Abstract
This study was designed to identify the blood lactate threshold (LT2) for the half squat (HS) and to examine cardiorespiratory and metabolic variables during a HS test performed at a work intensity corresponding to the LT2. Twenty-four healthy men completed 3 test sessions. In the first, their one-repetition maximum (1RM) was determined for the HS. In the second session, a resistance HS incremental-load test was performed to determine LT2. Finally, in the third session, subjects performed a constant-load HS exercise at the load corresponding to the LT2 (21 sets of 15 repetitions with 1 min of rest between sets). In this last test, blood samples were collected for lactate determination before the test and 30 s after the end of set (S) 3, S6, S9, S12, S15, S18 and S21. During the test, heart rate (HR) was telemetrically monitored and oxygen consumption (VO2), carbon dioxide production (VCO2), minute ventilation (VE), respiratory exchange ratio (RER), ventilatory equivalent for O2 (VE·VO2-1) and ventilatory equivalent for CO2 (VE·VCO2-1) were monitored using a breath-by-breath respiratory gas analyzer. The mean LT2 for the participants was 24.8 ± 4.8% 1RM. Blood lactate concentrations showed no significant differences between sets 3 and 21 of exercise (p = 1.000). HR failed to vary between S6 and S21 (p > 1.000). The respiratory variables VO2, VCO2, and VE·VCO2-1 stabilized from S3 to the end of the constant-load HS test (p = 0.471, p = 0.136, p = 1.000), while VE and VE·VO2-1 stabilized from S6 to S21. RER did not vary significantly across exercise sets (p = 0.103). The LT2 was readily identified in the incremental HS test. Cardiorespiratory and metabolic variables remained stable during this resistance exercise conducted at an exercise intensity corresponding to the LT2. These responses need to be confirmed for other resistance exercises and adaptations in these responses after a training program also need to be addressed.
Key points.
It can be identified lactate threshold at half-squat.
Exercise intensity is predominantly aerobic.
The duration of the half-squat can be maintained over time, ~30 min of discontinuous exercise (21 sets, 15 repetitions, 1 min rest).
Lactate threshold intensity may be suitable for older adults, sedentary individuals, patients or subjects with a lower functional capacity and even for resistance sports athletes.
Key words: Aerobic fitness/VO2max, anaerobic threshold, exercise physiology, strength training
Introduction
Resistance training (RT), besides providing gains in muscle strength, size, power and local endurance, may help avoid injury (Faude et al. 2009). However, to fulfill these objectives of RT, exercise performance needs to be optimized by objectively determining the ideal training load for each individual (González-Badillo and Sánchez-Medina, 2010). One way to do this is to calculate a relative load percentage of the one-repetition maximum (1RM), or maximal strength (Fry, 2004). Another way to determine the ideal exercise intensity is the use of movement velocity as an indicator of relative load in a given exercise, thus avoiding the need for a 1RM test (González-Badillo and Sánchez-Medina, 2010). As opposed to resistance exercise, in endurance sports such as running, cycling or swimming, the first and second thresholds of the triphasic model first described by Skinner and McLellan (1980) are often used as indicators of the training load and may be determined using several methods (Bosquet et al., 2002). In addition, a wide range of intensities is used in exercise prescription models for both healthy subjects and patients and these intensities are based on maximal heart rate (HR) and oxygen uptake (VO2) (Hofmann and Tschakert, 2011). This generates heterogeneous cardiovascular and metabolic responses, which makes it difficult to define adequate work zones and leads to errors. A solution to this problem could be the selection of training loads based on markers of submaximal exercise (thresholds or cutoffs) (Hofmann and Tschakert, 2011). One such method is to locate the lactate threshold (LT2), defined as the first significant sustained increase produced in blood lactate concentration above resting levels during incremental exercise (Meyer et al., 2005). The LT2 is considered a valid indicator of endurance performance (Faude et al., 2009) and is associated with a low/moderate exercise intensity in high-level endurance athletes (Lucía et al., 2001). In effect, this intensity reflected by the LT2 has been also described to help improve cardiorespiratory fitness in recreational sports (Faude et al., 2009), healthy adults and elderly persons (Ratamess et al., 2009; Simões et al., 2010; de Sousa et al., 2012), and patients (Meyer et al., 2005). The LT2 marks the upper