Abstract
Periostin is a mesenchymal cell marker predominantly expressed in collagen-rich fibrous connective tissues, including heart valves, tendons, perichondrium, periosteum, and periodontal ligament (PDL). Knockdown of periostin expression in mice results in early-onset periodontitis and failure of cardiac healing after acute myocardial infarction, suggesting that periostin is essential for connective tissue homeostasis and regeneration. However, its role(s) in periodontal tissues has not yet been fully defined. In this study, we describe a novel human isoform of periostin (PDL-POSTN). Isoform-specific analysis by reverse-transcription polymerase chain-reaction (RT-PCR) revealed that PDL-POSTN was predominantly expressed in the PDL, with much lower expression in other tissues and organs. A PDL cell line transfected with PDL-POSTN showed enhanced alkaline phosphatase (ALPase) activity and calcified nodule formation, compared with cells transfected with the full-length periostin isoform. A neutralizing antibody against integrin-αv inhibited both ALPase activity and calcified nodule formation in cells transfected with PDL-POSTN. Furthermore, co-immunoprecipitation assays revealed that PDL-POSTN bound to integrin αvβ3 more strongly than the common isoform of periostin, resulting in strong activation of the integrin αvβ3-focal adhesion kinase (FAK) signaling pathway. These results suggest that PDL-POSTN positively regulates cytodifferentiation and mineralization in PDL cells through integrin αvβ3.
Keywords: cell differentiation, cementoblasts, osteoblasts, periodontal tissues/periodontium, integrin, mineralization
Introduction
The periodontal ligament (PDL) secures teeth to the alveolar bone, thereby providing support, protection, and sensory input for the masticatory system. PDL tissues also contain multipotent mesenchymal stem cells that can differentiate into mineralized tissue-forming cells, such as osteoblasts and cementoblasts (Seo et al., 2004). Thus, the PDL is thought to play crucial roles not only in the homeostasis of periodontal tissues but also in bone remodeling, wound healing, and tissue regeneration (Beertsen et al., 1997).
Periostin is found within the PDL and is a key extracellular matrix protein involved in periodontal tissue homeostasis (Kii and Kudo, 2007; Anastasilakis et al., 2013; Romanos et al., 2013). Periostin knockout (KO) mice are unable to sustain normal physiological occlusal forces, resulting in a rapid deterioration of the functional and structural integrity of the periodontium, advanced alveolar bone loss, severe clinical attachment loss, and significant widening of the PDL region (Rios et al., 2005). However, the molecular mechanisms by which periostin functions in the PDL are yet to be delineated.
Periostin was originally identified in MC3T3-E1 cells, and its messenger ribonucleic acid (mRNA) undergoes alternative mRNA splicing, generating several C-terminal domain variants (Takeshita et al., 1993; Horiuchi et al., 1999). A heart-specific isoform of mouse periostin plays important roles in the regeneration of heart tissue (Shimazaki et al., 2008), while periostin-like factor (PLF), another isoform of mouse periostin, is expressed in bone, heart, and vascular smooth muscle (Litvin et al., 2004, 2005; Zhu et al., 2009). These reports suggest that tissue-specific isoforms of periostin play important roles in the regulation of tissue-specific functions.
In this study, we examine the expression and function of periostin in PDL cells. We demonstrate specific and strong expression of periostin in PDL tissues and cells in human and mouse samples and reveal that periostin expression is strongly induced during, and may therefore regulate, PDL cell differentiation. Interestingly, we found a novel isoform of periostin in human PDL cells, which is specific to the PDL, and we named this periodontal ligament-specific periostin (PDL-POSTN). The actions of PDL-POSTN indicate that it positively regulates cytodifferentiation and mineralization of PDL cells through integrin αvβ3.
Materials & Methods
RNA Extraction and Real-time PCR Analysis
The clinical aspects of this study were approved by the Institutional Ethics Committee, and informed consent was obtained from all volunteers for the use of their tissues. Details of RNA extraction and real-time PCR analysis are described in the online Appendix.
