Abstract
Musculoskeletal examinations provide informative and valuable quantitative insight into muscle and bone health. DXA is one mainstream tool used to accurately and reliably determine body composition components and bone mass characteristics in-vivo. Presently, whole body scan models separate the body into axial and appendicular regions, however there is a need for localised appendicular segmentation models to further examine regions of interest within the upper and lower extremities. Similarly, inconsistencies pertaining to patient positioning exist in the literature which influence measurement precision and analysis outcomes highlighting a need for standardised procedure. This paper provides standardised and reproducible: 1) positioning and analysis procedures using DXA and 2) reliable segmental examinations through descriptive appendicular boundaries. Whole-body scans were performed on forty-six (n = 46) football athletes (age: 22.9 ± 4.3 yrs; height: 1.85 ± 0.07 cm; weight: 87.4 ± 10.3 kg; body fat: 11.4 ± 4.5 %) using DXA. All segments across all scans were analysed three times by the main investigator on three separate days, and by three independent investigators a week following the original analysis. To examine intra-rater and inter-rater, between day and researcher reliability, coefficients of variation (CV) and intraclass correlation coefficients (ICC) were determined. Positioning and segmental analysis procedures presented in this study produced very high, nearly perfect intra-tester (CV ≤ 2.0%; ICC ≥ 0.988) and inter-tester (CV ≤ 2.4%; ICC ≥ 0.980) reliability, demonstrating excellent reproducibility within and between practitioners. Standardised examinations of axial and appendicular segments are necessary. Future studies aiming to quantify and report segmental analyses of the upper- and lower-body musculoskeletal properties using whole-body DXA scans are encouraged to use the patient positioning and image analysis procedures outlined in this paper.
Key points.
Musculoskeletal examinations using DXA technology require highly standardised and reproducible patient positioning and image analysis procedures to accurately measure and monitor axial, appendicular and segmental regions of interest.
Internal rotation and fixation of the lower-limbs is strongly recommended during whole-body DXA scans to prevent undesired movement, improve frontal mass accessibility and enhance ankle joint visibility during scan performance and analysis.
Appendicular segmental analyses using whole-body DXA scans are highly reliable for all regional upper-body and lower-body segmentations, with hard-tissue (CV ≤ 1.5%; R ≥ 0.990) achieving greater reliability and lower error than soft-tissue (CV ≤ 2.4%; R ≥ 0.980) masses when using our appendicular segmental boundaries.
Key words: Segmentation, muscle, bone, standardisation, composition, regional, method
Introduction
Biomechanical models and human movement data largely relies on obtained segmental values to estimate and replicate the true anatomical structure of its subjects (Durkin et al., 2002; Lee et al., 2009a; Pearsall and Costigan, 1999; Pearsall and Reid, 1994; Rao et al., 2006). In particular, body segment parameters and body composition components provide important data for biomechanists and exercise physiologists in the analysis of human movement and health status in both sporting and clinical contexts (Lee et al., 2009a; Pfeiffer et al., 2010; Pearsall and Reid, 1994; Rao et al., 2006). Biomechanically, segmental anthropometric parameters are routinely estimated for use within inverse dynamics equations in order to calculate intersegmental forces and net joint moments during gait, kicking, throwing and striking actions (Davids et al., 2000; Ganley and Powers, 2004a; 2004b; Lee et al., 2009b; Pearsall and Costigan, 1999; Putnam, 1991; 1993; Rao et al., 2006). Physiologically, body composition and tissue mass assessments of bone, muscle and fat are used to diagnose and monitor health status and changes in paediatric, athletic, adult and geriatric populations, while also used to examine the interventional efficacy of exercise, pharmacology or nutritional programs (Abrahamyan et al., 2008; Beaumesnil et al., 2011; Bridge et al., 2009;Fawzy et al., 2011; Pfeiffer et al., 2010; Veale et al., 2010; Miller et al., 2009).
Dual energy x-ray absorptiometry (DXA) is a commonly used two-dimensional in-vivo imaging technique which is able to capture full body projections of mass densities through x-ray technology (Pfeiffer et al., 2010; Durkin et al., 2002). In comparison with other imaging techniques, it is inexpensive, exposes the participant to substantially less radiation, is widely available for use, and produces minimal error when employed to ascertain body segment parameter measures (Durkin and Dowling, 2006; Heymsfield et al., 1990; Lee et al., 2009a; Pietrobelli et al., 1996). Specifically, DXA functions by emitting two collimated x-ray beams of alternating frequencies which pass through the individual being scanned (Durkin et al., 2002; Hologic, 2004), the resulting attenuation coefficients are used within a three-compartment model to differentiate between bone, fat and lean tissue mass (Ganley and Powers, 2001; 2004a; Pietrobelli et al., 1996). On completion, the scan creates a pixel-by-pixel, two-dimensional image reconstruction of the participant, allowing whole-body and regional analyses to be performed thereafter (Lee et al., 2009b; Ganley and Powers, 2004b).
