Abstract
The mechanism of pain in dentine hypersensitivity is poorly understood but proposed to result from the activation of dental sensory neurons in response to dentinal fluid movements. Odontoblasts have been suggested to contribute to thermal and mechanosensation in the tooth via expression of transient receptor potential (TRP) channels. However, a mechanism by which odontoblasts could modulate neuronal activity has not been demonstrated. In this study, we investigated functional TRP channel expression in human odontoblast-like cells and measured ATP release in response to TRP channel activation. Human immortalized dental pulp cells were driven toward an odontoblast phenotype by culture in conditioned media. Functional expression of TRP channels was determined with reverse transcription polymerase chain reaction and ratiometric calcium imaging with Fura-2. ATP release was measured using a luciferin-luciferase assay. Expression of mRNA for TRPA1, TRPV1, and TRPV4 but not TRPM8 was detected in odontoblasts by reverse transcription polymerase chain reaction. Expression of TRPV4 protein was detected by Western blotting and immunocytochemistry. The TRPA1 agonists allyl isothiocyanate and cinnamaldehyde and the TRPV4 agonist GSK1016790A caused a concentration-dependent increase in intracellular Ca2+ concentration that was inhibited by the selective antagonists HC030031, AP18, and HC067047, respectively. In contrast, exposure to the TRPV1 agonist capsaicin or the TRPM8 agonist icilin had no effect on intracellular Ca2+ concentration. Treatment with allyl isothiocyanate, cinnamaldehyde, or GSK1016790A caused an increase in ATP concentration in culture medium that was abolished by preincubation with TRP channel antagonists. These data demonstrate that activation of TRPA1 and TRPV4 channels in human odontoblast-like cells can stimulate ATP release. We were unable to confirm the presence of thermosensitive TRPV1 and TRPM8 that has previously been reported in odontoblasts.
Keywords: transient receptor potential channel, orofacial pain, signal transduction, dentin, sensation, ion channels
Introduction
Exposed dentine is painful when exposed to changes in temperature or osmolarity. Anatomic and neurophysiologic properties of the tooth suggest that these changes are not detected directly by sensory nerve endings. Instead, dentinal fluid movements are detected by mechanosensitive nociceptive trigeminal nerve endings, the “hydrodynamic theory” of dental pain (Linden and Brannstrom, 1967; Brannstrom and Johnson, 1970). However, direct mechanical activation of the sensory nerves has not been described, and there is doubt whether such small fluid pressure changes could directly activate high-threshold mechanoreceptive nociceptors (Fried et al., 2011). It is thus not clear whether there is direct mechanotransduction by neurons or if other cells (e.g., odontoblasts) may contribute to sensory processing.
The odontoblasts are situated between the dentine and the nerve plexus, containing the majority of dental sensory fiber terminals. They possess cell processes that extend into the dentine tubules (Magloire et al., 2004). Recent studies also suggest that odontoblasts express many ion channels commonly found in sensory neurons, such as mechanosensitive KCa and TREK-1 potassium channels (Allard et al., 2000; Magloire et al., 2003) and voltage-gated sodium channels (Byers and Westenbroek, 2011).
The transient receptor potential (TRP) family of cation channels play important roles in sensory transduction. The thermosensitive (>42°C) TRPV1, cool-sensitive (<22°C) TRPM8, cold/mechanosensitive TRPA1 and warm/mechanosensitive TRPV4 have received particular attention as a result of in vivo experiments, implicating them in nociception (Bevan and Andersson, 2009). Studies of human and rodent odontoblasts have identified expression of TRPV1, TRPM8, TRPA1, and TRPV4, but there is significant disagreement about whether all 4 channels are always expressed. Rat odontoblasts have been suggested to express TRPV1 (Okumura et al., 2005; Tsumura et al., 2012) and TRPA1 (Byers and Westenbroek, 2011), but another study failed to find either channel in reverse transcription polymerase chain reaction (RT-PCR) and Ca2+ imaging experiments (Yeon et al., 2009). TRPV1 and TRPV4 were identified in cultured mouse odontoblasts, but no evidence of TRPA1 or TRPM8 expression was identified (Son et al., 2009). TRPV1 and TRPV4 were proposed to mediate Ca2+ influx caused by hypotonic membrane stretching in these cells (Sato et al., 2013). Odontoblast-like cells derived from human teeth expressed TRPV1, TRPA1, and TRPM8 (El Karim et al., 2011), and immunostaining of human teeth identified odontoblast expression of TRPV4 (Sole-Magdalena et al., 2011).
The expression of sensory ion channels by odontoblasts suggests that they may contribute to sensation in the tooth. However, no evidence has been provided for a mechanism by which odontoblasts could modulate sensory neuron activity. In many tissues, mechanical stimulation of cells causes ATP release (Praetorius and Leipziger, 2009; Lazarowski, 2012). Sensory fibers in the dental pulp, in the subodontoblastic plexus of Raschkow, and within the odontoblast layer express ionotropic P2X3 receptors (Alavi et al., 2001; Renton et al., 2003), so a mechanically stimulated release of ATP from odontoblasts could directly activate sensory neurons.
We have previously isolated, immortalized, and characterized odontoblast-like cells from human teeth (Egbuniwe et al., 2011; Egbuniwe et al., 2013). In this study, we investigated functional TRP channel expression in cultured human odontoblasts. We hypothesized that odontoblasts express the mechanosensitive TRP channels TRPA1 and TRPV4 and that activation of these channels would stimulate the release of ATP.
