Abstract
Regularly attending adult patients are increasingly asymptomatic and not in need of treatment when attending for their routine dental examinations. As oral health improves further, using the general dental practitioner to undertake the “checkup” on regular “low-risk” patients represents a substantial and potentially unnecessary cost for state-funded systems. Given recent regulatory changes in the United Kingdom, it is now theoretically possible to delegate a range of tasks to hygiene-therapists. This has the potential to release the general dental practitioner’s time and increase the capacity to care. The aim of this study is to compare the diagnostic test accuracy of hygiene-therapists when screening for dental caries and periodontal disease in regularly attending asymptomatic adults who attend for their checkup. A visual screen by hygiene-therapists acted as the index test, and the general dental practitioner acted as the reference standard. Consenting asymptomatic adult patients, who were regularly attending patients at 10 practices across the Northwest of England, entered the study. Both sets of clinicians made an assessment of dental caries and periodontal disease. The primary outcomes measured were the sensitivity and specificity values for dental caries and periodontal disease. In total, 1899 patients were screened. The summary point for sensitivity of dental care professionals when screening for caries and periodontal disease was 0.81 (95% CI, 0.74 to 0.87) and 0.89 (0.86 to 0.92), respectively. The summary point for specificity of dental care professionals when screening for caries and periodontal disease was 0.87 (0.78 to 0.92) and 0.75 (0.66 to 0.82), respectively. The results suggest that hygiene-therapists could be used to screen for dental caries and periodontal disease. This has important ramifications for service design in public-funded health systems.
Keywords: health workforce, caries, periodontal diseases, dental hygienists, sensitivity and specificity, healthcare disparities
Background
Approximately 95% of the annual budget for National Health Service (NHS) dentistry in the United Kingdom is spent on the provision of services in primary care by general dental practitioners (GDPs). More than two-thirds of this activity relates to the routine dental “checkup” (Health and Social Care Information Centre 2014). A large proportion of regularly attending adult patients who attend for their checkup do not require any further appointments or active restorative intervention (Milsom et al. 2009), yet their care is delivered by the most expensive resource, the GDP. In contrast, just under half of the population do not attend the GDP, and this group tends to experience the majority of the disease (House of Commons Health Select Committee [HSC] 2008; Milsom et al. 2009). If unchallenged, this situation where those with the lowest need consume the majority of health care resources is likely to exacerbate further and increase oral health inequalities in the United Kingdom (Godson and Williams 2008).
For state-funded health systems, where taxation supports the cost of service provision, it is imperative to ensure that “the right number of people with the right skills are in the right place at the right time to provide the right services to the right people” (Birch 2002). One method of achieving this in primary dental care is to fully use all the members of the dental team and to explore new potential models of care (Steele 2009). The use of mid-level providers like hygiene-therapists (H-Ts) to screen for disease among regular and asymptomatic attenders could free up significant resources. In turn, these could be used to increase the capacity to care or be used to pay for population approaches to prevention (Nash et al. 2008; Steele 2009). Screening is defined as a “process of identifying apparently healthy people who may be at increased risk of a disease or condition” (National Screening Committee [NSC] 2013). In the United Kingdom, the levels of dental caries and periodontal disease in adults are falling and are predicted to continue to do so (Steele 2009; Health and Social Care Information Centre 2014). As a result, many patients who present for their routine checkup will not have disease, unlike a number of decades ago, where most presented with disease. This requires a paradigm shift in the way that services are organized so that dental teams move from a “cure” culture to a “care” culture (Glick et al. 2012).
Savings could be delivered in NHS dentistry by simply extending the recall interval with GDPs, as doubling this interval halves the cost. However, this does not account for the variation in risk of the disease across the practice population. This is important as research suggests that once patients develop disease, their risk of future disease is higher (Milsom et al. 2008; Tickle and Milsom 2008). As a result, it is important to maintain regular contact with patients according to their risk status to prevent dental disease from developing. Hence, there is a challenge for NHS dentistry to provide preventive care while delivering on quality, increasing productivity, releasing resources, and increasing the capacity to care for those who currently cannot or do not access services (Milsom et al. 2008).
In medicine, a systematic review (Laurant et al. 2009) found that services provided by nurse practitioners were associated with higher levels of patient compliance and satisfaction (Laurant et al. 2009). In 2003, a systematic review undertaken on the use of role delegation in dentistry concluded that H-Ts could screen for disease (Galloway et al. 2003); this was reiterated in a more recent rapid evidence review (Turner et al. 2012). However, many of the studies in these reviews were criticized for being of poor quality (Galloway et al. 2003; Dyer et al. 2014) while also failing to ascertain the efficacy of H-Ts when screening for common oral diseases (Innes and Evans 2013). There is evidence of efficacy for H-Ts in epidemiologic programs (Kwan et al. 1996; Kwan and Prendergast 1998; Patel et al. 2012) and technical procedures (Phillips and Shaefer 2013), but little research has been done in the context of screening in a practice environment.
