Abstract
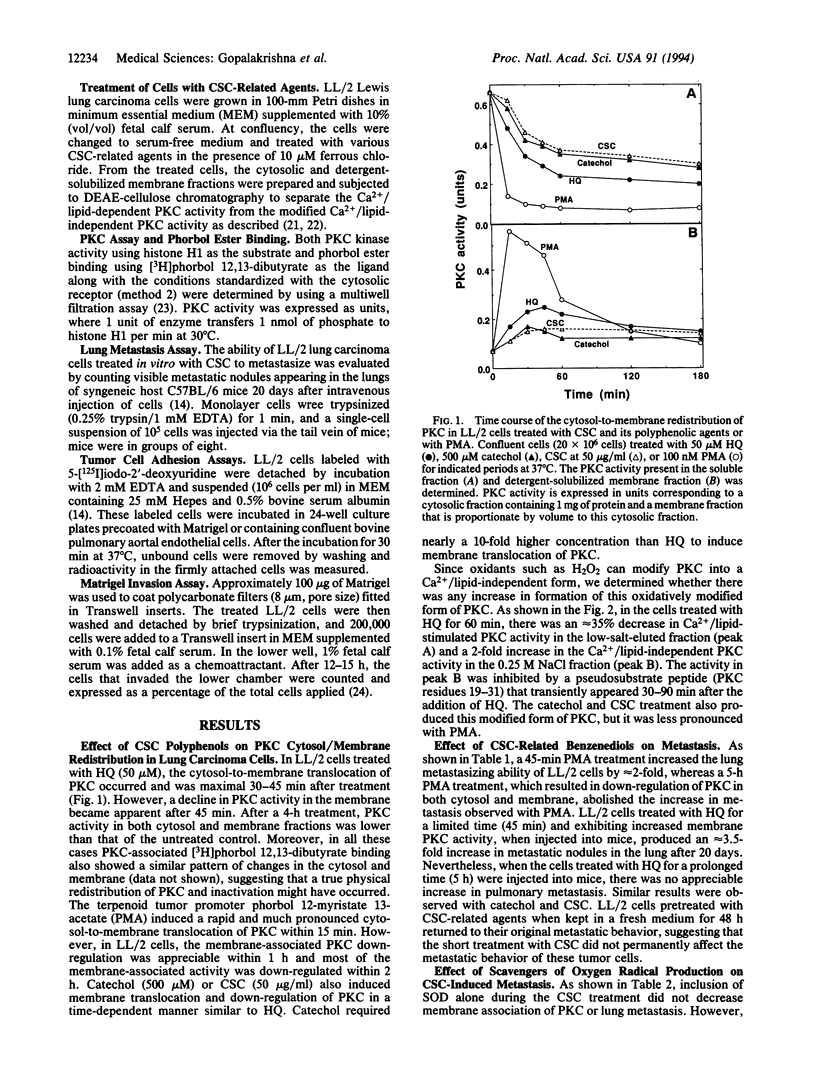
Cigarette smoke polyphenolic agents (catechol and hydroquinone) that generate oxidants have been shown to be tumor promoters. Furthermore, oxidants can influence protein kinase C (PKC)-mediated signal transduction. Since terpenoid tumor promoters, phorbol esters, increase invasion and metastasis by activating PKC, we have determined whether polyphenolic agents present in the cigarette smoke condensate (CSC) could also influence these events. Hydroquinone (50 microM), catechol (500 microM), or CSC (50 micrograms/ml) induced an initial cytosol-to-membrane translocation of PKC in LL/2 lung carcinoma cells, followed by a later down-regulation of the enzyme. LL/2 cells treated with these CSC-related agents for a limited time (45 min) and exhibiting high membrane-associated PKC activity, when injected into mice through the tail vein, produced an increase in metastatic nodules in the lungs after 20 days. However, cells treated with CSC-related agents for a prolonged period did not exhibit an increase in metastasis. Agents that decrease the rate of production of reactive oxygen species, such as catalase either alone or in combination with superoxide dismutase, and a cell-permeable iron-chelator, o-phenanthroline, inhibited CSC-mediated membrane association of PKC and metastasis. Prior treatment of CSC with tyrosinase to modify polyphenols resulted in a partial loss of CSC stimulation of metastasis. Furthermore, a cell-permeable Ca2+ chelator and diverse PKC inhibitors, such as calphostin C, hypericin, chelerythrine, and bisindolylmaleimide, inhibited CSC-enhanced metastasis. CSC increased in vitro tumor cell adhesion to endothelial monolayers and to reconstituted basement membrane (Matrigel) and also enhanced the invasion through Matrigel coated on the polycarbonate filters in Transwells. All these CSC effects were found to be temporary and were blocked by the above mentioned antioxidant systems and PKC inhibitors. Thus, these results suggest that the oxidants generated by autooxidation of polyphenolic agents present in tobacco smoke increase tumor cell invasion and metastasis, at least in part by activation of Ca2+/PKC signal transduction. Conceivably, cigarette smoke constituents not only promote tumorigenesis but also may increase the spread of cancer in the body.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Daniell H. W. Increased lymph node metastases at mastectomy for breast cancer associated with host obesity, cigarette smoking, age, and large tumor size. Cancer. 1988 Jul 15;62(2):429–435. doi: 10.1002/1097-0142(19880715)62:2<429::aid-cncr2820620230>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Dumont J. A., Jones W. D., Jr, Bitonti A. J. Inhibition of experimental metastasis and cell adhesion of B16F1 melanoma cells by inhibitors of protein kinase C. Cancer Res. 1992 Mar 1;52(5):1195–1200. [PubMed] [Google Scholar]
- Felder C. C., Ma A. L., Liotta L. A., Kohn E. C. The antiproliferative and antimetastatic compound L651582 inhibits muscarinic acetylcholine receptor-stimulated calcium influx and arachidonic acid release. J Pharmacol Exp Ther. 1991 Jun;257(3):967–971. [PubMed] [Google Scholar]