Tissue Preparation and Immunohistochemical Staining
All animal experiments were approved by the Institutional Animal Care and Use Committee. Eight-week-old C57BL/6 mice were acquired from SLC (Shizuoka, Japan) immediately prior to experimentation. Tissue preparation and immunohistochemical staining were performed as previously described (Hou et al., 2012). Details are described in the online Appendix.
Western Blot Analysis
Cells underwent lysis with RIPA lysis buffer (Millipore, Billerica, MA, USA). The protein concentrations of the cell lysates were measured by a Bradford-595 assay (Bio-Rad, Hercules, CA, USA). Aliquots of cell lysates (30 µg protein), cell culture supernatants (25 μL), or immunoprecipitates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes. Details are described in the online Appendix.
Cell Culture and Induction of PDL Cell Cytodifferentiation
Human PDL (HPDL) cells, dental pulp (HDP) cells, gingival fibroblasts (HGFs), and gingival epithelial cells (HGEs) were cultured according to previously described methods (Murakami et al., 2001, 2002; Shimabukuro et al., 2005, 2009). We previously established a mouse PDL cell line, MPDL22, and used this as described in our earlier publication (Yamada et al., 2007). Details are described in the online Appendix.
Isolation of Periostin Isoforms from Human PDL Cells
We performed RT-PCR using PrimeSTAR HS DNA Polymerase (Takara Bio, Shiga, Japan) to amplify the open-reading frame of periostin in cDNA derived from human PDL cells. The primers were: (sense) 5′-AGA GAC TCA AGA TGA TTC CCT-3′ and (FLAG®-tagged antisense) 5′-TCA TCA CTT GTC ATC GTC GTC CTT GTA ATC CTG AGA ACG ACC TTC CCT TA-3′. The PCR products were cloned into pGEM-T (Promega, Madison, WI, USA) and sequenced. The human PDL-POSTN sequences were deposited in GenBank under accession number AY918092.
Plasmids and Transfection
The adenovirus and plasmid constructs used are shown in the Appendix Fig. Details on vector construction and transfection are described in the online Appendix.
RNA Interference of Periostin
A small interfering RNA (siRNA) oligonucleotide against the mouse periostin 5′-region was designed to knock down all periostin transcripts, according to Reynolds et al. (2004). The target sequences included 5′-AAG GGT CAT ACA CGT ACT TCG-3′ (siRNA-1) and 5′-AAC CTG GAT TCT GAC ATT CGC-3′ (siRNA-2), which are located at nucleotides 410-430 and 453-474 of the mouse periostin gene, respectively. Details are described in the online Appendix.
Determination of Alkaline Phosphatase Activity and Calcified Nodule Formation
Alkaline phosphatase (ALPase) activity was measured as previously described (Yamada et al., 2007). Alizarin red staining of calcified nodules was performed by a method described by Dahl (1952).
Co-immunoprecipitation Assay with Conditioned Medium Containing Recombinant Periostin Isoforms
A 500-µL aliquot of conditioned medium containing recombinant FLAG-tagged PDL-POSTN or full-length periostin was incubated with 7.5 μL of anti-FLAG antibody-conjugated agarose (Santa Cruz Biotech, Santa Cruz, CA, USA) for 1 hr at 4°C. After being washed, 1.0 µg of recombinant integrin αvβ3 (R&D Systems, Minneapolis, MN, USA) was added and incubated for 1 hr at 4°C in 0.5 mL of binding buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, and complete protease inhibitor cocktail (Roche, Indianapolis, IN, USA)]. The precipitates were washed three times with binding buffer and subjected to 10% SDS-PAGE for Western blot analysis by standard techniques. Details on the preparation of conditioned medium containing recombinant FLAG-tagged PDL-POSTN, full-length periostin, or β-galactosidase (as a control) are in the online Appendix.