limit of almost exclusively aerobic work, as an increase in blood lactate is related to a predominant anaerobic metabolism (Meyer et al., 2005). Thus, working at an individual's LT2 has the benefit that exercise can be performed over several hours (Faude et al., 2009). In addition, working at the LT2 could also help improve local muscular endurance through aerobic metabolism (Ratamess et al., 2009; Simões et al., 2010; de Sousa el al., 2012). Despite these implications of the LT2, its use as an indicator of exercise intensity in RT has been little addressed (Barros et al., 2004; de Sousa et al., 2011; 2012; Moreira et al., 2008; Oliveira et al., 2006; Simões et al., 2010; 2013). A possible reason for this could be that RT conducted at low or moderate loads (40%-70% 1RM) is dependent on anaerobic metabolism with muscle glycogen used as the main energy source (Collins et al., 1989) and it is difficult to determine the LT2 during RT, since blood lactate levels are elevated from the onset of exercise. In addition, RT induces the recruitment of fast twitch muscle fibers reducing the activity of oxidative enzymes (Dudley, 1988) and may also lead to a decrease in mitochondrial volume and capillary density, and thus limit aerobic performance (Kraemer et al., 1988). Such a predominance of anaerobic work will also impair the detection of the LT2. To address these difficulties in LT2 detection, a new method has been recently introduced that periodizes intensities in RT at the LT2 intensity (de Sousa et al., 2011). To develop this method, the authors identified the LT2 in the leg press (LP) (Barros et al., 2004; Moreira et al., 2008; Oliveira et al., 2006; Simões et al., 2010; 2013), biceps curl (elbow flexion) (Barros et al., 2004), and bench press (Oliveira et al., 2006; Moreira et al., 2008), and examined responses to constant-load exercise at the LT2 intensity (de Sousa et al., 2012). The findings of this last study indicate that cardiorespiratory and metabolic variables stabilize when the intensity is ~ 30% 1RM. Compared to LP, the leg movement in HS probably requires greater muscular activity. In a study comparing LP and squats, the latter were found to elicit greater muscle activation and achieved greater strength gains (Escamilla et al., 2001). According to Garhammer (1981), free-weight resistance exercise like squat shows more neuromuscular specificity and may be appropriate for transfer to sports activities. However, the literature lacks similar studies examining the LT2 in other forms of resistance exercise such as half squat (HS). This type of exercise is especially suitable for improving patterns of intra- and intermuscular coordination and resembles specific tasks involving an individual's own body weight (Ratamess et al., 2009), which are, in turn, essential for executing activities of daily living.
The aim of this study was thus to determine the exercise intensity corresponding to LT2 in a HS incremental resistance test. Once the LT2 had been established, we assessed the effects on metabolic and cardiorespiratory variables of an exercise protocol conducted at a constant exercise intensity equivalent to the LT2. The working hypothesis was that stable acute responses would persist over time.
Methods
Experimental approach to the problem
Each participant completed three HS exercise sessions, each separated by a rest period of 48 hours. In the first session, the individual’s 1RM was determined. In the second session, LT2 was determined in an incremental test, in which the load was increased in small percentage steps relative to the 1RM. Then, using the load corresponding to the LT2, an exercise protocol was designed for each subject to include periods of work and rest; this protocol was executed for approximately 31 min in the third session. Participants were instructed on the following established protocol for this exercise: 1) each repetition should last 2 s, 1 s for the eccentric and 1 s for the concentric phase (except the 1RM test); these phases were identified through visual and acoustic signals emitted by a metronome (except the 1RM test), 2) for the HS movement, the knees should be flexed to an angle of 90° (monitored using a goniometer).
Subjects
24 healthy young men (age 21.5±1.8 years; height 180.1±5.2 cm; weight 81.9±8.7 kg) were recruited among the undergraduates of the degree course in Physical Activity and Sport Sciences. Subjects were excluded if they were elite athletes or took any sort of medication or performance-enhancing drugs. The subjects were required to obtain a 1RM test result of at least 150 kg in the HS (mean 1RM = 203.7 ± 42.2 kg) (Table 1). The minimum experience in resistance training of the study participants was 6 months and they were all accustomed to HS exercise.
Table 1.