As a result, the aim of this study was to address this paucity of evidence and use diagnostic test methodologies to determine whether H-Ts could be used in a screening role in a practice environment.
Methods
Ethical Approval
Ethical approval was given by the National Research Ethics Service Committee North-West (Cheshire) on February 19, 2013 (13/NW/0010; 118638), and the study protocol was published (Macey et al. 2013).
Participants and Procedure
The study was based on diagnostic test accuracy (DTA) methodology and was designed to satisfy the Standard for Reporting of Diagnostic (STARD) tests (Bossuyt et al. 2003). Dental practices were sampled purposively according to the following eligibility criteria:
Employment of at least one H-T for the provision of routine dental care within the NHS
Large throughput of NHS patients
Minimum practice size of 3 surgeries
Supported by a practice manager
Registered with the Care Quality Commission
Evidence of a robust clinical governance system in place (e.g., BDA Good Practice Scheme; membership or fellowship of the Faculty of General Dental Practice [United Kingdom])
The inclusion criteria for the patients in the study were as follows:
An NHS patient
A minimum of 18 y of age
Asymptomatic
Patient attending for a routine inspection (checkup)
Willingness to consent to study
Patients in pain or requiring active intervention were excluded.
Patients due for a routine checkup were contacted by the dental practice with an introductory letter and an information sheet explaining the study. A dedicated and trained member of the practice followed up this initial contact by telephone to ascertain whether they were willing to take part in the study. Upon arrival for their checkup, the trained and dedicated member of the practice checked the patient’s eligibility and gained informed consent. Participants were free to withdraw from the trial at any time, without explanation. The study population was recruited consecutively.
Index test: screen by H-T for dental caries and periodontal disease
Reference test: screen by GDP for dental caries and periodontal disease
Both H-Ts and GDPs attended a compulsory training day, which covered recruitment, consenting, screening process, and patient record form completion. Calibration was conducted using stock photographs of carious and noncarious teeth (Irwig et al. 2002). GDP training was considered successful when values for sensitivity exceeded 85%, the threshold set by the World Health Organization (1997). Levels for specificity were set lower at 65%, based on the results of an earlier in vitro study (Brocklehurst et al. 2012).
The diagnostic threshold for dental caries was defined as any tooth in the patient’s mouth that showed evidence of frank cavitation or shadowing and opacity that would indicate dental caries into the dentine (when clean and dried). The diagnostic threshold for periodontal disease was any pocket in the patient’s mouth where the black-band of a basic periodontal examination (BPE) probe (3.5 to 5.5 mm) partially or totally disappeared. This is equivalent to a BPE score of 3 or above and was chosen as it represents the point where a full pocket charting is thought to be necessary (British Society of Periodontology [BSP] 2011).
For both dental caries and periodontal disease, the H-T and the GDP were independently asked to answer a hypothetical question on a record form based on their findings at a patient level: “Does the patient require any further investigation?” (Brocklehurst et al. 2011). Any tooth or any periodontal pocket in the patient’s mouth that exceeded the diagnostic threshold was considered to represent a positive test for the respective disease. Given the variation among clinicians when assessing risk, clinicians were asked to avoid any subjective assessment of risk and simply focus on whether the patient exceeded the diagnostic thresholds for caries and periodontal disease.
To reduce order effects, each practice was randomly allocated using a permuted block sampling (Matts and Lachin 1988) system to either H-T first or GDP first. As such, half of the patients received the index test first and half received the reference standard first. The H-T and GDP examinations were performed independently, and clinicians were blind to each other’s score; the results were shared only with the research team that performed the statistical analysis (see Appendix Figure). Participating practices used the same H-T/GDP pairing for all patients, who were recruited on a consecutive basis.
Sample Size
Flahault’s sample size method was used as the prevalence of both diseases was anticipated to be below 50% (Flahault et al. 2005). This requires information on the expected sensitivity values of the new diagnostic test and the minimum acceptable lower confidence limit. Brocklehurst et al. (2012) found that the sensitivity of different clinicians to assess photographs of potentially carious teeth was 85%. As clinicians were expected to find the judgment task in vivo easier, this was set at 90%. The acceptable lower confidence limit was set at 80% to align with the World Health Organization (1997). The number of patients required to satisfy a power of 0.8 was 235. Of the 2 diseases being studied, dental caries has the lower prevalence of 20% (Sheiham and Sabbah 2010) and so was the constraining factor for the sample size calculation. This means 4 × 235 = 940 (since [(1 – Prev)/Prev] = 4) cases are needed without caries, giving a total of 1,175 cases. To allow for pooling of the data within GDP/H-T pairings, a target of 2,000 patients was set.