3H-thymidine Incorporation Assay
We assessed the proliferation activity of transfected MPDL22 cells by measuring 3H-thymidine incorporation. Details are described in the online Appendix.
Intracellular Signaling Assay by Immobilized PDL-POSTN Stimulation
Aliquots of conditioned medium (500 µL) containing recombinant FLAG-tagged PDL-POSTN or full-length periostin were incubated in 12-well culture dishes for 12 hrs at 4°C to facilitate protein immobilization. The culture dishes were washed three times with PBS, and MPDL22 cells were then plated into the dishes (1×106 cells/well). After stimulation at the indicated time-points, cell lysates were subjected to SDS-PAGE.
Statistical Analysis
Data are presented as the mean ± standard deviation (mean ± SD). Statistical analyses were performed by Student’s t test (for paired comparisons) and one-way ANOVA followed by Bonferroni’s post hoc comparison test for multiple comparisons. A value of p < .05 was considered statistically significant.
Results
Specific and High Levels of Periostin Expression in the Periodontal Ligament
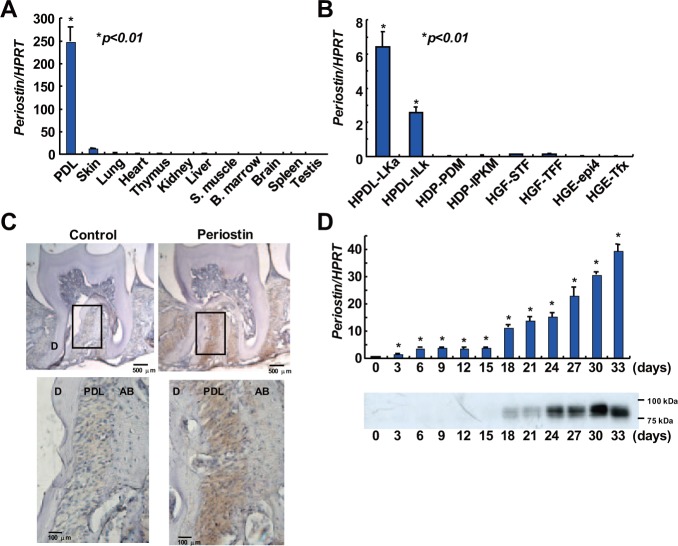
We used real-time PCR analysis to determine periostin mRNA levels in various human tissues (Fig. 1A). The highest level of periostin expression was seen in the PDL compared with other tissues, including skin and lung, which are recognized as periostin-positive tissues. We then assessed periostin expression in various human cell lines derived from human oral tissues (Fig. 1B). PDL cells showed higher levels of periostin mRNA compared with dental pulp cells, gingival fibroblasts, and gingival epithelial cells. Immunohistochemical analyses of mouse maxilla specimens with an anti-periostin polyclonal antibody reactive for the periostin common region showed strong and specific expression of periostin in PDL tissues (Fig. 1C).
Figure 1.
Periostin is specifically expressed in periodontal ligament tissue and cells and is induced during PDL cell differentiation. Real-time PCR analysis of periostin in various human tissues (A) and human oral-tissue-derived cell lines (B). Values represent the mean ± SD of triplicate assays. *p < .01, compared with other tissues. **p < .01, compared with other cell lines. (C) Specific expression of periostin in PDL tissues in vivo. Periostin protein was detected in 8-week-old mouse periodontium by immunohistochemical staining with an anti-periostin antibody. D, dentin; PDL, periodontal ligament; AB, alveolar bone. (D) Induction of periostin mRNA and protein during PDL cell differentiation. Human PDL cells were cultured in mineralization-inducing medium. Periostin expression was assessed by real-time PCR analysis (upper panel) and Western blot analysis (lower panel) on every third day of culture. Values represent the mean ± SD of triplicate assays. *p < .01, compared with day 0.