Methods & Materials
Odontoblast Isolation and Culture
Immortalized human odontoblast-like cells (hOBs) were obtained from extracted human teeth, and an odontoblast phenotype was confirmed by expression and function of specific markers (e.g., collagen 1, alkaline phosphatase), as previously described (Egbuniwe et al., 2011; Egbuniwe et al., 2013). All experiments were approved by the local research ethics committee of King’s College London and King’s College Hospital Foundation Trust (reference 08/H0808/105).
The hOBs were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2,500 U/mL of penicillin, 2.5 mg/mL of streptomycin, 50 µg/mL of ascorbic acid, 10mM β-glycerophosphate, and 100nM dexamethasone (all from Sigma, UK).
Reverse Transcription Polymerase Chain Reaction
hOBs were grown to confluence, and total cellular RNA was isolated with an RNeasy Plus Mini Kit (Qiagen, UK) according to the manufacturer’s instructions. Cellular RNA from Chinese hamster ovary (CHO) cells overexpressing rat TRPM8 was kindly supplied by Prof. Stuart Bevan, King’s College London, and 1 µg of total RNA was reverse transcribed to cDNA using a High Capacity RNA-to-cDNA Kit (Applied Biosystems, UK). Products were expanded from cDNA by PCR with a Masterplus Sybr Green I dye detection system (Roche, UK) and specific primers (Appendix Table). Products of PCR were visualized with GelRed (Biotium Inc., USA) under ultraviolet light following 1.5% agarose gel electrophoresis.
Western Blotting
hOBs were lysed in phosphate buffered saline (PBS) containing 1% SDS, pH 7.4. Mouse urothelium was surgically separated from TRPV4 wild-type and knockout bladders and homogenized in RIPA buffer (50mM Tris-HCl, 150mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1mM EDTA, pH 7.4) with protease inhibitors (Roche). Lysate (30 µg) was separated by SDS-PAGE (8% acrylamide), transferred to nitrocellulose membranes, and blocked in TBS with 0.1% Tween-20 and 5% powdered milk for 60 min. Membranes were incubated with rabbit anti-TRPV4 (1:250, AbCam) and mouse anti-GAPDH (anti–glyceraldehyde 3-phosphate dehydrogenase; 1:6,000, Applied Biosystems) overnight at 4°C. Membranes were washed and then incubated with donkey anti-rabbit-IRDye 800 (1:15,000, LI-COR, UK) and goat anti-mouse-Alexa Fluor 680 (Molecular Probes, UK) for 1 hr at room temperature. Immunoreactive proteins were detected with an Odyssey Infrared Imaging System (LI-COR; Grant et al., 2007; Alexander et al., 2013).
Immunocytochemistry
hOBs were fixed in 4% paraformaldehyde for 60 min at 4°C. Coverslips were washed in PBS. Cells were incubated overnight at 4°C with rabbit anti-TRPV4 (1:200, AbCam, UK). Coverslips were washed in PBS containing 0.1% Triton X-100; then, donkey anti-rabbit-Alexa 546 (1:1,000) was added and incubated for 2 hr at room temperature. Coverslips were washed in PBS containing 0.1% Triton X-100 and then mounted on slides with Vectashield with DAPI (Vector Labs, UK), and images were collected with an Axioplan 2 microscope (Zeiss, UK).
Measurement of Intracellular [Ca2+]
hOBs were grown to confluence in black-walled 96-well plates. Cells were loaded with Fura-2-AM (2µM) in the presence of probenecid (1mM) for 1 hr at 30°C in Hank’s balanced salt solution with 10mM HEPES. Cells were washed, and the mean fluorescence across each well was measured alternately at 340/380-nm excitation and 520-nm emission with a Flexstation (Molecular Devices, Wokingham, UK). Each experimental run lasted 180 s, with agonist treatments at 20 s. In a further series of experiments, cells were pretreated for 15 min with TRP channel antagonists before the start of Ca2+ imaging, and the antagonist remained in contact with the cells throughout. Experiments in the absence of external Ca2+ were performed with Ca2+/Mg2+–free Hank’s balanced salt solution, supplemented with 10mM HEPES, 1mM Mg2+, and 1mM EGTA.
Measurement of ATP Release
hOBs were grown to confluence in 4-well plates. Cell culture medium was replaced 2 hr before experiments with fresh medium alone or medium containing TRP channel antagonists. In one set of experiments, 100-µL samples of medium were collected 1, 5, or 15 min after the medium was replaced with warmed fresh medium. In another, cells were treated for 15 min with TRP channel agonists, and then 100-µL samples of medium were collected. ATP content in culture medium was assayed with the CellTiter-Glo kit (Promega, UK) according to the manufacturer’s protocol, with an ATP standard curve (1nM-1µM). Duplicate measurements were made from each well and an average taken of the measurements from 2 wells to give n = 1.
Materials
Allyl isothiocyanate (AITC), capsaicin, cinnamaldehyde (CA), GSK1016790A, HC030031, HC067047, icilin, and probenecid were all obtained from Sigma. AP18 was obtained from Tocris, UK. Fura-2-AM was obtained from Molecular Probes (Invitrogen, UK). All compounds were dissolved in DMSO and diluted in culture medium / reaction buffer as appropriate.