Presently, full-body DXA scans generate automated and mandatory regions of interest in accordance with a standardised whole body model which separates the subject into basic axial and appendicular sections (Durkin et al., 2002; Heymsfield et al., 1990). While this provides useful diagnostic information in clinical applications, more specific detail is usually required under scientific research and rehabilitative contexts to determine body segment parameters and provide localised composite measures (Ganley and Powers, 2004a; Lee et al., 2009a). Specifically, the reliable quantification of upper and lower extremity segmental masses is highly important for use in sensitive biomechanical models and human movement research when examining normal and abnormal gait (Lee et al. 2009b; Holmes et al., 2005; Pearsall and Costigan, 1999; Rossi et al., 2013), or to objectively examine more specific and localised changes in composition during longitudinal exercise interventions as a mechanism to assess program effectiveness (Mueller et al., 2013; Sillanpää et al., 2013; Wood et al., 2010).
Unfortunately, no definitive boundaries have been reliably described to manually and uniformly distinguish between individual segments of the upper and lower extremities using scan technology. While several authors have attempted to define individual segments using DXA (Burkhart et al., 2009; Chambers et al., 2011; Hart et al., 2013; 2014; Rossi et al., 2013), numerous methodological inconsistencies exist. Variations in subject positioning and segmental boundaries employed during the scan and analysis process leads to further complications when interpreting and comparing data sets in the literature, and may exacerbate other inherent limitations of DXA. Due to DXA’s capability to accurately quantify the mass of defined regions (Glickman et al., 2004; Haarbo et al., 1991; Kohrt, 1998; Lee et al., 2009b), it is the purpose of this paper to: 1) present a standardised methodological approach to assess and analyse in-vivo appendicular segmental mass through positioning and analysis considerations; and 2) determine the between-day intra-tester and inter-tester reliability of the proposed regional boundaries and segmental cross-sections using DXA technology.
Methods
Participants
Forty-six football athletes (age: 22.9 ± 4.3 yrs; height: 1.85 ± 0.07 cm; mass: 87.4 ± 10.3 kg; body fat: 11.4 ± 4.5 %) were recruited from the Australian Football League (AFL) and Western Australian Football League (WAFL) for participation in this study. All participants were absent of injury and contraindication and were required to wear minimal, light clothing free of metallic material. Any participants with metallic surgical implants were excluded from this study. Ethics approval was obtained from the University Human Research Ethics Committee. Participants were notified of potential risks and provided written informed consent. Data collection and management procedures conformed to the Code of Ethics (World Medical Association), Declaration of Helsinki.
Anthropometry
Standing height was recorded to the nearest 0.1 centimetre using a wall-mounted stadiometer (Model 222, Seca, Hamburg, DE), with body mass recorded to the nearest 0.1 kilogram using an electronic weighing scale (AE Adams CPW Plus-200, Adam Equipment Inc, CT, USA). All anthropometric measures were performed by the same accredited exercise scientist. Standing height was assessed three times for each participant, with the average of the three trials retained for analysis. All measures were reliably performed by the same accredited exercise scientist (CV ≤ 0.20%; ICC ≥ 0.998).
Scan procedure
Whole body scans were performed using DXA (Hologic Discovery A, Waltham, MA) in order to quantify the magnitude and quality of full-body mass distribution (lean, fat, bone and total). Participants assumed a stationary, supine position on the scanning bed with both arms pronated by their side. To ensure consistent and reproducible positioning, the DXA operator manually assisted participants in order to: 1) straighten the head, neck and torso parallel to the long-axis of the scan bed; 2) position the shoulders and pelvis perpendicular to the long-axis of the scan bed; 3) place both arms in pronation by their side; 4) place legs at shoulder width with 45° internal rotation; and 5) fixate feet together using strapping tape to minimise incidental movement and for the participants comfort, within the DXA scanning zone (Figure 1). This has been shown to produce a scan-rescan coefficient of variation under 1% in our laboratory for body composition components (Pfeiffer et al, 2010).
Figure 1.
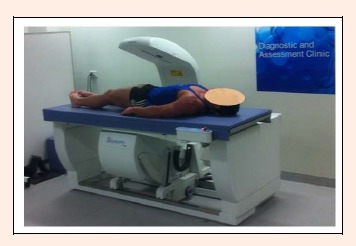
Subject positioning for use during whole-body DXA scans, with the head, neck and torso positioned parallel to the long-axis of the scan bed; the shoulders and pelvis positioned perpendicular to the long-axis of the scan bed; arms pronated by the side; and legs internally rotated to 45° and fixated together to minimise incidental movement.