Statistical Methods
Data were analyzed using the Metandi package in STATA 13 (StataCorp LP, College Station, TX, USA) and RevMan 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The H-T screening (index test) was compared with the GDPs (reference test) to determine true-positive, false-positive, false-negative, and true-negative values. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic odds ratios were calculated, and a 95% confidence interval was applied where appropriate. A meta-analysis was performed according to the methods of the Cochrane DTA Handbook (Macaskill et al. 2010). Summary points, the confidence region, and prediction region were calculated using bivariate model parameters for the sensitivity and specificity of each practice and plotted in receiver operating characteristic (ROC) space to investigate heterogeneity.
Results
The study ran from June 2013 to August 2014. A total of 1,899 patients consented to the study; no patients withdrew, and no adverse events were experienced. In total, 996 patients were randomly allocated to see the GDP first, and 903 attended the H-T first. All recruited patients received both the index and reference tests. The interval between index and reference tests was never greater than 21 min. Patient and practice characteristics are presented in Table 1. There were no missing results regarding the outcome of a positive or negative screening decision, but 2 practices failed to collect data on smoking and dentures; these have been classified as “not reported” (NR) in Table 1 and therefore are not included in the smoking and dentures data. The patient’s mean age was 49.15 y, and 53.3% of patients were women. In total, 287 patients were smokers, 207 had dentures, and 55% of patients had 1 to 9 restorations. The mean time taken for the screening check was 5 min and 25 s for H-Ts and 4 min and 26 s for GDPs.
Table 1.
Characteristics and Results According to Practice
| Practice |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Number | 200 | 201 | 200 | 116 | 187 | 200 | 201 | 200 | 199 | 195 | 1,899 |
| Age, mean (SD), y | 48.6 (15.2) | 48.2 (17.3) | 49.2 (17.3) | 58.2 (14.6) | 47.8 (16.1) | 39.6 (14.8) | 51.7 (16.0) | 48.6 (16.4) | 50.9 (16.8) | 52.3 (16.8) | 49.2a (16.7) |
| Sex, female, % | 50.5 | 51.3 | 51.8 | 41.4 | 55.2 | 55.0 | 56.0 | 58.8 | 54.6 | 58.5 | 53.3a |
| No. of pregnant patients | 2 | 3 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 6 | 17 |
| No. (%) of smokers | 33 (16) | 29 (14) | 38 (19) | 8 (7) | 41 (22) | 64 (32) | 36 (18) | 38 (19) | NR | NR | 287 (19) |
| No. (%) of patients with dentures | 19 (9) | 14 (7) | 29 (15) | 23 (19) | 34 (18) | 21 (11) | 43 (21) | 24 (12) | NR | NR | 207 (14) |
| Kappa values of H-T and GDP calibration | 0.50 | 0.85 | 0.76 | 0.81 | 0.74 | 0.72 | 0.82 | 0.79 | 0.39 | 0.69 | 0.71a |
| No. (%) of patients within each band of restorations and crowns | |||||||||||
| 0 | 7 (4) | 11 (5) | 22 (11) | 1 (1) | 3 (2) | 23 (12) | 10 (5) | 9 (5) | 20 (10) | 5 (3) | 111 (6) |
| 1 to 9 | 107 (54) | 116 (58) | 114 (57) | 56 (48) | 107 (57) | 122 (61) | 114 (57) | 120 (60) | 94 (47) | 92 (46) | 1,042 (55) |
| 10+ | 86 (43) | 74 (37) | 64 (32) | 59 (51) | 77 (41) | 55 (28) | 77 (39) | 71 (35) | 84 (43) | 98 (49) | 745 (39) |
| Mean time taken by practitioner for screening, min:s | |||||||||||
| H-T | 3:39 | 3:36 | 4:52 | 4:14 | 7:12 | 3:15 | 3:39 | 8:07 | 12:01 | 3:31 | 5:25a |
| GDP | 3:10 | 2:30 | 4:43 | 3:45 | 8:26 | 3:14 | 3:55 | 9:22 | 2:06 | 3:16 | 4:27a |
| Caries results | |||||||||||
| Positive predictive value (95% CI) | 0.91 | 0.65 | 0.69 | 0.34 | 0.80 | 0.90 | 0.92 | 0.73 | 0.57 | 0.66 | 0.75 (0.72 to 0.80) |