- Ferson M., Edwards A., Lind A., Milton G. W., Hersey P. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer. 1979 May 15;23(5):603–609. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch Biochem Biophys. 1991 Mar;285(2):382–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Barsky S. H. Tumor promoter-induced membrane-bound protein kinase C regulates hematogenous metastasis. Proc Natl Acad Sci U S A. 1988 Jan;85(2):612–616. doi: 10.1073/pnas.85.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Chen Z. H., Gundimeda U. Irreversible oxidative inactivation of protein kinase C by photosensitive inhibitor calphostin C. FEBS Lett. 1992 Dec 14;314(2):149–154. doi: 10.1016/0014-5793(92)80962-g. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Chen Z. H., Gundimeda U., Wilson J. C., Anderson W. B. Rapid filtration assays for protein kinase C activity and phorbol ester binding using multiwell plates with fitted filtration discs. Anal Biochem. 1992 Oct;206(1):24–35. doi: 10.1016/s0003-2697(05)80006-5. [DOI] [PubMed] [Google Scholar]
- Greenlee W. F., Sun J. D., Bus J. S. A proposed mechanism of benzene toxicity: formation of reactive intermediates from polyphenol metabolites. Toxicol Appl Pharmacol. 1981 Jun 30;59(2):187–195. doi: 10.1016/0041-008x(81)90189-7. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Maffrand J. P. Tumor cell adherence to cultured capillary endothelial cells is promoted by activators of protein kinase C. Biochem Pharmacol. 1991 Jun 21;42(1):163–170. doi: 10.1016/0006-2952(91)90695-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Hecht S. S., Wynder E. L. Tumor promoters and cocarcinogens in tobacco carcinogenesis. Environ Health Perspect. 1983 Apr;50:247–257. doi: 10.1289/ehp.8350247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn K. V., Onoda J. M., Pampalona K., Battaglia M., Neagos G., Taylor J. D., Diglio C. A., Sloane B. F. Inhibition by dihydropyridine class calcium channel blockers of tumor cell-platelet-endothelial cell interactions in vitro and metastasis in vivo. Biochem Pharmacol. 1985 Jan 15;34(2):235–241. doi: 10.1016/0006-2952(85)90130-3. [DOI] [PubMed] [Google Scholar]
- Isakov N., Gopas J., Priel E., Segal S., Altman A. Effect of protein kinase C activating tumor promoters on metastases formation by fibrosarcoma cells. Invasion Metastasis. 1991;11(1):14–24. [PubMed] [Google Scholar]
- Johnston-Early A., Cohen M. H., Minna J. D., Paxton L. M., Fossieck B. E., Jr, Ihde D. C., Bunn P. A., Jr, Matthews M. J., Makuch R. Smoking abstinence and small cell lung cancer survival. An association. JAMA. 1980 Nov 14;244(19):2175–2179. [PubMed] [Google Scholar]
- Kensler T. W., Trush M. A. Role of oxygen radicals in tumor promotion. Environ Mutagen. 1984;6(4):593–616. doi: 10.1002/em.2860060412. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Iizasa T., Momiki S., Garcia-Palazzo I., Caamano J., Metcalf R., Welsh J., Harris C. C. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6693–6697. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak B., Whale C., Kerbel R. S. Possible involvement of Ca2+ mobilization and protein kinase C activation in the induction of spontaneous metastasis by mouse mammary adenocarcinoma cells. Cancer Res. 1989 May 15;49(10):2597–2602. [PubMed] [Google Scholar]
- Lafrenie R., Shaughnessy S. G., Orr F. W. Cancer cell interactions with injured or activated endothelium. Cancer Metastasis Rev. 1992 Nov;11(3-4):377–388. doi: 10.1007/BF01307188. [DOI] [PubMed] [Google Scholar]