PDL cells can differentiate into hard-tissue-forming cells, leading to the formation of mineralized nodules in in vitro cultures. To assess the possible association of periostin expression with PDL cell differentiation, we analyzed periostin expression in PDL cells during the cell differentiation process. As shown in Fig. 1D, the abundance of both periostin mRNA and protein increased during cell differentiation.
Identification of a Periodontal-ligament-specific Isoform of Periostin
A previous study reported that mouse periostin has several isoforms that vary in their C-termini (Horiuchi et al., 1999). Interestingly, by using an anti-periostin common region polyclonal antibody, we observed multiple periostin proteins of different sizes in human PDL cells (Fig. 1D), suggesting the existence of several human periostin isoforms, similar to those seen in mice. To identify the periostin isoforms present in human tissues, we performed RT-PCR analysis of the periostin 3′-region in cDNA from human PDL cells. Multiple PCR amplicons were generated, indicating several isoforms that include this region (Fig. 2A). We then attempted to isolate and analyze the full-length mRNAs of these alternatively spliced isoforms. We completed RT-PCR using universal primers that anneal to the open-reading frame of human periostin mRNA. We then cloned the amplified products into plasmid vectors. We sequenced these cDNA clones and made a profile of the periostin isoforms present in human PDL cells (Fig. 2A). From this, we identified an isoform whose strong expression in PDL cells was entirely novel. We have named this isoform periodontal-ligament-specific periostin, or PDL-POSTN. RT-PCR analysis with specific primers for the PDL-POSTN isoform revealed that it was expressed only in PDL tissues (Fig. 2B) and human PDL cells (Fig. 2C). The low sensitivity of conventional RT-PCR analysis might be the cause of differences in the expression ratio of the full-length periostin isoform (termed Type I) and PDL-POSTN, as seen in Figs. 2A, 2B, and 2C.
Figure 2.
A novel isoform of periostin isolated from human PDL cells shows specific expression in PDL tissue. (A) Periostin isoforms isolated from human PDL cells. RT-PCR analysis of the 3′-region of periostin generated multiple amplicons. This illustration of the C-terminal region of isolated periostin isoforms shows exon-intron arrangement, expression frequency, and numbers of amino acids. MW, molecular weight; HPDL, human periodontal ligament cells; RD1, repeated domain 1; RD2, repeated domain 2; RD3, repeated domain 3; RD4, repeated domain 4; Frequency, expression frequency in the cDNA profile; AA, numbers of amino acids in the isoform. Isoform-specific RT-PCR analysis of periostin in mRNA from the indicated tissues (B) and human oral-tissue-derived cell lines (C). RT-PCR primers annealing to exons a and c′ were used. The size of each amplified isoform is indicated on the right of the gel image. The number of PCR cycles is shown above each lane in (B). The number of PCR cycles in (C) is 30. HPRT, hypoxanthine phosphoribosyl transferase. Results shown are representative of three independent experiments.