| Negative predictive value (95% CI) | 0.91 | 0.97 | 0.96 | 0.89 | 0.80 | 0.88 | 0.76 | 0.96 | 0.89 | 0.82 | 0.90 (0.88 to 0.91) |
| Positive likelihood ratio (95% CI) | 27.04 | 8.36 | 7.0 | 2.53 | 4.44 | 4.75 | 9.89 | 20.52 | 3.22 | 9.10 | 6.14 (3.68 to 10.26) |
| Negative likelihood ratio (95% CI) | 0.27 | 0.16 | 0.14 | 0.62 | 0.27 | 0.08 | 0.27 | 0.32 | 0.30 | 1.80 | 0.29 (0.15 to 0.30) |
| Diagnostic odds ratio (95% CI) | 101.43 | 53.98 | 49.00 | 4.05 | 16.47 | 61.58 | 36.88 | 65.14 | 10.89 | .20 | 28.10 (15.5 to 50.95) |
| Periodontal disease results | |||||||||||
| Positive predictive value (95% CI) | 0.84 | 0.84 | 0.93 | 0.81 | 0.88 | 0.83 | 0.86 | 0.93 | 0.59 | 0.66 | 0.82 (0.80 to 0.84) |
| Negative predictive value (95% CI) | 0.88 | 0.91 | 0.89 | 0.74 | 0.61 | 0.91 | 0.82 | 0.73 | 0.82 | 0.84 | 0.84 (0.81 to 0.86) |
| Positive likelihood ratio (95% CI) | 7.48 | 6.68 | 7.90 | 1.67 | 2.60 | 4.30 | 2.76 | 5.54 | 2.12 | 2.42 | 3.63 (2.58 to 5.12) |
| Negative likelihood ratio (95% CI) | 0.19 | 0.13 | 0.07 | 0.14 | 0.23 | 0.08 | 0.10 | 0.15 | 0.32 | 0.23 | 0.15 (0.11 to 0.19) |
| Diagnostic odds ratio (95% CI) | 39.43 | 50.96 | 107.94 | 12.30 | 11.49 | 51.94 | 28.20 | 37.04 | 6.58 | 10.49 | 24.99 (14.59 to 42.82) |
CI, confidence interval; DCP, ; GDP, general dental practitioner; H-T, hygiene-therapist; NR, not recorded by dental practice; SD, standard deviation.
Mean values.
Figures 1 and 2 show the sensitivity and specificity results for caries and periodontal disease. Table 1 reports positive predictive value, negative predictive value, and diagnostic odds ratio for both periodontal disease and dental caries. The Appendix Table states the results in 2 × 2 table format to highlight the level of agreement between the H-T and GDP. Figures 1 and 2 show the variation in performance between practices.
Figure 1.
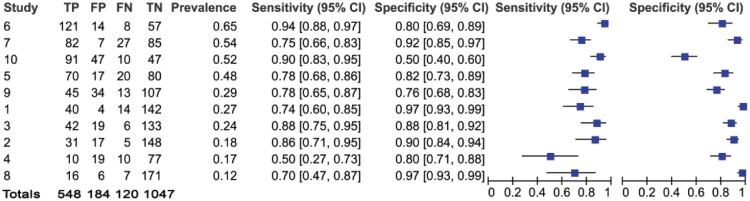
Results and forest plots of caries data, per practice. CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Figure 2.
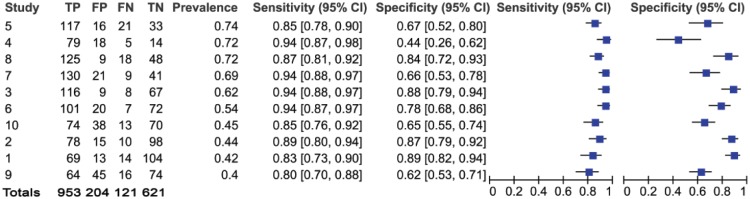
Results and forest plots of periodontal disease data, per practice. CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Dental Caries
In total, 668 patients had evidence of caries identified by the GDP, resulting in a prevalence of 0.35. The H-T classified 548 of these as positive and correctly identified 1,047 of the 1,231 patients whom the GDP screened as negative for caries. The summary points for sensitivity and specificity were 0.81 and 0.87, respectively. This means that in a sample of 100 patients, in whom 35 experienced dental caries (prevalence of 0.35), 7 individuals would remain undetected if screened by an H-T (false negative) and 8 individuals without caries would be referred for treatment (false positive).
Periodontal Disease
In total, 1,074 patients were identified by the GDP as having at least one pocket exceeding 3.5 mm in depth and therefore being screened positive for periodontal disease, resulting in a prevalence of 0.57. Of these, 953 were correctly identified by the H-T. Of the 825 patients whom the GDP screened as negative, the H-T reached the same screening decision for 621 patients. The summary points for sensitivity and specificity were 0.89 and 0.75, respectively. This means that in a sample of 100 patients, in whom 57 had periodontal disease (based on the prevalence of 0.57), 6 individuals would remain undetected if screened by an H-T (false negatives), and 11 individuals without periodontal disease would be referred for treatment (false positives).