- Leanderson P., Tagesson C. Cigarette smoke-induced DNA damage in cultured human lung cells: role of hydroxyl radicals and endonuclease activation. Chem Biol Interact. 1992 Jan;81(1-2):197–208. doi: 10.1016/0009-2797(92)90034-i. [DOI] [PubMed] [Google Scholar]
- Leanderson P., Tagesson C. Cigarette smoke-induced DNA-damage: role of hydroquinone and catechol in the formation of the oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chem Biol Interact. 1990;75(1):71–81. doi: 10.1016/0009-2797(90)90023-g. [DOI] [PubMed] [Google Scholar]
- Liu B., Timar J., Howlett J., Diglio C. A., Honn K. V. Lipoxygenase metabolites of arachidonic and linoleic acids modulate the adhesion of tumor cells to endothelium via regulation of protein kinase C. Cell Regul. 1991 Dec;2(12):1045–1055. doi: 10.1091/mbc.2.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. P., Mossman B. T. Role of asbestos and active oxygen species in activation and expression of ornithine decarboxylase in hamster tracheal epithelial cells. Cancer Res. 1991 Jan 1;51(1):167–173. [PubMed] [Google Scholar]
- Nakayama T., Church D. F., Pryor W. A. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med. 1989;7(1):9–15. doi: 10.1016/0891-5849(89)90094-4. [DOI] [PubMed] [Google Scholar]
- Nassi-Calò L., Mello-Filho C., Meneghini R. o-phenanthroline protects mammalian cells from hydrogen peroxide-induced gene mutation and morphological transformation. Carcinogenesis. 1989 Jun;10(6):1055–1057. doi: 10.1093/carcin/10.6.1055. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Picardo M., Passi S., Nazzaro-Porro M., Breathnach A., Zompetta C., Faggioni A., Riley P. Mechanism of antitumoral activity of catechols in culture. Biochem Pharmacol. 1987 Feb 15;36(4):417–425. doi: 10.1016/0006-2952(87)90345-5. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993 May 28;686:12–28. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- Qian M. W., Eaton J. W. Tobacco-borne siderophoric activity. Arch Biochem Biophys. 1989 Nov 15;275(1):280–288. doi: 10.1016/0003-9861(89)90374-3. [DOI] [PubMed] [Google Scholar]
- Repesh L. A. A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis. 1989;9(3):192–208. [PubMed] [Google Scholar]
- Robertson M. L., Eastmond D. A., Smith M. T. Two benzene metabolites, catechol and hydroquinone, produce a synergistic induction of micronuclei and toxicity in cultured human lymphocytes. Mutat Res. 1991 Jul;249(1):201–209. doi: 10.1016/0027-5107(91)90147-g. [DOI] [PubMed] [Google Scholar]
- Schwartz G. K., Redwood S. M., Ohnuma T., Holland J. F., Droller M. J., Liu B. C. Inhibition of invasion of invasive human bladder carcinoma cells by protein kinase C inhibitor staurosporine. J Natl Cancer Inst. 1990 Nov 21;82(22):1753–1756. doi: 10.1093/jnci/82.22.1753. [DOI] [PubMed] [Google Scholar]
- Stenius U., Warholm M., Rannug A., Walles S., Lundberg I., Högberg J. The role of GSH depletion and toxicity in hydroquinone-induced development of enzyme-altered foci. Carcinogenesis. 1989 Mar;10(3):593–599. doi: 10.1093/carcin/10.3.593. [DOI] [PubMed] [Google Scholar]
- Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Hirose M., Fukushima S., Hasegawa R., Ito N. Modification by catechol and resorcinol of upper digestive tract carcinogenesis in rats treated with methyl-N-amylnitrosamine. Cancer Res. 1989 Nov 1;49(21):6015–6018. [PubMed] [Google Scholar]