Characteristics of the study participants (n = 24). Data are means (±SD).
| Age (years) | 21.5 (1.8) |
| Weight (kg) | 81.9 (8.7) |
| Height (m) | 1.80 (.05) |
| IMC (kg·m2-1) | 25.2 (2.1) |
| 1RM (kg) | 203.7 (42.2) |
BMI = Body mass index; 1RM = one-repetition maximum.
Participants were informed of the experimental procedures and signed a consent form before performing the tests. During the study, subjects refrained from participating in other strength or endurance sports. They were also instructed not to eat, smoke or drink tea or coffee in the two hours prior to the tests (they were allowed some water). Participants performed all tests at the same time each day. The study protocol received institutional review board approval (Department of Physical Activity and Sport Sciences) and was conducted according to the tenets of the Declaration of Helsinki.
Session 1: one-repetition maximum
The 1RM for the HS was determined using the method described by Baechle et al. (2000). After a general warm-up (10 min of stretching and joint mobility exercises), subjects undertook a HS warm-up protocol (1 set of 3-5 repetitions lifting a weight equivalent to their body weight). After a 2-min recovery period, the participants started the 1RM test in which increasing weights were lifted in 3 to 5 exercise sets of 1 repetition each. In the first set, the load used was 50% of the estimated 1RM; in the second and third sets, it was 70% and 90% of this value, respectively. For subsequent sets, the weight was adjusted until the 1RM, recorded as the heaviest load lifted by the subject in one repetition. Rest intervals between sets were 4 min.
Session 2: graded incremental test
The incremental resistance test (IRT) used was that described by de Sousa et al. (2012), Garnacho-Castaño et al. (2015a) and (2015b) in which the starting load is 10% 1RM and this is increased in small steps until 40% 1RM (20, 25, 30, 35, 40% 1RM). Subsequently, the load lifted was increased in steps of 10% of 1RM until exhaustion. This enables detection of the point at which blood lactate starts to rise from resting levels. The test was preceded by a general warm-up consisting of 5 min of gentle running followed by 5 min of ballistic stretching and upper and lower extremity joint mobility exercises. Each step of the test performed at the different loads (% 1RM) lasted 1 min, and included 30 repetitions of 2 s each (1 s eccentric, 1 s concentric). Between each load increase, blood samples were collected during a 2-min recovery period. These recovery periods were necessary for several reasons: 1) so that the weights on the barbell could be replaced increasing the workload by 5% of the 1RM each time, 2) so that blood samples could be obtained by finger pricking for lactate determination, 3) to more easily detect the workload at which blood lactate starts to rise. Using smaller stepwise increases in workloads without rest periods makes it more difficult to detect the LT2.
Blood samples (5 µl) were collected 30 s after the end of each step of the incremental load test, and blood lactate concentrations determined using a portable digital Lactate Pro meter (Lactate Pro LT-1710, Arkray Factory Inc., KDK Corporation, Siga, Japan) (McNaughton et al., 2002, Mclean et al., 2004).
The LT2 intensity was identified as an exponential rise in blood lactate concentration (Wasserman et al., 1964) using the algorithm adjustment method (de Sousa et al., 2011). This method detects the intersection point of the blood lactate and load segments through computerized 2-segment regression analysis. The 2 linear regression equations were set equal to each other and the work rate at the LT2 resolved for x. Data were extracted using Matlab version 7.4 software (Math-Works, Natick, MA, USA).
Session 3: constant-load test
A constant-load HS test (CLT) was executed at the load corresponding to the LT2. The idea behind this test was that being of low intensity and of long duration, metabolic and cardiorespiratory variables would eventually reach a point of stability. It was therefore essential to control the time of exercise and recovery time. Thus, after 48 h of rest followed by the same warm-up as for the incremental test, and using the work intensity of the LT2 identified in the previous test, participants performed 30 min and 30 s of HS, divided into 21 sets of 30 s. In each set, 15 repetitions of 2 s each (1 s eccentric, 1 s concentric) were completed. The duration of passive recovery between sets was 1 min. The objective pursued was that subjects would be able to perform a resistance exercise at the LT2 intensity over 30 minutes. In preliminary tests, the optimal ratio between exercise duration and rest time was determined as 1:2 to avoid excessive anaerobic glycolysis. Blood samples were drawn and assayed using the portable lactate analyzer at rest and 30 s after the end of set (S) 3, S6, S9, S12, S15, S18 and S21.