Scan analysis
Upon scan completion, a two-dimensional image was automatically generated for scan analysis purposes. Using the in-built scan analysis software (Version 12.4; QDR for Windows, Hologic, Waltham, MA), the full-body images were separated into axial and appendicular regions using the predefined and mandatory whole body model as required by the software (Hologic, 2004). Further analysis was subsequently performed to manually identify and assess appendicular segmental masses. Specifically, using the sub-region analysis tool, customised regions-of-interest (ROI) were drawn to capture twelve segments: the left upper arm, right upper arm, left forearm, right forearm, left hand, right hand, left thigh, right thigh, left shank, right shank, left foot and right foot regions (Figure 2). Explicit descriptions of the proximal and distal boundaries used to identify these segments are provided (Table 1).
Figure 2.
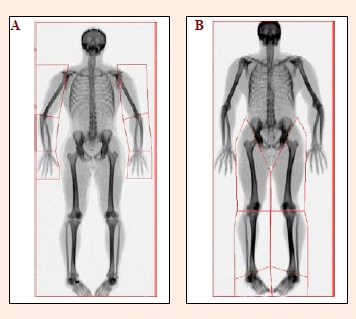
Example illustrations showing applied (A) upper body regions and (B) lower body regions during the scan analysis process. Specifically, internal rotation and fixation of the lower-limbs allows greater visibility of the talocrural joint for more accurate and reliable segmentation.
Table 1.
Appendicular definitions for each segment outlining proximal and distal boundaries with descriptive landmarks.
| Segment | Proximal boundary | Distal boundary | Basic description a |
|---|---|---|---|
| Upper Arm | Glenohumeral Joint | Humeroulnar Joint | Commences between the head of the Humerus and glenoid fossa of the Scapula, separating the upper arm from the trunk; Ending at the elbow axis, noted by the trochlea of the Humerus and olecranon process of the Ulna. |
| Forearm | Humeroulnar Joint | Ulnocarpal Joint | Commences at the elbow axis (described above); Ending through the wrist axis, noted by the styloid process of the Ulna, and the base of the Pisiform, Lunate and Scaphoid carpal bones. |
| Hand | Ulnocarpal Joint | Distal Phalanges | Commences through the wrist axis (described above); Ending at the most distal point of the phalanges of the hand. |
| Thigh | Femoral Head | Tibiofemoral Joint | Commences beneath the head of the Femur along the line of the anterior superior iliac spine (ASIS) and inferior ramus of the pubis; Ending through knee axis, noted as the ‘tibial plateau’ (space between the femoral and tibial condyles). |
| Shank | Tibiofemoral Joint | Talocrural Joint | Commences through the knee axis (described above); Ending through the ankle junction, noted as the articulation between the Talus, Tibia and Fibula, spanning beneath the medial and lateral malleoli. |
| Foot | Talocrural Joint | Distal Phalanges | Commences through the ankle junction (described above); Ending at the most distal point of the phalanges of the foot. |
a Mass of pelvis is not included
Sub-region creation
During sub-region creation, zones can be repositioned and manipulated by modifying the length, angle and location of lines, providing operators with full sub-region customisation. As composite mass is assigned to the scan image on a pixel-by-pixel basis, consideration must be given to the manner in which lines cross required segment and joint. In particular, lines must: 1) dissect the point of articulation within a joint, 2) capture all bones and soft tissue within the segment, and 3) not encroach on other sub-regions. This enables the prevention of regional loss or overlap, protecting against inflated or deflated segmental outputs, while also enabling consistent standards for analysis across scans within and between research studies.
Statistical analysis
All twelve appendicular segments for all forty-six scans were analysed three times by the same investigator on two separate days, and were re-analysed by three other independent investigators following the initial analysis. All investigators were required to follow the sub-region analysis process described earlier in order to assess the between-day intra-tester and inter-tester reliability for appendicular segmentation. Intra-class correlation coefficients (ICC) and coefficients of variation (CV) were calculated using statistical analysis software (SPSS, Version 17.0; Chicago, IL). The strength of reliability for ICC coefficients was classified in accordance with Hopkins (2002): R ≥ 0.3 is moderate; R ≥ 0.5 is strong; R ≥ 0.7 is very strong; R ≥ 0.9 is nearly perfect; and R = 1.0 is perfect. Coefficients of variation below 5% were considered highly reliable (Hopkins, 2002).
