzbacher F. Deep-tissue light delivery via optrode arrays. J Biomed Opt. 2014;19(1):015006–015006. doi: 10.1117/1.JBO.19.1.015006. [DOI] [PubMed] [Google Scholar]
- 154.Abaya TVF, Diwekar M, Blair S , et al. Characterization of a 3D optrode array for infrared neural stimulation. Biomed Opt Express. 2012;3(9):2200–2219. doi: 10.1364/BOE.3.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Normann RA, Dowden BR, Frankel MA , et al. Coordinated, multi-joint, fatigue-resistant feline stance produced with intrafascicular hind limb nerve stimulation. J Neural Eng. 2012;9(2):026019. doi: 10.1088/1741-2560/9/2/026019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abaya TVF, Diwekar M, Blair S, Tathireddy P, Rieth L, Solzbacher F. 3D waveguide penetrating arrays for optical neural stimulation. In Optical MEMS and Nanophotonics (OMN) 2012 International Conferen; 2012; pp. 216–217. [Google Scholar]
- 157.Poher V, Grossman N, Kennedy GT , et al. Micro-LED arrays a tool for two-dimensional neuron stimulation. J Phys D Appl Phys. 2008;41(9):094014. [Google Scholar]
- 158.Goßler C, Bierbrauer C, Moser R , et al. GaN-based micro-LED arrays on flexible substrates for optical cochlear implants. J Phys D Appl Phys. 2014;47(20):205401. [Google Scholar]
- 159.Keller MD, Stafford JW, Stafford RC , et al. Laser source development for infrared neural stimulation. In Electronics Communications and Photonics Conference (SIECPC) 2013 Saudi Internat; 2013; pp. 1–4. [Google Scholar]
- 160.Thompson AC. Hawthorn Australia: Swinburne University of Technology.; 2014. Investigation of Infrared Neural Stimulation in the Cochlea [PhD Thesis] [Google Scholar]
- 161.Bernstein JG, Han X, Henninger MA , et al. Prosthetic systems for therapeutic optical activation and silencing of genetically-targeted neurons. Proceedings - SPIE. 2008;6854:68540H. doi: 10.1117/12.768798. [DOI] [PMC free article] [PubMed] [Google Scholar]