Discussion
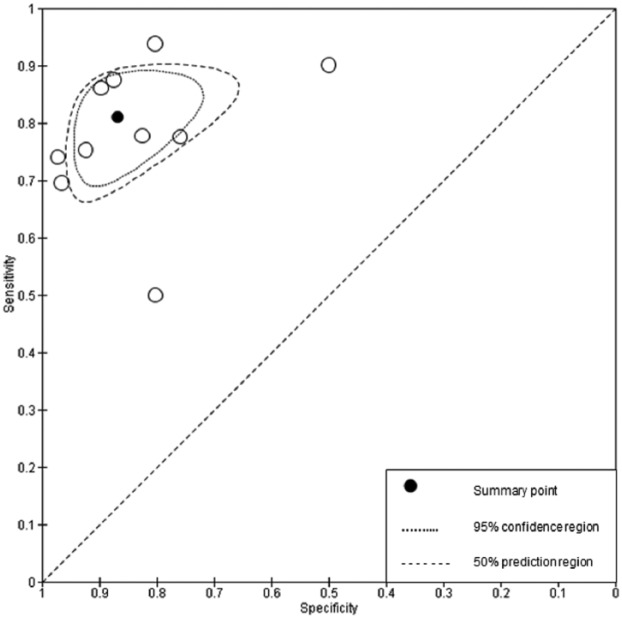
The summary sensitivity point (Figs. 3 and 4) for H-Ts screening for dental caries and periodontal disease suggest that H-Ts are capable of identifying disease in patients, where it exists. According to the criteria of Deeks and Altman (2004), as the diagnostic odds ratio was in excess of 20, with the positive likelihood ratio in excess of unity and the negative likelihood ratio being less than unity, the results would suggest that H-Ts are capable of screening for both dental caries and periodontal disease (Deeks and Altman 2004). There is a high certainty that when either disease exists, the H-T will correctly classify the patient as screen positive. The summary specificity point for dental caries also confirms their ability to correctly classify screen negatives in presenting patients (i.e., health).
Figure 3.
Caries; sensitivity and specificity per practice, plotted in receiver operating characteristic space.
Figure 4.
Periodontal disease; sensitivity and specificity per practice, plotted in receiver operating characteristic space.
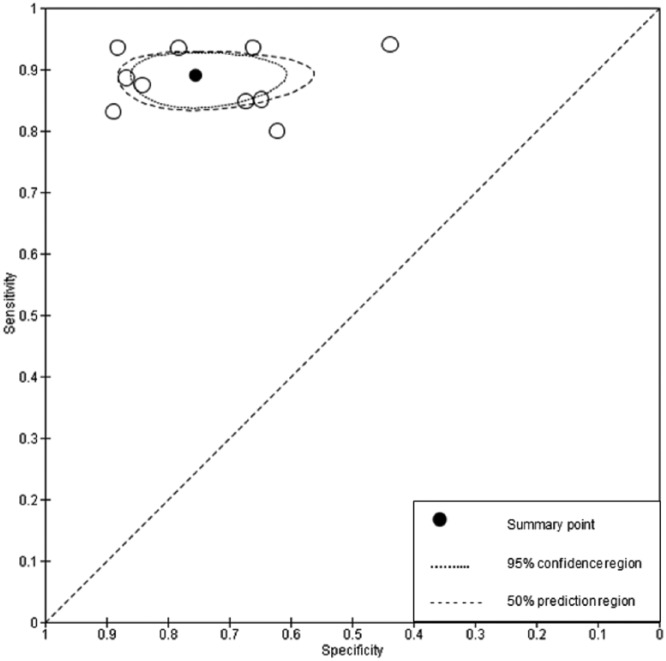
The summary specificity point for periodontal disease is lower than dental caries and suggests that there was greater difficulty in classifying healthy patients. This may be surprising as it is often thought that H-Ts are well skilled in the management of periodontal disease, as this currently reflects the majority of their workload (Godson et al. 2009). The results of this study would result in 204 patients of the 1,899 being falsely classified as screen positive (Fig. 2). The reasons for this could be that in borderline cases where the periodontal pocket was close to the 3.5-mm threshold, H-Ts were inclined to report a positive index test result to ensure that nothing would be missed, a trend seen in other studies where, if in doubt, the patient is referred on for further investigation (Brocklehurst et al. 2012). Alternatively, this may result from different judgment criteria being applied by GDPs and H-Ts when probing periodontal pockets (Velden and Vries 1980; Freed et al. 1983). Equally, it could be that the GDP was incorrectly classifying diseased patients as negative; it has been suggested that H-Ts could be better than GDPs at periodontal examinations, given the greater emphasis placed in this clinical area during their training (Rowbotham et al. 2009).
This leads to the main weakness of this study: the use of a clinician as a reference standard. The reference standard should be the best possible method of determining whether the patient presents with the disease being assessed (Manchikanti et al. 2009) and should be objective rather than subjective (Bossuyt et al. 2003). However, the clinical diagnostic test for dental caries is problematic (Wenzel and Hintze 1999), and it is widely accepted that different dentists often reach different diagnostic decisions (Bader and Shugars 1995). Means to overcome this would have been to use trained epidemiologists, consensus from a committee of dentists, or the use of radiographs to inform the decision. However, all of these options would have been problematic—the former two because of additional time constraints and the potential discomfort of multiple examinations, the latter being unethical for healthy patients. This was a pragmatic study and therefore used one GDP as the reference standard and ensured that the study was achievable within a reasonable time frame and had a minimal impact on the patient. It also reflected the potential model that could be used in large dental teams, should H-Ts be used in this screening role.