Results
Intra-tester reliability
Reliability coefficients for between-day, intra-tester scan analysis across all appendicular segments are provided (Table 2). Regional analysis demonstrated nearly perfect intra-tester reliability for segmental upper body soft-tissue (R = 0.988 to 0.998) and hard-tissue (R = 0.995 to 0.998) masses; as well as segmental lower body soft-tissue (R = 0.989 to 0.998) and hard-tissue (R = 0.992 to 0.999) masses. Further, intra-tester coefficients of variation demonstrated very high reliability for segmental upper body soft-tissue (CV = 1.5 to 2.0) and hard-tissue (CV = 1.1 to 1.3) masses; as well as segmental lower body soft-tissue (CV = 0.8 to 2.0) and hard-tissue (CV = 0.6 to 1.4) masses.
Table 2.
Intra-tester and inter-tester reliability coefficients of appendicular scan analysis procedures for the upper body and lower body for hard-tissue (bone), soft tissue (fat, lean) and total mass.
| Sub-Regions | Intra-tester Coefficients | Inter-tester Coefficients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat (g) | Lean (g) | Bone (g) | Total (g) | Fat (g) | Lean (g) | Bone (g) | Total (g) | |||||||||
| CV | ICC | CV | ICC | CV | ICC | CV | ICC | CV | ICC | CV | ICC | CV | ICC | CV | ICC | |
| Upper Body | ||||||||||||||||
| Left Upper Arm | 1.7 | .998 | 1.7 | .995 | 1.2 | .997 | 1.6 | .991 | 2.2 | .983 | 2.1 | .987 | 1.4 | .995 | 2.2 | .983 |
| Left Forearm | 1.7 | .995 | 1.6 | .993 | 1.3 | .995 | 1.7 | .988 | 1.9 | .991 | 1.8 | .990 | 1.4 | .993 | 1.9 | .987 |
| Left Hand | 1.8 | .997 | 1.6 | .992 | 1.2 | .997 | 1.5 | .994 | 2.3 | .987 | 2.2 | .985 | 1.3 | .995 | 2.0 | .990 |
| Right Upper Arm | 1.8 | .993 | 1.9 | .989 | 1.1 | .998 | 1.7 | .989 | 2.0 | .991 | 2.0 | .986 | 1.2 | .996 | 2.1 | .986 |
| Right Forearm | 1.9 | .992 | 1.8 | .990 | 1.3 | .995 | 1.6 | .990 | 2.1 | .989 | 1.9 | .992 | 1.5 | .991 | 2.0 | .989 |
| Right Hand | 2.0 | .988 | 1.9 | .990 | 1.3 | .996 | 1.6 | .992 | 2.4 | .980 | 2.2 | .985 | 1.3 | .992 | 2.0 | .991 |
| Lower Body | ||||||||||||||||
| Left Thigh | 1.9 | .991 | 2.0 | .989 | 1.4 | .992 | 1.8 | .991 | 2.1 | .990 | 2.2 | .987 | 1.4 | .991 | 2.0 | .988 |
| Left Shank | .8 | .997 | 0.9 | .998 | .7 | .999 | 1.0 | .995 | .9 | .998 | 1.0 | .996 | 1.0 | .996 | 1.1 | .995 |
| Left Foot | 2.0 | .989 | 1.8 | .992 | 1.0 | .997 | 1.9 | .989 | 2.1 | .987 | 1.9 | .990 | 1.1 | .995 | 2.2 | .986 |
| Right Thigh | 1.8 | .993 | 2.0 | .990 | 1.3 | .994 | 1.8 | .989 | 1.9 | .991 | 2.1 | .988 | 1.4 | .992 | 2.1 | .989 |
| Right Shank | .7 | .998 | 0.9 | .997 | .6 | .998 | .9 | .996 | .9 | .996 | .9 | .995 | .9 | .994 | 1.0 | .995 |
| Right Foot | 1.9 | .992 | 1.9 | .993 | 1.1 | .993 | 1.8 | .990 | 1.8 | .993 | 2.0 | .991 | 1.2 | .990 | 2.1 | .988 |
Inter-tester reliability
Reliability coefficients for inter-tester scan analysis across all appendicular segments are provided (Table 2). Regional analysis demonstrated nearly perfect inter-tester reliability for segmental upper body soft-tissue (R = 0.980 to 0.992) and hard-tissue (R = 0.991 to 0.996) masses; as well as segmental lower body soft-tissue (R = 0.987 to0.998) and hard-tissue (R = 0.990 to 0.996) masses. Further, inter-tester coefficients of variation also demonstrated very high reliability for segmental upper body soft-tissue (CV = 1.8 to 2.4) and hard-tissue (CV = 1.2 to 1.5) masses; as well as lower body soft-tissue (CV = 0.9 to 2.2) and hard-tissue (CV = 0.9 to 1.4) masses.