Analysis of Data
Graphical data are presented as mean ± SEM. TRP channel agonist responses in the Ca2+ imaging experiments were calculated as maximum Δ ratio in the presence of the agonist minus the mean baseline ratio. Sigmoidal curves were fitted to the concentration-response data via GraphPad Prism software. ATP release following medium change was compared with a nonparametric Kruskal-Wallis test, followed by Dunn’s posttest. ATP release in response to TRP channel agonists was compared with unpaired t tests.
Results
TRP Channel Expression in Cultured Human Odontoblasts
Expression of TRP channels in cultured hOBs was initially examined by RT-PCR. Specific primers amplified products for GAPDH and the TRP channels TRPA1 and TRPV1 from total cellular cDNA (Figure 1a). A doublet band was observed with primers specific to TRPV4 (Grant et al., 2007), whereas no band was seen for TRPM8 (Figure 1a). Expansion of products with primers for 2 phenotypic markers of odontoblasts—alkaline phosphatase and collagen 1—was also observed (Figure 1b). To confirm the ability of the TRPM8 primers to amplify a product, a further RT-PCR reaction was run with cDNA from hOBs and CHO-TRPM8 cells. A band for TRPM8 was detected only with CHO-TRPM8 cDNA.
Figure 1.
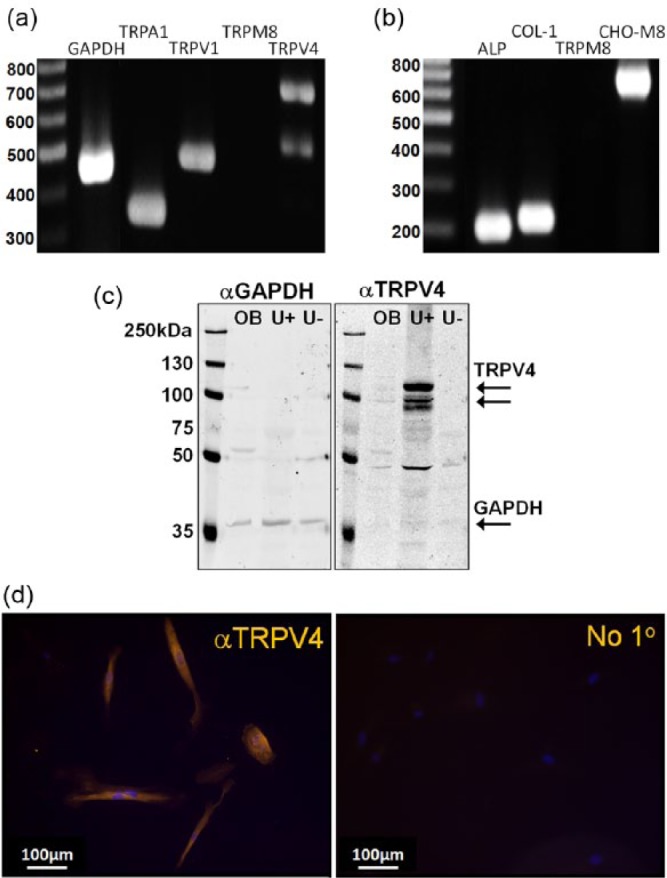
Expression of transient receptor potential channels in cultured human odontoblast-like cells (hOBs). (a) Reverse transcription polymerase chain reaction with primers selective for GAPDH (product size = 425 bp), TRPA1 (381 bp), TRPV1 (452 bp), and TRPV4 (512/692 bp) produced bands, but no product of TRPM8 cDNA was expanded. (b) Primers selective for alkaline phosphatase (ALP; 196 bp) and collagen 1 (COL-1; 213 bp) produced bands from hOB cDNA, whereas primers selective for TRPM8 produced a product only from CHO-TRPM8 cDNA (681 bp) but not from hOBs. (c) Lysates (30 µg of total protein) from hOBs (OB), wild-type mouse urothelium (U+), and TRPV4 –/– mouse urothelium (U–) showed expression of GAPDH (37 kDa, left blot). Lysates from hOBs and wild-type mouse urothelium showed the double band corresponding to TRPV4 (98 and 104 kDa, right blot), but it was absent in TRPV4 –/– mouse urothelium. (d) Immunostaining of hOBs with rabbit anti-TRPV4 (1:200) and donkey anti-rabbit-Alexa 546 (1:1,000) revealed positive TRPV4 immunoreactivity that was not absent in the “no primary” cells. Images are representative of data obtained from n = 3 independent experiments.
Expression of TRPV4 protein was studied in hOB lysates by Western blotting. To confirm antibody specificity, urothelial (a tissue strongly expressing TRPV4; Mochizuki et al., 2009) lysates from wild-type and TRPV4 knockout mice were also assayed. A band at 37 kDa, corresponding to GAPDH, was seen in all lysates (Figure 1c). A relatively weak double band (98 and 104 kDa) corresponding to TRPV4 was seen in the hOB lysate, and stronger bands of the same size were observed in wild-type urothelial lysate. No bands at these sizes were seen in urothelial lysates from TRPV4 knockout mice (Figure 1c). Immunostaining of hOBs with anti-TRPV4 also demonstrated expression of protein, and specificity of antibody binding was further supported by the absence of fluorescence in cells exposed to secondary antibody alone (Figure 1d).
We attempted to extend these experiments to investigate expression of TRPA1 and TRPV1 protein in hOBs. Various commercially available antibodies were tested by Western blotting on lysates of dorsal root ganglia from wild-type, TRPA1 knockout, and TRPV1 knockout mice. We were unable to identify any antibodies that specifically recognized bands corresponding to the molecular weights of these proteins.