Figure 1 identifies that 120 of 1,899 patients were misclassified as false negative for caries (121 for periodontal disease; Fig. 2). Despite the relatively high sensitivity and negative predictive values, any false negatives are concerning. If these patients had been seen as part of a model of care in which they only saw an H-T, these patients with disease would have been misclassified as healthy. However, if these adult patients were being regularly screened, they would be seen again within a relatively short time frame. As such, the impact of false negatives would be ameliorated to a large degree, given that the progression of caries is relatively slow in low-risk adult populations and that they would be likely to be identified at the next screening appointment (Broadbent et al. 2008; van Gemert-Schriks et al. 2008; Stephenson et al. 2010). It also assumes that GDPs never misdiagnose, which is unlikely.
Of greater clinical significance are the positive and negative predictive values (Pretty and Maupome 2004). The negative predictive value for caries is particularly high and shows that when an H-T identifies a caries-free patient, there is a 90% likelihood that they do not have the disease. Conversely, the lower positive predictive value suggests that when in doubt, H-Ts refer on. This could be due to the difficulties in diagnosing borderline cases (i.e., H-Ts may have less experience in screening for caries). This would appear reasonable given the difficulties in caries diagnosis at all levels of the profession (Bader et al. 2001) and the proliferation of adjunctive aids to inform this decision. The forest plots (Figs. 1 and 2) and ROC space plots (Figs. 3 and 4) show a greater variation between practices for dental caries than periodontal disease, suggesting that the diagnostic task for the former is more difficult than the latter. The prevalence has been used to order the practices within the forest plots to assess any impact prevalence may have. The confidence intervals can be seen to widen for sensitivity (Fig. 1) when the prevalence is low, suggesting greater uncertainty in the screening decision when a low number of caries is present in the population.
A further issue is the artificial nature of the judgment ecology. The diagnostic thresholds used in this study would mean that a 75-year-old patient with a periodontal pocket of 3.6 mm on a single molar would be classed as diseased, when in reality, a risk assessment would be undertaken in conjunction with an assessment of the patient’s history to assess whether treatment was necessary. This study did not allow for an assessment of risk or aim to assess treatment planning decisions. Instead, it aimed to assess the ability of H-Ts to make correct screening decisions. This was deliberate given the potential variation that is observed when clinicians are asked to record risk (Petersson and Bratthall 2000; Persson et al. 2003).
Despite these issues, the results of the study suggest that H-Ts could be used more innovatively within new models of care to screen for disease in public-funded systems. Data on the number of attending asymptomatic patients within the United States are difficult to ascertain. However, data from the United Kingdom suggest that around half of all NHS clinical activity relates to routine examinations alone (Health and Social Care Information Centre [HSCIC] 2014) and less than a quarter of NHS patients are classified as being at high risk of disease (Department of Health 2014). This means that of the 13 million routine adult dental examinations conducted in the NHS from 2013 to 2014 (HSCIC 2014), 10 million could theoretically have been conducted by an H-T at significantly lower cost. Recommendations have been made to shift the dental paradigm from a “cure” to a “care” culture, and again, H-Ts could play a crucial role here (Gallagher and Wilson 2009). With H-Ts demonstrating an ability to screen for common oral diseases, their utility in new models of “care” with an emphasis on prevention appears promising. In turn, this has the potential to release resources at a practice level in public-funded systems to address the changes in population need and target care more effectively to reduce oral health inequalities.
Author Contributions
R. Macey, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Glenny, contributed to conception, design, and data interpretation, critically revised the manuscript; T. Walsh, H. Worthington, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; M. Tickle, contributed to conception and design, critically revised the manuscript; J. Ashley, contributed to design, critically revised the manuscript; P. Brocklehurst, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank the dental practices and patients who took part in this study.
Footnotes
Interim results were presented at the 2014 IADR and final results at PER.