Discussion
Despite biomechanical and physiological relevance, precise quantifications of segmental inertial properties and composite measures of the appendicular skeleton remain largely inadequate (Durkin and Dowling, 2006; Heymsfield et al., 1990; Wells, 2009). While current in-vivo techniques provide broad insight into axial and appendicular regions (Fuller et al., 1992; Heysmfield et al., 1990; Visser et al., 1999), no definitive boundaries have been reliably described to further distinguish between individual segments of the upper and lower extremities using scan technology. Several authors have attempted to further define appendicular segments (Durkin and Dowling, 2006; Lee et al., 2009a; 2009b; Ganley and Powers, 2004a; 2004b; Visser et al., 1999). Unfortunately, numerous methodological irregularities exist in the literature pertaining to frontal plane subject positioning and segmental analyses employed during scan procedures, with scarce procedural reliability data reported. This study therefore aimed to: 1) present a standardised methodological approach to assess and analyse in-vivo appendicular segmental mass through positioning and analysis considerations; and 2) determine the between-day intra-tester and inter-tester reliability of the proposed regional boundaries and segmental cross-sections using DXA technology.
Positioning and analysis methodologies presented in this paper resulted in very high, nearly perfect reliability within and between researchers when examining hard- and soft- tissue masses across all segments of the upper and lower extremity (CV ≤ 2.4%; R ≥ 0.980). While no observable difference in reliability was evident between upper-body and lower-body segments; hard-tissue (CV ≤ 1.5%; R ≥ 0.990) achieved greater reliability than soft-tissue (CV ≤ 2.4%; R ≥ 0.980) masses through-out. In addition, the scan-rescan coefficient of variation for this subject positioning procedure has routinely produced a score below 1% (Hologic, 2004; Pfeiffer et al, 2010), with our reported manual segmentation procedure producing an average coefficient of variation below 1.6% across all appendicular segments and tissue masses. This is important as body segment parameters obtained using DXA technology are routinely used in biomechanical models that are highly sensitive to data input fluctuations (Ganley and Powers, 2004a; Lenzi et al., 2003; Lee et al., 2009b; Pearsall and Costigan, 1999; Rao et al., 2006); and are further used to measure and monitor physiological changes in segmental mass during cross-sectional investigations and longitudinal interventions.
Patient positioning prior to scan commencement requires considerable attention as DXA is only capable of uniplanar measurement (Rossi et al, 2013; Wells, 2009), thus limited to quantifying frontal mass distribution. As a result, any limb rotations, or lack thereof can influence the scan re-scan accuracy of DXA (Wells, 2009), and subsequently also influence the visibility of joints required by practitioners to accurately apply regional segmentations during post-scan analysis (Lee and Gallagher, 2008). While upper body positioning has been satisfactorily described in the literature (Durkin and Dowling, 2002; 2003), substantially different lower body scan positions have been used with various levels of external (Ganley and Powers, 2004a; 2004b), neutral (Durkin and Dowling, 2006; Rossi et al, 2013) or internal rotations (Hart et al, 2013; Hart et al, 2014; Lee et al, 2009a; 2009b) of the thigh in conjunction with neutral or plantar-flexed feet. The very low error provided by our standardised methodological approach stems from the careful consideration given to subject positioning prior to scan commencement. This study explicitly prescribes internal rotation of the thigh with neutral foot position. The advantages of internally rotating the thigh whilst maintaining neutral foot position is three-fold: 1) It maximises the available surface area of fat and fat-free mass of the thigh, shank and foot segments viewable in the frontal plane, 2) provides greater visibility of the calcaneus (heel) and talocrural joint (ankle) required for lower-body segmentation precision, and 3) allows the operator to fixate the subjects feet together within the scanning zone in order to prevent unwarranted or incidental movement or disruption during the scanning process. This positioning and segmentation protocol is in agreement with numerous recent papers in the field (Hart et al, 2013; 2014; Lee et al, 2009a; 2009b) who did not report methodological reliability.
Scan analysis and regional segmentations are reliant upon image quality which is a product of subject positioning during the scanning process. As composite mass is assigned to the scanned image on a pixel-by-pixel basis (Ganley and Powers, 2004b; Lee et al., 2009a), outcomes are influenced by the quantity and distribution of mass viewable in the frontal plane (Durkin et al., 2002; Lee et al., 2009b). Regional boundaries are applied to the resultant two dimensional scan image to define the limits of each area for analysis. Presently, no model exists for appendicular segmental analyses of the upper and lower extremities using DXA. Several authors have attempted to differentiate between segments of the extremities (Durkin and Dowling, 2006; Ganley and Powers, 2004a; 2004b; Lee et al., 2009a; 2009b; Visier et al., 1999). However, anatomical inconsistencies exist in regarding locations for body segmentation, further confounded by inadequate descriptions for the purpose of reproduction in practical or research contexts. Our reported methodology provides clear graphical and written guidance for practitioners to adequately and consistently produce segmental appendicular musculoskeletal examinations with very high reliability and very low error. This study explicitly defined appendicular boundaries using clear anatomical descriptors that: 1) are easily visible and accessible through images produced by DXA; 2) capture the maximum amount of segmental tissue as possible; and 3) are consistently reproducible through scan re-scan, and analysis re-analysis procedures. The anatomical and segmental boundaries recommended by this study are largely in agreement with two recent papers in the field (Burkhart et al., 2009; Chambers et al., 2011).