PDL-POSTN Enhances the Course of PDL Cell Differentiation
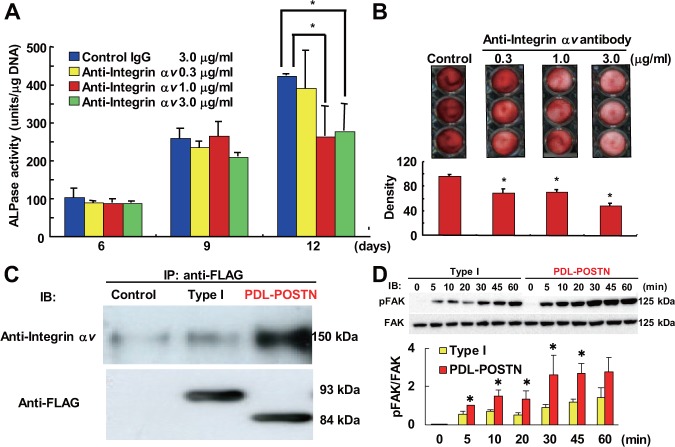
To identify the function(s) of PDL-POSTN, we transfected the mouse periodontal ligament cell line, MPDL22, with a PDL-POSTN expression vector. We also transfected MPDL22 cells with the full-length periostin isoform (Type I) expression vector or with a LacZ expression vector as a negative control. We cultured the transfected MPDL22 cells in mineralization-inducing medium and measured the ALPase activity (Fig. 3A). ALPase activity was significantly enhanced in the PDL-POSTN transfectants. Furthermore, Alizarin red staining of calcified nodules showed that the formation of these nodules was significantly elevated in the PDL-POSTN transfectants compared with the two controls (cells expressing no periostin or expressing periostin Type I). Transfected cells were stimulated with 10% fetal calf serum (FCS), and their proliferative activity was assessed (Fig. 3C). Cells transfected with PDL-POSTN and full-length periostin had proliferative responses equivalent to those of FCS stimulation. Because it is difficult to knock down PDL-POSTN specifically in human PDL cells, we examined the effects of general periostin knockdown in MPDL22 cells on the course of cell mineralization. We established MPDL22-transfected cells containing shRNA for common sequences of mouse periostin genes. We obtained two different transfectant cell lines, shRNA-1 and shRNA-2. These cell lines showed reduced periostin expression at the mRNA and protein levels (Fig. 3D). We cultured shRNA-1 and shRNA-2 in mineralization-inducing medium and measured ALPase activity (Fig. 3E). ALPase activities were significantly inhibited in the shRNA-1 and shRNA-2 cells compared with the negative control cells.
Figure 3.
PDL-POSTN induces PDL cell differentiation more strongly than the full-length isoform of periostin. (A) PDL-POSTN enhances ALPase activities. MPDL22 cells transfected with control vector, the Type I isoform of periostin, or PDL-POSTN were cultured in mineralization-inducing medium, and ALPase activities were measured on every third day of culture. (B) PDL-POSTN enhanced calcified nodule formation. Alizarin red staining was performed on day 12. The image illustrates the alizarin red staining in each group, with the staining densities quantified in the graph below. (C) PDL-POSTN did not influence proliferation activity. [3H]-thymidine incorporation into transfected MPDL22 cells after stimulation with 10% FCS is shown. Values are the mean ± SD of triplicate assays. (D) Confirmation of stable knockdown of periostin at the mRNA level by real-time PCR analysis (left) and at the protein level by Western blot analysis of the culture supernatants (right). Periostin shRNA-transfected MPDL22 cells cultured in mineralization-inducing medium were analyzed on day 9. (E) Stable knockdown of periostin decreased ALPase activities. Control, MPDL22 cells transiently transfected with a control vector; Type I, MPDL22 cells transiently transfected with a vector expressing periostin type I isoform; PDL-POSTN, MPDL22 cells transiently transfected with a vector expressing PDL-POSTN; shRNA-1, MPDL22 cells stably transfected with mouse periostin siRNA-1 sequences; shRNA-2, MPDL22 cells stably transfected with mouse periostin siRNA-2 sequences; shControl, MPDL22 cells stably transfected with control siRNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. This figure is available in color online at http://jdr.sagepub.com.
PDL-POSTN Positively Regulates PDL Cell Differentiation via Direct Interaction with Integrin αvβ3
To clarify how PDL-POSTN regulates PDL cell differentiation, we cultured the PDL-POSTN transfectants with mineralization-inducing medium in the presence or absence of anti-integrin αv neutralizing antibody. Anti-integrin αv neutralizing antibody significantly inhibited ALPase activity and calcified nodule formation in PDL-POSTN-transfected cells (Figs. 4A and 4B, respectively). Immunoprecipitation experiments with the recombinant FLAG-tagged PDL-POSTN protein and integrin αvβ3 demonstrated that integrin αvβ3 co-immunoprecipitated with periostin (Fig. 4C). Interestingly, PDL-POSTN showed a stronger interaction with integrin αvβ3 than did the full-length periostin isoform. We then assessed whether PDL-POSTN activated focal adhesion kinase (FAK), which is downstream of integrin αvβ3 in this intracellular signaling pathway (Schaller et al., 1992). Western blot analysis with anti-phosphorylated FAK antibody revealed that PDL-POSTN stimulation induced a significantly stronger phosphorylation of FAK in MPDL22 cells than did the full-length periostin isoform (Fig. 4D).