Identification of Functional TRP Channel Expression in Odontoblasts by Ca2+ Fluorimetry
Exposure of cultured hOBs to the TRPV4 agonist GSK1016790A caused a concentration-dependent increase in intracellular Ca2+ concentration, with EC50 = 30nM (Figure 2a). The TRPA1 agonists AITC and CA also caused concentration-dependent increases in [Ca2+]i, with EC50 = 6µM and 36µM, respectively (Figure 2b and c). In contrast, the TRPV1 agonist capsaicin and TRPM8 agonist icilin, both at concentrations up to 10µM, had no effect on intracellular Ca2+ concentration (Figure 2d and e).
Figure 2.
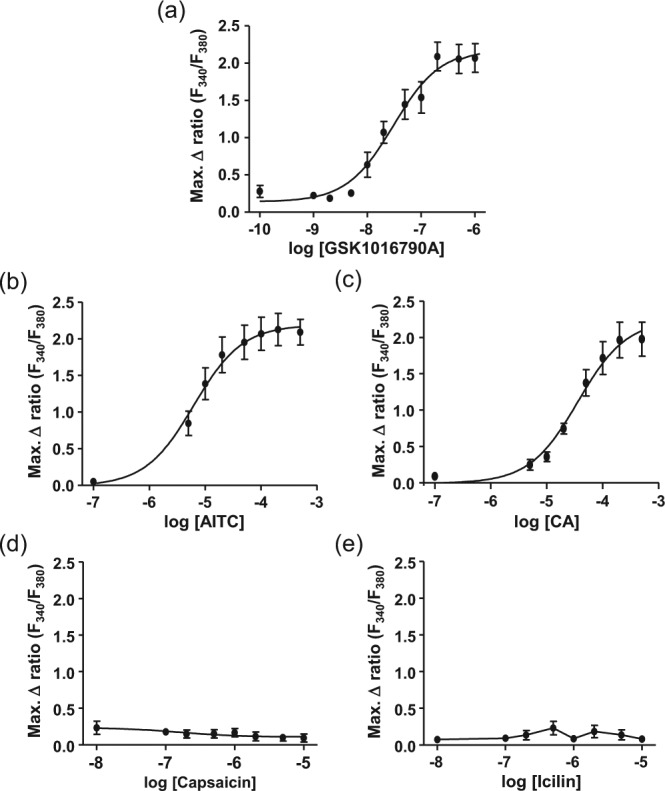
The effect of agonists of TRPV4, TRPA1, TRPV1, and TRPM8 on [Ca2+]i in cultured human odontoblast-like cells. (a) The TRPV4 agonist GSK1016790a (0.1nM-1µM) and the TRPA1 agonists (b) allyl isothiocyanate (AITC; 0.1-500µM) and (c) cinnamaldehyde (CA; 0.1-500µM) caused a concentration-dependent increase in intracellular Ca2+ concentration. (d) The TRPV1 agonist capsaicin (10nM-10µM) and (e) the TRPM8 agonist icilin (10nM-10µM) had no effect on intracellular Ca2+ concentration. n = 3-7 triplicate measurements.
Preincubation of hOBs for 15 min with the TRPV4 antagonist HC067047 caused a concentration-dependent reduction in the increase in [Ca2+]i caused by 200nM GSK1016790A, with IC50 = 100nM (Figure 3a). The TRPA1 antagonist HC030031 caused concentration-dependent inhibition of the increase in [Ca2+]i to 200µM AITC or CA with IC50 = 67µM and 30µM, respectively (Figure 3b and c). Similarly, the TRPA1 antagonist AP18 caused concentration-dependent inhibition of the increase in [Ca2+]i to 200µM AITC or CA with IC50 = 6µM and 3µM, respectively (Figure 3d and e).
Figure 3.
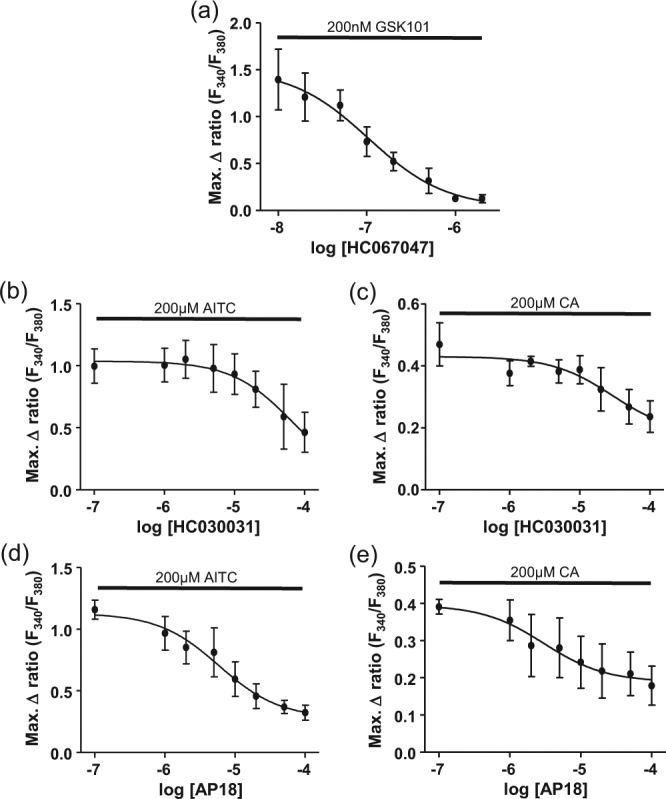
The effect of pretreatment with transient receptor potential channel antagonists on changes in [Ca2+]i in cultured human odontoblast-like cells induced by TRPV4 and TRPA1 agonists. (a) The TRPV4 antagonist HC067047 (10nM-2µM) caused a concentration-dependent reduction in the increase in intracellular Ca2+ concentration caused by 200nM GSK1016790A. The TRPA1 antagonist HC030031 (0.1-100µM) caused a concentration-dependent reduction in the increase in intracellular Ca2+ concentration caused by (b) 200µM AITC and (c) 200µM CA. The TRPA1 antagonist AP18 (0.1-100µM) caused a concentration-dependent reduction in the increase in intracellular Ca2+ concentration caused by (d) 200µM allyl isothiocyanate (AITC) and (e) 200µM cinnamaldehyde (CA). n = 4 triplicate measurements.