Conclusion
Positioning and analysis methodologies presented in this paper provide practitioners with a standardised, reproducible and robust procedure to quantify appendicular segmental masses using DXA technology (CV ≤ 2.4%; ICC ≥ 0.980). Owing to the uniplanar, two-dimensional nature of DXA, careful consideration of patient positioning specific to limb rotations is necessary to optimise tissue mass accessibility in the frontal plane during measurement, and joint visibility for segmentation during regional analysis. Practitioners are encouraged to use this methodological procedure to examine localised appendicular segmental information. It is recommended that familiarisation and training is provided prior to using this methodological approach to ensure very high reliability and very low error produced by this standardised protocol. Practitioners wishing to cross-sectionally examine or longitudinally monitor changes in appendicular musculoskeletal segmental masses are encouraged to use this standardised methodological procedure, and to provide detailed methodological illustrations and descriptions of subject positioning and scan analysis when disseminating research outcomes.
Acknowledgments
The authors would like to thank the ECU Health and Wellness Institute and Vario Wellness Clinic for the use of their facility and equipment. The authors would also like to thank Jason Weber, Michael Dobbin and Chris Dorman for the use of their athletes.
Biographies

Nicolas H. HART
Employment
Research Fellow, Exercise Oncology
Degree
PhD, CSCS, ESSAM.
Research interests
Exercise as medicine; Injury prevention; Musculoskeletal screening, training and adaptation; Bone strength and disease.
E-mail: n.hart@ecu.edu.au

Sophia NIMPHIUS
Employment
Senior Lecturer, Strength & Conditioning
Degree
PhD, CSCS*D.
Research interests
Profiling and long-term training adaptations of youth and elite athletes; Measurement and development of strength and power; Gender differences in athletic performance.
E-mail: s.nimphius@ecu.edu.au

Tania SPITERI
Employment
Lecturer, Motor Control
Degree
PhD
Research interests
Agility, Change of direction, Athlete profiling; Skill acquisition; Gender differences in athletic performance; Strength and power development.
E-mail: tania.spiteri@nd.edu.au

Jodie COCHRANE
Employment
Senior Lecturer, Biomechanics
Degree
PhD
Research interests
Sports biomechanics; Neuromuscular training and injury prevention
E-mail: j.wilkie@ecu.edu.au

Robert NEWTON
Employment
Foundation Professor, Exercise and Sport Science.
Degree
PhD, CSCS*D, AEP, FESSA, FNSCA
Research interests
Muscular strength and power; athletic profiling and long-term monitoring; Exercise as medicine; Exercise oncology.
E-mail: r.newton@ecu.edu.au
References
- Abrahamyan D.O., Gazarian A., Braillon P.M. (2008) Estimation of stature and length of limb segments in children and adolescents from whole-body dual-energy X-ray absorptiometry scans. Pediatric Radiology 38(3), 311-315. [DOI] [PubMed] [Google Scholar]
- Beaumesnil M., Chaillou E., Wagner A.C., Rouquette A., Audran M., Ginies J.L. (2011) Body composition analysis in patients with cystic fibrosis. Comparison of 3 methods: dual energy x-ray absorptiometry, bioelectrical impedance analysis, and skinfold measurements. Archives of Paediatrics 18(4), 370-375. [DOI] [PubMed] [Google Scholar]
- Bridge P., Pocock N.A., Nguyen T., Munns C., Cowell C.T., Thompson M.W. (2009) Prediction of appendicular skeletal and fat mass in children: excellent concordance of dual-energy X-ray absorptiometry and magnetic resonance imaging. Journal of Pediatric Endocrinology and Metabolism 22(9), 795-804. [DOI] [PubMed] [Google Scholar]
- Burkhart T.A., Arthurs K.L., Andrews D.M. (2009) Manual segmentation of DXA scan images results in reliable upper and lower extremity soft and rigid tissue mass estimates. Journal of Biomechanics 42(8), 1138-1142. [DOI] [PubMed] [Google Scholar]
- Chambers A.J., Sukits A.L., McCrory J.L., Cham R. (2011) Differences in geriatric anthropometric data between DXA-based subject-specific estimates and non-age-specific traditional regression models. Journal of Applied Biomechanics 27(3), 197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids K., Lees A., Burwitz L. (2000) Understanding and measuring coordination and control in kicking skills in soccer: Implications for talent identification and skill acquisition. Journal of Sports Sciences 18(9), 703-714. [DOI] [PubMed] [Google Scholar]