Figure 4.
PDL-POSTN positively regulates PDL cell differentiation through a direct interaction with integrin αvβ3. (A) MPDL22 cells transfected with PDL-POSTN were cultured in mineralization-inducing medium in the presence of a neutralizing antibody against integrin αv. ALPase activity of these cells was measured on the indicated day of culture. Values are the mean ± SD of triplicate assays. (B) Cells prepared the same way as in (A) were treated with an anti-integrin αv neutralizing antibody to investigate its effect on calcified nodule formation induced by PDL-POSTN. Alizarin red staining was performed on day 12. The image illustrates alizarin red staining, while the chart below shows the quantification of this staining. Values are the mean ± SD of triplicate assays. (C) FLAG-tagged recombinant periostin was incubated with recombinant integrin αvβ3 and immunoprecipitated with an anti-FLAG antibody. Co-precipitated recombinant integrin αvβ3 was detected with an anti-integrin αv antibody (upper panel). Input recombinant periostin was detected with anti-FLAG antibody (lower panel). (D) MPDL22 cells were stimulated with immobilized Type I periostin or PDL-POSTN at the indicated times, and phosphorylated FAK (pFAK) was detected by Western blot analysis. Quantitative analysis is shown as the ratios of phosphorylated-FAK to total FAK, determined by densitometric analysis. Values represent the mean ± SD of three independent assays. *p < .05 compared with Type I periostin. Control, conditioned medium derived from MPDL22 cells infected with a LacZ-expressing adenovirus; Type I, conditioned medium derived from MPDL22 cells infected with an adenovirus expressing the periostin type I isoform; PDL-POSTN, conditioned medium derived from cells infected with an adenovirus expressing PDL-POSTN. This figure is available in color online at http://jdr.sagepub.com.
Discussion
In this study, we have identified a novel isoform of periostin that is specifically expressed in the PDL, and have named it PDL-POSTN. We have demonstrated that PDL-POSTN positively regulates PDL cell differentiation through a direct interaction with integrin αvβ3. These findings suggest that PDL-POSTN influences the differentiation of PDL cells into hard-tissue-forming cells such as cementoblasts and osteoblasts, which function in the homeostasis and repair of PDL tissue.
Litvin et al. (2004) reported that periostin-like-factor (PLF), a novel alternatively spliced isoform of periostin, promotes osteogenesis. PLF is expressed in periosteal mesenchymal cells and in trabecular bone osteoblasts, where it accelerates proliferation and differentiation. Although both PDL-POSTN and PLF promote the differentiation of the cells that form hard tissues, PLF and PDL-POSTN are specifically expressed in bone and PDL tissues, respectively. We also confirmed that PDL-POSTN and PLF are independent isoforms. Notably, Western blot analysis with the anti-periostin common region polyclonal antibody detected only a single band in MPDL22 cells (Fig. 3D). Because MPDL22 cells are a monoclonal cell line (Yamada et al., 2007), this indicates that this single cell clone produces only a single periostin isoform. These findings suggest that tissue-specific transcriptional regulation of periostin is tightly controlled in each tissue, organ, and cell type.