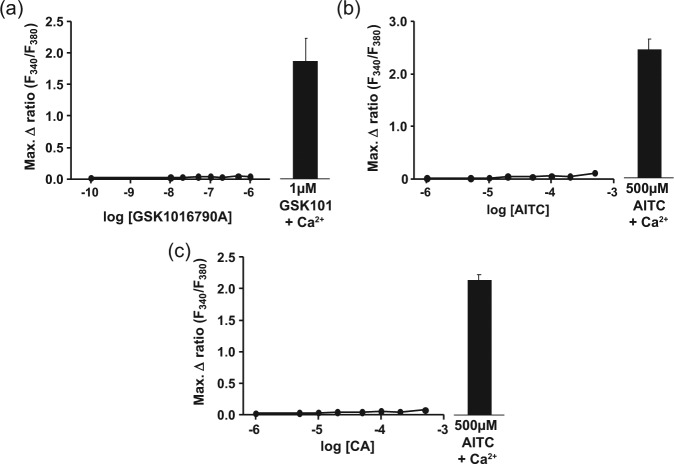
In the absence of external Ca2+, no increase in intracellular Ca2+ concentration occurred following exposure to GSK101679 0A, AITC, or CA. In the same experiment, cells exposed to 1µM GSK1016790A, 500µM AITC, or 500µM CA in the presence of external Ca2+ showed a robust increase in [Ca2+]i (Figure 4).
Figure 4.
Removal of extracellular Ca2+ abolished the increase in [Ca2+]i induced by (a) GSK1016790A (0.1nM-1µM), (b) allyl isothiocyanate (AITC; 1-500µM), and (c) cinnamaldehyde (CA; 1-500µM) in cultured human odontoblast-like cells. The highest doses of each agonist induced a robust increase in [Ca2+]i in the same cells when extracellular Ca2+ was present. n = 3 triplicate measurements.
Activation of TRPA1 and TRPV4 in Odontoblasts Stimulates ATP Release
Changing the hOB cell culture medium caused a release of ATP, with the concentrations remaining elevated in cell culture medium 1 and 5 min after medium change. The concentration had returned to its basal value by 15 min (Figure 5a). Thus, to avoid any artifact from mechanical stimulation of cells during pipetting, the effects of TRP channel agonists on ATP concentration were assessed 15 min after application.
Figure 5.
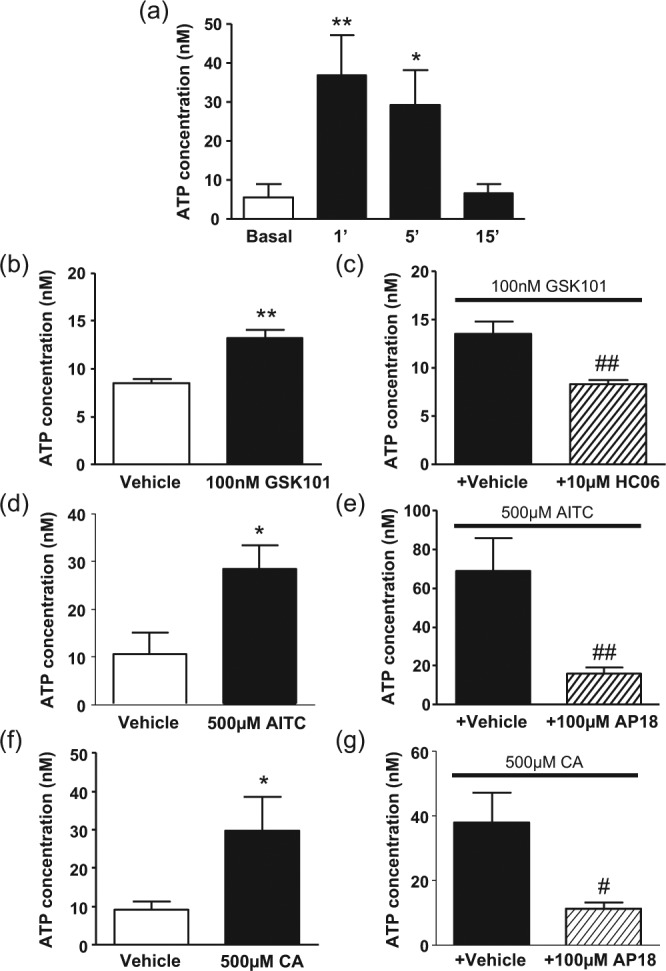
Release of ATP from cultured human odontoblast-like cells. (a) Changing the cell culture medium caused an increase in extracellular ATP concentration 1 and 5 min afterward, which returned to basal levels by 15 min. (b) 100nM GSK1016790A, (d) 500µM allyl isothiocyanate (AITC), and (f) 500µM CA caused a significant increase in extracellular ATP concentration, measured 15 min after agonist administration. These increases were significantly inhibited by pretreatment with (c) 10µM HC067047 or (e, g) 100µM AP18. *p < .05, **p < .01 vs. vehicle/basal groups. #p < .05, ##p < .01 vs. agonist + antagonist vehicle groups. n = 8-10 groups of paired duplicate measurements.