- Dempster W.T. (1955) Space requirements of the seated operator. WADC Technical Report (TR-59-159). OH: Wright-Patterson Air Force Base. [Google Scholar]
- Durkin J.L., Dowling J.J. (2003) Analysis of body segment parameter differences between four human populations and the estimation errors of four popular mathematical models. Journal of Biomechanical Engineering 125(4), 515-522. [DOI] [PubMed] [Google Scholar]
- Durkin J.L., Dowling J.J. (2006) Body segment parameter estimation of the human lower leg using an elliptical model with validation from DEXA. Annals of Biomedical Engineering 34(9), 1483-1493. [DOI] [PubMed] [Google Scholar]
- Durkin J.L., Dowling J.J., Andrews D.M. (2002) The measurement of body segment inertial parameters using dual energy X-ray absorptiometry. Journal of Biomechanics 35(12), 1575-1580. [DOI] [PubMed] [Google Scholar]
- Fawzy T., Muttappallymyalil J., Sreedharan J., Ahmed A., Alshamsi S.O., Al Ali M.S., Balooshi K.A.A. (2011). Association between Body Mass Index and Bone Mineral Density in Patients Referred for Dual-Energy X-Ray Absorptiometry Scan in Ajman, UAE. Journal of Osteoporosis Article ID 876309, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller N.J., Laskey M.A., Elia M. (1992) Assessment of the composition of major body regions by dual-energy X-ray absorptiometry (DEXA), with special reference to limb muscle mass. Clinical Physiology 12(3), 253-266. [DOI] [PubMed] [Google Scholar]
- Ganley K.J., Powers C.M. (2001) The use of dual energy X-ray absorptiometry in determining subject-specific anthropometric measures for kinetic analysis during gait. Gait and Posture 13, 271-272. [Google Scholar]
- Ganley K.J., Powers C.M. (2004a) Anthropometric parameters in children: a comparison of values obtained from dual energy x-ray absorptiometry and cadaver-based estimates. Gait and Posture 19(2), 133-140. [DOI] [PubMed] [Google Scholar]
- Ganley K.J., Powers C. M. (2004b) Determination of lower extremity anthropometric parameters using dual energy X-ray absorptiometry: the influence on net joint moments during gait. Clinical Biomechanics 19(1), 50-56. [DOI] [PubMed] [Google Scholar]
- Glickman S.G., Marn C.S., Supiano M.A., Dengel D.R. (2004) Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. Journal of Applied Physiology 97(2), 509-514. [DOI] [PubMed] [Google Scholar]
- Haarbo J., Gotfredsen A., Hassager C., Christiansen C. (1991) Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clinical Physiology 11(4), 331-341. [DOI] [PubMed] [Google Scholar]
- Hart N.H., Nimphius S., Spiteri T., Newton R.U. (2014) Leg strength and lean mass asymmetry influences kicking performance in Australian Football. Journal of Sports Science & Medicine 13(1), 157. [PMC free article] [PubMed] [Google Scholar]
- Hart N.H., Nimphius S., Cochrane J.L., Newton R.U. (2013) Leg mass characteristics of Accurate and Inaccurate kickers – an Australian Football perspective. Journal of Sports Sciences 31(15), 1647-1655. [DOI] [PubMed] [Google Scholar]
- Heymsfield S.B., Smith R., Aulet M., Bensen B., Lichtman S., Wang J., Pierson R.N. (1990) Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. American Journal of Clinical Nutrition 52(2), 214-218. [DOI] [PubMed] [Google Scholar]
- Hologic. (2004) QDR for Windows XP Reference Manual. Hologic Inc. [Google Scholar]
- Hopkins W. (2000) Measures of reliability in sports medicine and science. Sports Medicine 30(1), 1-15. [DOI] [PubMed] [Google Scholar]
- Hopkins W. (2002) A scale of magnitudes for effect statistics. Available from URL: http://www.sportsci.org/resource/stats/effect-mag.html [Google Scholar]
- Kohrt W.M. (1998) Preliminary evidence that DEXA provides an accurate assessment of body composition. Journal of Applied Physiology 84(1), 372-377. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Gallagher D. (2008) Assessment methods in human body composition. Current Opinion in Clinical Nutrition and Metabolic Care 11(5), 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.K., Koh M., Fang A.C., Le S.N., Balasekaran G. (2009a) Estimation of Body Segment Parameters Using Dual Energy Absorptiometry and 3-D Exterior Geometry. 13th International Conference on Biomedical Engineering 23(5), 1777-1780. [Google Scholar]