This trial was funded by the National Institute for Health Research (NIHR) Clinician Scientist Award (NIHR/CS/010/004) and will be published in full in Health Technology Assessment. We acknowledge the support of the National Institute of Health Research Clinical Research Network (NIHR CRN). The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health. NHS support cost and NHS treatment costs were met by the Great Manchester Comprehensive Research Network. The University of Manchester acted as the sponsor.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bader JD, Shugars DA. 1995. Variation in dentists’ clinical decisions. J Public Health Dent. 55(3):181–188. [DOI] [PubMed] [Google Scholar]
- Bader JD, Shugars DA, Bonito AJ. 2001. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 65(10):960–968. [PubMed] [Google Scholar]
- Birch S. 2002. Health human resource planning for the new millennium: inputs in the production of health, illness, and recovery in populations. Can J Nurs Res. 33(4):109–114. [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC; Standards for Reporting of Diagnostic Accuracy Group. 2003. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Croat Med J. 44(5):635–638. [PubMed] [Google Scholar]
- Broadbent J, Thomson W, Poulton R. 2008. Trajectory patterns of dental caries experience in the permanent dentition to the fourth decade of life. J Dent Res. 87(1):69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst P, Ashley J, Walsh T, Tickle M. 2012. Relative performance of different dental professional groups in screening for occlusal caries. Community Dent Oral Epidemiol. 40(3):239–246. [DOI] [PubMed] [Google Scholar]
- Brocklehurst PR, Ashley JR, Tickle M. 2011. Patient assessment in general dental practice—risk assessment or clinical monitoring? Br Dent J. 210(8):351–354. [DOI] [PubMed] [Google Scholar]
- British Society of Periodontology (BSP). 2011. Basic periodontal examination (BPE) [accessed 2014 Dec 15]. http://www.bsperio.org.uk/publications/downloads/39_143748_bpe2011.pdf. [Google Scholar]
- Deeks JJ, Altman DG. 2004. Diagnostic tests 4: likelihood ratios. BMJ. 329(7458):168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health. 2014. NHS dental contract pilots—learning after first two years of piloting. [accessed 2014 Dec 15]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/282760/Dental_contract_pilots_evidence_and_learning_report.pdf.
- Dyer TA, Brocklehurst P, Glenny AM, Davies L, Tickle M, Issac A, Robinson PG. 2014. Dental auxiliaries for dental care traditionally provided by dentists. Cochrane Database Syst Rev. 8:CD010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flahault A, Cadilhac M, Thomas G. 2005. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol. 58(8):859–862. [DOI] [PubMed] [Google Scholar]
- Freed HK, Gapper RL, Kalkwarf KL. 1983. Evaluation of periodontal probing forces. J Periodontol. 54(8):488–492. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Wilson N. 2009. The future dental workforce? Br Dent J. 206(4):195–199. [DOI] [PubMed] [Google Scholar]
- Galloway J, Gorham J, Lambert M, Richards D, Russell D, Russell I, Welshman J. 2003. The professionals complementary to dentistry: systematic review and synthesis. [accessed 2014 Dec 15]. http://www.thedentalelf.net/wp-content/uploads/2012/03/PCD-Review-complete.pdf.
- Glick M, Monteiro da, Silva O, Seeberger GK, Xu T, Pucca G, Williams DM, et al. 2012. FDI Vision 2020: shaping the future of oral health. Int Dent J. 62(6):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson JH, Williams SA. 2008. Inequalities in health and oral health in the UK. Dent Update. 35(4):243–246, 248–250. [DOI] [PubMed] [Google Scholar]
- Godson JH, Williams SA, Csikar JI, Bradley S, Rowbotham JS. 2009. Dental therapy in the United Kingdom: part 2. A survey of reported working practices. Br Dent J. 207(9):417–423. [DOI] [PubMed] [Google Scholar]
- Health and Social Care Information Centre (HSCIC). (2014). NHS dental statistics for England—2013–14. [accessed 2014 Dec 15]. http://www.hscic.gov.uk/catalogue/PUB14738.
- House of Commons Health Select Committee (HSC). 2008. Dental Services—fifth report of session 2007–08. [accessed 2014 Dec 15]. http://www.publications.parliament.uk/pa/cm200708/cmselect/cmhealth/289/289i.pdf.