Application of 100nM GSK1016790A to hOBs caused a significant increase in ATP concentration in the cell culture medium (Figure 5b). This was prevented by preincubation with 10µM HC067047 (Figure 5c). Application of 500µM AITC or 500µM CA to hOBs also caused an increase in ATP concentration in the cell culture medium (Figure 5d and f). Preincubation with 100µM AP18 prevented this increase in both cases (Figure 5e and g). Application of 10µM capsaicin (vehicle: 2.91 ± 1.41nM vs. capsaicin: 2.92 ± 0.72nM) or 10µM icilin (vehicle: 2.61 ± 0.87nM vs. icilin: 3.26 ± 1.50nM) had no effect on the concentration of ATP in the cell culture medium.
Discussion
In this study, we have identified functional expression of the ion channels TRPA1 and TRPV4 in cultured hOBs. We were unable to find evidence for expression of TRPV1 or TRPM8. We have shown for the first time that hOBs can release ATP following activation of TRPA1 and TRPV4, suggesting a possible contribution to sensation in the tooth.
Identification of alkaline phosphatase and collagen 1 expression supports our previous finding that these cells maintain an odontoblast phenotype in culture (Egbuniwe et al., 2011; Egbuniwe et al., 2013). Expression of TRPV4 (mRNA and protein) and TRPA1 (mRNA) was also identified in these cells. Expression of TRPV4 protein was identified by Western blotting and immunocytochemistry. The TRPV4 agonist GSK1016790A caused a concentration-dependent increase in [Ca2+]i with EC50 = 32nM, a similar order of magnitude to the 43nM that we previously observed in a human keratinocyte cell line (Alexander et al., 2013). The increase in [Ca2+]i was inhibited in a concentration-dependent manner by the TRPV4 antagonist HC067047. Removal of Ca2+ from the extracellular media prevented any increase in [Ca2+]i following exposure to GSK1016790A, suggesting that it occurs via entry of Ca2+ through TRPV4. Together, the presence of TRPV4 mRNA and protein, as well as calcium increases following exposure to a TRPV4 agonist, suggests that cultured hOBs express functional TRPV4. Immunohistochemical identification of TRPV4 in odontoblasts in human teeth (Sole-Magdalena et al., 2011) suggests that this expression also occurs in situ.
The TRPA1 antagonists AITC and CA both caused concentration-dependent increases in [Ca2+]i. Two different TRPA1 antagonists inhibited the increase in [Ca2+]i caused by either AITC or CA. Removing Ca2+ from the extracellular media prevented any increase in [Ca2+]i following exposure to AITC or CA, suggesting that each was triggering an influx of extracellular Ca2+ through TRPA1. Together, the presence of TRPA1 mRNA and calcium increases following exposure to TRPA1 agonists suggests that cultured hOBs express functional TRPA1. Odontoblasts in human teeth were positive for TRPA1 expression following immunohistochemical staining, suggesting that this expression is also present in situ (Kim et al., 2012).
Although TRPV1 mRNA was identified by RT-PCR, no increase in [Ca2+]i followed exposure to the TRPV1 agonist capsaicin up to a concentration of 10µM, well above the EC50 value of 300nM at hTRPV1 (McNamara et al., 2005). Furthermore, exposure of hOBs to 10µM capsaicin had no effect on ATP release. These findings suggest that TRPV1 mRNA is not translated into mature protein or that the mature protein does not reach the cell surface. Another explanation could be that the TRPV1 mRNA is translated into a capsaicin-insensitive splice variant of the channel, several of which have been described. One variant was identified in murine sensory neurons, where it negatively regulated the function of the capsaicin-sensitive isoform (Wang et al., 2004). An N-terminal variant TRPV1 was also proposed to mediate depolarization of magnocellular neurosecretory cells by thermal and hyperosmotic stimuli in mice (Sudbury et al., 2010). Further investigation of TRPV1 expression and cellular localization is necessary to understand this discrepancy between expression and function data.
In contrast to our findings, several previous studies have identified functional capsaicin-sensitive TRPV1 in odontoblasts (e.g., Okumura et al., 2005; El Karim et al., 2011; Sato et al., 2013), although another study failed to do so (Yeon et al., 2009). Species differences in the odontoblast expression of TRP channels may explain some of this variability. Additionally, some data supporting an expression of TRPV1 are based on immunohistochemical staining, which must be interpreted with caution as TRPV1 antibodies bind nonspecifically to tissues lacking TRPV1 (Everaerts et al., 2009). However, El Karim et al. (2011) studied human odontoblasts isolated and cultured with a methodology similar to that used in this study and identified increased [Ca2+]i following exposure to capsaicin or noxious heat. It is notable that the mean fluorescence changes to capsaicin and heat in their study are very small with a large variability and the number of experimental repeats is not described, so it is possible that these were just nonspecific fluctuations in [Ca2+]i in a few cells that coincided with capsaicin or heat application. Cells isolated from dental pulps also exhibit neuronal phenotypes (D’Anto et al., 2006), so there is the possibility that the studies identifying odontoblast TRPV1 have actually isolated precursors displaying a mix of neuronal and odontoblast characteristics. A recent study using a TRPV1 reporter mouse to overcome antibody specificity problems identified only TRPV1 expression in neurons and a small subset of vascular smooth muscle (Cavanaugh et al., 2011), so expression of TRPV1 in odontoblasts would be surprising.