- Lee M.K., Le N.S., Fang A.C., Koh M.T. (2009b) Measurement of body segment parameters using dual energy X-ray absorptiometry and three-dimensional geometry: an application in gait analysis. Journal of Biomechanics 42(3), 217-222. [DOI] [PubMed] [Google Scholar]
- Lenzi D., Cappello A., Chiari L. (2003) Influence of body segment parameters and modeling assumptions on the estimate of center of mass trajectory. Journal of Biomechanics 36(9), 1335-1341. [DOI] [PubMed] [Google Scholar]
- Mueller S.M., Anliker E., Knechtle P., Knechtle B., Toigo M. (2013) Changes in body composition in triathletes during an Ironman race. European Journal of Applied Physiology 113(9), 2343-2352. [DOI] [PubMed] [Google Scholar]
- Miller A., Strauss B.J., Mol S., Kyoong A., Holmes P.H., Finlay P., Bardin P.G., Guy P. (2009) Dual-energy X-ray absorptiometry is the method of choice to assess body composition in COPD. Respirology 14(3), 411-418. [DOI] [PubMed] [Google Scholar]
- Pavol M.J., Owings T.M., Grabiner M.D. (2002) Body segment inertial parameter estimation for the general population of older adults. Journal of Biomechanics 35(5), 707-712. [DOI] [PubMed] [Google Scholar]
- Pearsall D.J., Costigan P.A. (1999) The effect of segment parameter error on gait analysis results. Gait and Posture 9(3), 173-183. [DOI] [PubMed] [Google Scholar]
- Pearsall D.J., Reid J.G. (1994) The study of human body segment parameters in biomechanics. An historical review and current status report. Sports Medicine 18(2), 126-140. [DOI] [PubMed] [Google Scholar]
- Pfeiffer J.J., Galvao D.A., Gibbs Z., Smith K., Turner D., Foster J., Martins R., Newton R.U. (2010) Strength and functional characteristics of men and women 65 years and older. Rejuvenation Research 13(1), 75-82. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A., Formica C., Wang Z., Heymsfield S.B. (1996) Dual-energy X-ray absorptiometry body composition model: review of physical concepts. American Journal of Physiology 271(6 Pt 1), E941-951. [DOI] [PubMed] [Google Scholar]
- Putnam C.A. (1991) A segment interaction analysis of proximal-to-distal sequential segment motion patterns. Medicine and Science in Sports and Exercise 23(1), 130-144. [PubMed] [Google Scholar]
- Putnam C.A. (1993) Sequential motions of body segments in striking and throwing skills: descriptions and explanations. Journal of Biomechanics 26(Suppl. 1), 125-135. [DOI] [PubMed] [Google Scholar]
- Rao G., Amarantini D., Berton E., Favier D. (2006) Influence of body segments' parameters estimation models on inverse dynamics solutions during gait. Journal of Biomechanics 39(8), 1531-1536. [DOI] [PubMed] [Google Scholar]
- Reid J.G., Jensen R.K. (1990) Human body segment inertia parameters: a survey and status report. Exercise and Sport Sciences Reviews 18(1), 225-241. [PubMed] [Google Scholar]
- Rossi M., Lyttle A., El-Sallam A., Benjanuvatra N., Blanksby B. (2013) Body Segment Inertial Parameters of elite swimmers Using DXA and indirect Methods. Journal of Sports Science and Medicine 12(4), 761-775. [PMC free article] [PubMed] [Google Scholar]
- Sillanpää E., Häkkinen A., Häkkinen K. (2013) Body composition changes by DXA, BIA and skinfolds during exercise training in women. European Journal of Applied Physiology 113(9), 2331-2341. [DOI] [PubMed] [Google Scholar]
- Veale J.P., Pearce A.J., Buttifant D., Carlson J.S. (2010) Anthropometric profiling of elite junior and senior Australian football players. International Journal of Sports Physiology and Performance 5(4), 509-520. [DOI] [PubMed] [Google Scholar]
- Visser M., Fuerst T., Lang T., Salamone L., Harris T.B. (1999) Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. Journal of Applied Physiology 87(4), 1513-1520. [DOI] [PubMed] [Google Scholar]
- Wells J. (2009) The limitations of DXA for body composition assessment. Endocrine Abstracts 19, S78. [Google Scholar]
- Winter D.A. (1990) Biomechanics and Motor Control of Human Movement. 2nd edition Toronto: John Wiley & Sons, Inc. [Google Scholar]
- Wood P.S., Krüger P.E., Grant C.C. (2010) DEXA-assessed regional body composition changes in young female military soldiers following 12-weeks of periodised training. Ergonomics 53(4), 537-547. [DOI] [PubMed] [Google Scholar]