It has been reported elsewhere that periostin is a ligand for integrin αvβ3 and acts during cancer metastasis and heart myoblast regeneration after myocardial infarction (Bao et al., 2004; Kuhn et al., 2007; Shimazaki et al., 2008). Integrin αvβ3 is involved in many biological functions, including cell adhesion, proliferation, tissue repair, and bone metabolism (Danhier et al., 2012), notably acting in concert with BMP2 to promote osteoblast differentiation (Lai and Cheng, 2005). Furthermore, integrin αvβ3 acts on osteoblasts to accelerate cell differentiation (Boutahar et al., 2004). In this study, we demonstrated that PDL-POSTN interacts directly with integrin αvβ3 in PDL cells to stimulate the integrin αvβ3-FAK signaling pathway and to mediate the cell differentiation process, and that it does so more strongly than full-length periostin. The amino acid sequence of exon A in periostin contains an RGS (Arg-Gly-Ser) motif similar to the RGD (Arg-Gly-Asp) motif known to interact with integrin family proteins (Danhier et al., 2012), and we speculate that periostin may interact with integrin αvβ3 through this RGS motif. However, all of the periostin isoforms isolated in this study contain this RGS motif, so the fact that PDL-POSTN binds more strongly to integrin αvβ3 compared with full-length periostin may indicate conformational differences in the protein structures of the periostin isoforms. It is also possible that other molecules, such as collagen type I, may bind to periostin and contribute to the periostin and integrin αvβ3 interaction. Further study is required to investigate the molecular basis of the interaction between PDL-POSTN and integrin αvβ3.
The C-terminal region of periostin is involved in cancer metastasis, and its deletion in cancer cells results in suppression of cell mobility and proliferation, suggesting a role for this region in regulating periostin protein functions (Yoshioka et al., 2002). In this study, we demonstrated that differences in the C-terminal sequence affected the level of cell differentiation but not the level of cell proliferation in PDL cells. This indicates that the C-terminal region may underlie differences in molecular function of the various periostin isoforms. Further studies to investigate the key functional motifs within the C-terminal of PDL-POSTN are ongoing.
Rios et al. (2008) clearly demonstrated that periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. Functional analyses in our study indicated that PDL-POSTN may be involved in the homeostasis of the mature periodontal ligament tissues in vivo. We speculate that PDL-POSTN is induced by the cellular response to tissue damage, and participates in the regeneration of periodontal ligament tissues as a positive regulator of PDL cell differentiation into osteoblasts or cementoblasts. Mechanical stress induces periostin expression through TGF-β in PDL cells (Rios et al., 2008; Wen et al., 2010), while Sidhu et al. (2010) reported that periostin forms homodimers that may be involved in the cross-linking of collagen fibers and/or collagen fibrillogenesis in bronchial epithelial cells. PDL-POSTN may also form homodimers or heterodimers with other periostin isoforms in the PDL. Further studies are required to explore PDL-POSTN functions regarding the response to mechanical stress, dimer formation, and/or collagen fibrillogenesis in the PDL. However, the involvement of PDL-POSTN in periodontal development of the tooth germ is poorly understood. Further studies to identify the murine counterpart of PDL-POSTN and to determine its expression during tooth development and/or to generate PDL-POSTN transgenic animals would provide invaluable information to clarify the role of PDL-POSTN in development.