We were also unable to find evidence for expression of TRPM8 mRNA or protein in the hOBs. Confirmation that our TRPM8 primers were working correctly was provided by their amplification of the correct product from cDNA from CHO-TRPM8 cells. As with TRPV1, this was in contrast to some studies (e.g., El Karim et al., 2011) but supporting the observation of others (e.g., Son et al., 2009). Similar to the putative TRPV1 responses, the putative TRPM8 responses to cold and icilin described by El Karim et al. (2011) are small in magnitude with a high degree of variability, so they may have been nonspecific fluctuations in cytoplasmic calcium. Most pertinently, none of the studies proposing expression of TRPV1 and TRPM8 as odontoblast thermosensors have explained how this would fit with the hydrodynamic theory. It is widely accepted that thermal changes are transduced indirectly via changes in dentine fluid volume that activate mechanosensory proteins on sensory cells (neurons, odontoblasts, or both). TRPV4 and TRPA1 activation by mechanical stimuli has been described (Suzuki et al., 2003; Kwan et al., 2006; Brierley et al., 2011), indicating that odontoblasts with these channels have the potential to detect pressure changes caused by changes in dentine fluid volume.
Although expression of TRPV4 and TRPA1 suggests that odontoblasts are mechanosensitive, they could not contribute to dental pain without some means to modulate the activity of sensory neurons. In many tissues, mechanical stimulation of cells induces ATP release, so we investigated whether a similar release could be triggered by stimulation of hOBs. Aspirating and replacing the cell culture medium to mechanically stimulate the cells caused an increase in ATP concentration 1 and 5 min later, but this returned to basal levels by 15 min. To ensure that observed changes in ATP concentration in TRP agonist experiments were due to chemical activation of TRP channels rather than inadvertent mechanical stimulation of cells, we measured the ATP concentration in the culture medium 15 min after agonist administration.
Application of GSK1016970A to hOBs caused an increase in ATP concentration in the culture medium, which was inhibited by the TRPV4 antagonist HC067047. Similarly, exposure to either AITC or CA caused an increase in extracellular ATP concentration that was inhibited by the TRPA1 antagonist AP18. This suggests that activation of TRPV4 or TRPA1 triggers a release of ATP from hOBs. Basal and evoked concentrations of ATP in the nanomolar range are similar in magnitude to those described in bulk phase in previous studies (Praetorius and Leipziger, 2009). TRPV4-induced release of ATP has been described in urothelial cells (Mochizuki et al., 2009) and keratinocytes (Mihara et al., 2011). Mechanical activation of TRPV4 by urothelial stretch and consequent release of ATP to activate P2X3 receptors on sensory neurons plays a key role in detecting bladder filling (Gevaert et al., 2007). Dental sensory neurons adjacent to the odontoblasts express P2X3 receptors (Alavi et al., 2001; Renton et al., 2003). The cell surface enzyme nucleoside triphosphate diphosphohydrolase 2, which hydrolyzes extracellular ATP, is expressed by sensory neurons and odontoblasts (Liu et al., 2012). The ability of hOBs to release ATP, the presence of ATP-sensitive receptors on dental sensory neurons, and the presence of enzymes necessary to terminate ATP signaling all suggest that odontoblasts could play a physiologic role in modulating sensory neuron activity in the tooth.