- Innes NP, Evans DJ. 2013. Evidence of improved access to dental care with direct access arrangements. Evid Based Dent. 14(2):36–37. [DOI] [PubMed] [Google Scholar]
- Irwig L, Bossuyt P, Glasziou P, Gatsonis C, Lijmer J. 2002. Evidence base of clinical diagnosis: designing studies to ensure that estimates of test accuracy are transferable. BMJ. 324(7338):669–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S, Prendergast MJ, Williams SA. 1996. The diagnostic reliability of clinical dental auxiliaries in caries prevalence surveys—a pilot study. Commun Dent Health. 13(3):145–149. [PubMed] [Google Scholar]
- Kwan SY, Prendergast MJ. 1998. The use of clinical dental auxiliaries as examiners in caries prevalence surveys in the United Kingdom: a feasibility study. Community Dent Oral Epidemiol. 26(3):194–200. [DOI] [PubMed] [Google Scholar]
- Laurant M, Harmsen M, Wollersheim H, Grol R, Faber M, Sibbald B. 2009. The impact of nonphysician clinicians: do they improve the quality and cost-effectiveness of health care services? Med Care Res Rev. 66(6):36S–89S. [DOI] [PubMed] [Google Scholar]
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. 2010. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 090. London (UK): The Cochrane Collaboration. [Google Scholar]
- Macey R, Walsh T, Glenny AM, Worthington H, Tickle M, Ashley J, Brocklehurst P. 2013. Protocol for diagnostic test accuracy study: the efficacy of screening for common dental diseases by dental care professionals. BMC Oral Health. 13(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Derby R, Wolfer L, Singh V, Datta S, Hirsch JA. 2009. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 5. Diagnostic accuracy studies. Pain Physician. 12(3):517–540. [PubMed] [Google Scholar]
- Matts JP, Lachin JM. 1988. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 9(4):327–344. [DOI] [PubMed] [Google Scholar]
- Milsom KM, Blinkhorn AS, Tickle M. 2008. The incidence of dental caries in the primary molar teeth of young children receiving National Health Service funded dental care in practices in the North West of England. Br Dent J. 205(7):E14; discussion 384–385. [DOI] [PubMed] [Google Scholar]
- Milsom KM, Jones C, Kearney-Mitchell P, Tickle M. 2009. A comparative needs assessment of the dental health of adults attending dental access centres and general dental practices in Halton & St Helens and Warrington PCTs 2007. Br Dent J. 206(5):257–261. [DOI] [PubMed] [Google Scholar]
- Nash DA, Friedman JW, Kardos TB, Kardos RL, Schwarz E, Satur J, Berg DG, Nasruddin J, Mumghamba EG, Davenport ES, et al. 2008. Dental therapists: a global perspective. Int Dent J. 58(2):61–70. [DOI] [PubMed] [Google Scholar]
- National Screening Committee (NSC). 2013. What is screening? UK National Screening Committee. [accessed 2014 Dec 15]. http://www.screening.nhs.uk/screening.#fileid7942.
- Patel R, Sprod A, Harwood P, Drugan C. 2012. The use of dental therapists as examiners in dental epidemiological surveys. Commun Dent Health. 29(3):195–197. [PubMed] [Google Scholar]
- Persson GR, Mancl L, Martin J, Page R. 2003. Assessing periodontal disease risk. J Am Dent Assoc. 134(5):575–582. [DOI] [PubMed] [Google Scholar]
- Petersson G, Bratthall D. 2000. Caries risk assessment: a comparison between the computer program ‘Cariogram’, dental hygienists and dentists. Swed Dent J. 24(4):129–137. [PubMed] [Google Scholar]
- Phillips E, Shaefer HL. 2013. Dental therapists: evidence of technical competence. J Dent Res. 92(7 Suppl):S11–S15. [DOI] [PubMed] [Google Scholar]
- Pretty IA, Maupome G. 2004. A closer look at diagnosis in clinical dental practice: Part 2. Using predictive values and receiver operating characteristics in assessing diagnostic accuracy. J Can Dent Assoc. 70(7):470–474. [PubMed] [Google Scholar]
- Rowbotham JS, Godson JH, Williams SA, Csikar JI, Bradley S. 2009. Dental therapy in the United Kingdom: Part 1. Developments in therapists’ training and role. Br Dent J. 207(8):355–359. [DOI] [PubMed] [Google Scholar]
- Sheiham A, Sabbah W. 2010. Using universal patterns of caries for planning and evaluating dental care. Caries Res. 44(2):141–150. [DOI] [PubMed] [Google Scholar]
- Steele J. 2009. NHS dental services in England. London (UK): Department of Health. [Google Scholar]
- Stephenson J, Chadwick BL, Playle RA, Treasure ET. 2010. Modelling childhood caries using parametric competing risks survival analysis methods for clustered data. Caries Res. 44(1):69–80. [DOI] [PubMed] [Google Scholar]
- Tickle M, Milsom K. 2008. The whole population approach to caries prevention in general dental practice. Br Dent J. 205(10):521–521. [DOI] [PubMed] [Google Scholar]
- Turner S, Tripathee S, MacGillivray S. 2012. Benefits and risks of direct access to treatment by dental care professionals: a rapid evidence review. Final report to the General Dental Council. [accessed 2014 Dec 15]. http://www.gdc-uk.org/newsandpublications/research/documents/final%20version%20of%20literature%20review%20on%20direct%20access.pdf.
- van Gemert-Schriks MC, van Amerongen WE, ten Cate JM, Aartman IH. 2008. The effect of different dental treatment strategies on the oral health of children: a longitudinal randomised controlled trial. Clin Oral Investig. 12(4):361–368. [DOI] [PubMed] [Google Scholar]
- Velden U, Vries J. 1980. The influence of probing force on the reproducibility of pocket depth measurements. J Clin Periodontol. 7(5):414–420. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Hintze H. 1999. The choice of gold standard for evaluating tests for caries diagnosis. Dentomaxillofac Radiol. 28(3):132–136. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 1997. Oral health surveys—basic methods. 4th ed. Geneva (Switzerland): WHO; [accessed 2014 Dec 15]. http://www.paho.org/hq/dmdocuments/2009/OH_st_Esurv. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.