In conclusion, this study reveals that PDL-POSTN is a periodontal-ligament-specific molecule that promotes the course of PDL cell differentiation through a direct interaction with integrin αvβ3. These findings suggest that PDL-POSTN is a key factor in PDL tissue homeostasis in vivo.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (Nos. 23390478 and 23249086).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Anastasilakis AD, Polyzos SA, Makras P, Savvides M, Sakellariou GT, Gkiomisi A, et al. (2013). Circulating periostin levels do not differ between postmenopausal women with normal and low bone mass and are not affected by zoledronic acid treatment. Horm Metab Res 46:145-149. [DOI] [PubMed] [Google Scholar]
- Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. (2004). Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5:329-339. [DOI] [PubMed] [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J. (1997). The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13: 20-40. [DOI] [PubMed] [Google Scholar]
- Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. (2004). Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem 279:30588-30599. [DOI] [PubMed] [Google Scholar]
- Dahl LK. (1952). A simple and sensitive histochemical method for calcium. Proc Soc Exp Biol Med 80:474-479. [DOI] [PubMed] [Google Scholar]
- Danhier F, Le Breton A, Preat V. (2012). RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm 9:2961-2973. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. (1999). Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239-1249. [DOI] [PubMed] [Google Scholar]
- Hou J, Yamada S, Kajikawa T, Ozaki N, Awata T, Yamaba S, et al. (2012). Role of ferritin in the cytodifferentiation of periodontal ligament cells. Biochem Biophys Res Commun 426:643-648. [DOI] [PubMed] [Google Scholar]
- Kii I, Kudo A. (2007). Periostin function in the periodontal ligament and the periosteum. Clin Calcium 17:202-208 [article in Japanese]. [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. (2007). Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13:962-969. [DOI] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. (2005). Alphavbeta integrins play an essential role in BMP-2 induction of osteoblast differentiation. J Bone Miner Res 20:330-340. [DOI] [PubMed] [Google Scholar]
- Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, et al. (2004). Expression and function of periostin isoforms in bone. J Cell Biochem 92:1044-1061. [DOI] [PubMed] [Google Scholar]
- Litvin J, Zhu S, Norris R, Markwald R. (2005). Periostin family of proteins: therapeutic targets for heart disease. Anat Rec A Discov Mol Cell Evol Biol 287:1205-1212. [DOI] [PubMed] [Google Scholar]
- Murakami S, Hashikawa T, Saho T, Takedachi M, Nozaki T, Shimabukuro Y, et al. (2001). Adenosine regulates the IL-1 beta-induced cellular functions of human gingival fibroblasts. Int Immunol 13:1533-1540. [DOI] [PubMed] [Google Scholar]
- Murakami S, Yoshimura N, Koide H, Watanabe J, Takedachi M, Terakura M, et al. (2002). Activation of adenosine-receptor-enhanced iNOS mRNA expression by gingival epithelial cells. J Dent Res 81:236-240. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. (2004). Rational siRNA design for RNA interference. Nat Biotechnol 22:326-330. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, et al. (2005). Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios H, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, et al. (2008). Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol 79:1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos GE, Asnani KP, Hingorani D, Deshmukh VL. (2013). Periostin: role in formation and maintenance of dental tissues. J Cell Physiol 229:1-5. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. (1992). pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA 89:5192-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155. [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Ichikawa T, Takayama S, Yamada S, Takedachi M, Terakura M, et al. (2005). Fibroblast growth factor-2 regulates the synthesis of hyaluronan by human periodontal ligament cells. J Cell Physiol 203:557-563. [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Ueda M, Ozasa M, Anzai J, Takedachi M, Yanagita M, et al. (2009). Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J Endod 35:1529-1535. [DOI] [PubMed] [Google Scholar]
- Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, et al. (2008). Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. (2010). Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 107:14170-14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Kikuno R, Tezuka K, Amann E. (1993). Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294(Pt 1):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Chau E, Jackson-Boeters L, Elliott C, Daley TD, Mamilton DW.(2010). TGF-β1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res 89:1439-1443. [DOI] [PubMed] [Google Scholar]
- Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, et al. (2007). PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem 282:23070-23080. [DOI] [PubMed] [Google Scholar]
- Yoshioka N, Fuji S, Shimakage M, Kodama K, Hakura A, Yutsudo M, et al. (2002). Suppression of anchorage-independent growth of human cancer cell lines by the TRIF52/periostin/OSF-2 gene. Exp Cell Res 279:91-99. [DOI] [PubMed] [Google Scholar]
- Zhu S, Barbe MF, Liu C, Hadjiargyrou M, Popoff SN, Rani S, et al. (2009). Periostin-like-factor in osteogenesis. J Cell Physiol 218:584-592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.