In conclusion, we have shown that hOBs express functional TRPV4 and TRPA1 and that activation of these channels triggers ATP release. This mechanism may contribute to dentine hypersensitivity by allowing odontoblasts to sense changes in dentine fluid pressure and release ATP to activate adjacent sensory neurons. However, direct mechanical activation of TRPV4 and TRPA1 in human odontoblasts, with subsequent ATP release, still remains to be identified. While this article was under revision, Shibukawa et al. (2014) published a report describing TRP channel–dependent release of ATP from cultured rat odontoblasts in response to mechanical stimulation, suggesting that this mechanism may be common to dental nociception in humans and rodents.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
These studies were supported by a Biomedical Research Centre award to the Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London, a training grant from the Royal College of Surgeons (England), and a Capacity Building Award in Integrative Mammalian Biology funded by the Biotechnology and Biological Sciences Research Council, British Pharmacological Society, Higher Education Funding Council for England, Knowledge Transfer Network, Medical Research Council.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alavi AM, Dubyak GR, Burnstock G. (2001). Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res 80:476-483. [DOI] [PubMed] [Google Scholar]
- Alexander R, Kerby A, Aubdool AA, Power AR, Grover S, Gentry C, et al. (2013). 4alpha-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol 168:761-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Couble ML, Magloire H, Bleicher F. (2000). Characterization and gene expression of high conductance calcium-activated potassium channels displaying mechanosensitivity in human odontoblasts. J Biol Chem 275:25556-25561. [DOI] [PubMed] [Google Scholar]
- Bevan S, Andersson DA. (2009). TRP channel antagonists for pain: opportunities beyond TRPV1. Curr Opin Investig Drugs 10:655-663. [PubMed] [Google Scholar]
- Brannstrom M, Johnson G. (1970). Movements of the dentine and pulp liquids on application of thermal stimuli: an in vitro study. Acta Odontol Scand 28:59-70. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, et al. (2011). TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 589(Pt 14):3575-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MR, Westenbroek RE. (2011). Odontoblasts in developing, mature and ageing rat teeth have multiple phenotypes that variably express all nine voltage-gated sodium channels. Arch Oral Biol 56:1199-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. (2011). Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31:5067-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anto V, Cantile M, D’Armiento M, Schiavo G, Spagnuolo G, Terracciano L, et al. (2006). The HOX genes are expressed, in vivo, in human tooth germs: in vitro cAMP exposure of dental pulp cells results in parallel HOX network activation and neuronal differentiation. J Cell Biochem 97:836-848. [DOI] [PubMed] [Google Scholar]
- Egbuniwe O, Idowu BD, Funes JM, Grant AD, Renton T, Di Silvio L. (2011). P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle 10:3912-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbuniwe O, Grant AD, Renton T, Di Silvio L. (2013). Phenotype-independent effects of retroviral transduction in human dental pulp stem cells. Macromol Biosci 13:851-859. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, et al. (2011). Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain 152:2211-2223. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Sepulveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. (2009). Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379:421-425. [DOI] [PubMed] [Google Scholar]
- Fried K, Sessle BJ, Devor M. (2011). The paradox of pain from tooth pulp: low-threshold “algoneurons”? Pain 152:2685-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, et al. (2007). Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. (2007). Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578(Pt 3):715-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Jung HK, Kwon TK, Kim CS, Cho JH, Ahn DK, et al. (2012). Expression of transient receptor potential ankyrin 1 in human dental pulp. J Endod 38:1087-1092. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. (2006). TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277-289. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER. (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8:359-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden L, Brannstrom M. (1967). Fluid movements in dentine and pulp. An in vitro study of flow produced by chemical solutions on exposed dentine. Odontol Revy 18:227-236. [PubMed] [Google Scholar]
- Liu X, Yu L, Wang Q, Pelletier J, Fausther M, Sévigny J, et al. (2012). Expression of ecto-ATPase NTPDase2 in human dental pulp. J Dent Res 91:261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire H, Lesage F, Couble ML, Lazdunski M, Bleicher F. (2003). Expression and localization of TREK-1 K+ channels in human odontoblasts. J Dent Res 82:542-545. [DOI] [PubMed] [Google Scholar]
- Magloire H, Couble ML, Romeas A, Bleicher F. (2004). Odontoblast primary cilia: facts and hypotheses. Cell Biol Int 28:93-99. [DOI] [PubMed] [Google Scholar]
- McNamara FN, Randall A, Gunthorpe MJ. (2005). Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol 144:781-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. (2011). Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol 589(Pt 14):3471-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, et al. (2009). The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284:21257-21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R, Shima K, Muramatsu T, Nakagawa K, Shimono M, Suzuki T, et al. (2005). The odontoblast as a sensory receptor cell? The expression of TRPV1 (VR-1) channels. Arch Histol Cytol 68:251-257. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. (2009). ATP release from non-excitable cells. Purinergic Signal 5:433-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. (2003). Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain 17:245-250. [PubMed] [Google Scholar]
- Sato M, Sobhan U, Tsumura M, Kuroda H, Soya M, Masamura A, et al. (2013). Hypotonic-induced stretching of plasma membrane activates transient receptor potential vanilloid channels and sodium-calcium exchangers in mouse odontoblasts. J Endod 39:779-787. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, et al. (2014). Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch. [Epub ahead of print 6/18/2014] [DOI] [PubMed] [Google Scholar]
- Sole-Magdalena A, Revuelta EG, Menenez-Diaz I, Calavia MG, Cobo T, Garcia-Suarez O, et al. (2011). Human odontoblasts express transient receptor protein and acid-sensing ion channel mechanosensor proteins. Microsc Res Tech 74:457-463. [DOI] [PubMed] [Google Scholar]
- Son AR, Yang YM, Hong JH, Lee SI, Shibukawa Y, Shin DM. (2009). Odontoblast TRP channels and thermo/mechanical transmission. J Dent Res 88:1014-1019. [DOI] [PubMed] [Google Scholar]
- Sudbury JR, Ciura S, Sharif-Naeini R, Bourque CW. (2010). Osmotic and thermal control of magnocellular neurosecretory neurons: role of an N-terminal variant of trpv1. Eur J Neurosci 32:2022-2030. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. (2003). Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664-22668. [DOI] [PubMed] [Google Scholar]
- Tsumura M, Sobhan U, Muramatsu T, Sato M, Ichikawa H, Sahara Y, et al. (2012). TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium-calcium exchangers in rat odontoblasts. Cell Calcium 52:124-136. [DOI] [PubMed] [Google Scholar]
- Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. (2004). An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem 279:37423-37430. [DOI] [PubMed] [Google Scholar]
- Yeon KY, Chung G, Shin MS, Jung SJ, Kim JS, Oh SB. (2009). Adult rat odontoblasts lack noxious thermal sensitivity. J Dent Res 88:328-332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.