During the constant-load HS test, gas exchange data were collected continuously using an automated breath-by-breath system which was previously calibrated (Vmax spectra 29, Sensormedics Corp., Yorba Linda, California, USA) to determine minute ventilation (VE), oxygen consumption (VO2), carbon dioxide production (VCO2), ventilatory equivalent for O2 (VE·VO2-1), ventilatory equivalent for CO2 (VE·VCO2-1), and the respiratory exchange ratio (RER). Heart rate (HR) was monitored by telemetry (RS-800CX, Polar Electro OY; Kempele, Finland). Variables were expressed as the means recorded for each of the established 30-s time points (S3, S6, S9, S12, S15, S18 and S21).
Statistical analysis
The normal distribution of the data was checked by the Shapiro-Wilk test. To detect significant differences in cardiorespiratory and metabolic variables during the constant-load exercise at the different time points, analysis of variance (ANOVA) with repeated measures was used for the time factor, and contrasted with the Mauchley sphericity test. If the hypothesis of sphericity was rejected, we used the univariate F statistic with correction through the Greenhouse-Geisser correction index. If significant differences were detected between variables for different time points, a post-hoc test with Bonferroni correction was used. We also determined the effect size (ES) and statistical power of the data (1-β ≥ 0.95 and α = 0.05). To compare the data recorded in the IRT and CLT a t-test for paired samples was used. All data are expressed as the mean (M) and standard deviation (SD). All statistical tests were performed using the SPSS program version 18.0 (SPSS, Chicago, III). Significance was set at p < 0.05.
Results
Descriptive data for the participants are provided in Table 1.
Incremental resistance test
LT2 for each subject (n = 24) was identified in the incremental load test. Using the algorithm adjustment method, the mean exercise intensity corresponding to LT2 was estimated at 24.8 ± 4.8% 1RM (Figure 1). The different variables recorded at the LT2 intensity during the IRT are provided in Table 2.
Figure 1.

Determination of the lactate threshold (LT2) using the algorithm adjustment method. The LT2 value shown is the mean obtained for the study participants (n = 24).
Table 2.
Cardiorespiratory and metabolic data recorded at the intensity of the lactate threshold (LT1) in the incremental HS test and the constant-load test in the study participants (n=24). Data are means (±SD).
| Variable | IRT at LT1 | Mean recorded in the CLT | T | P | % of IRT value | Maximum recorded in the CLT | T | P | % of IRT value |
|---|---|---|---|---|---|---|---|---|---|
| Lactate (mmol·L-1) | 4.58 (1.50) | 3.26 (.83) | -5.09 | .000 | 71.2 % | 3.45 (.70) (S18) | -4.208 | .000 | 75.3% |
| HR (bpm) | 144(16) | 144(18) | -0.45 | .964 | 100.1% | 150(19) (S21) | 1.586 | .126 | 104.3% |
| VO2 (L·min-1) | 2.06 (.34) | 1.59 (.20) | -8.99 | .000 | 77.5 % | 1.66 (.30) (S21) | -7.513 | .000 | 80% |
| VCO2 (L·min-1) | 1.84 (.34) | 1.47 (.20) | -5.84 | .000 | 79.7 % | 1.52 (.20) (S21) | -5.193 | .000 | 82.4 % |
| VE (L·min-1) | 52.72 (11.94) | 42.75 (6.70) | -5.25 | .000 | 81 % | 45.60 (7.30) (S21) | -3.629 | .001 | 86.5 % |
| RER | .91 (.07) | .93 (.04) | 1.37 | .184 | 102.2 % | .95 (.05) (S6) | 2.476 | .021 | 104.2 % |
| VE·VO2-1 (L·min-1) | 25.98 (2.83) | 27.39 (2.06) * | 2.87 | .009 | 105.4 % | 28.00 (2.10) (S21) | 3.825 | .001 | 107.8 % |
| VE·VCO2-1 (L·min-1) | 28.56 (1.81) | 29.39 (2.04) | 1.86 | .076 | 102.9% | 30.00 (2.00) (S21) | 2.953 | .007 | 105 % |
| Kilograms | 49.9 (16.15 ) |
IRT = incremental resistance test; CLT = constant load test; HR = heart rate; VO2 = oxygen consumption; VCO2 = carbon dioxide production; VE = minute ventilation; RER = respiratory exchange ratio; VE·VO2-1 = ventilatory equivalent for O2; VE·VCO2-1 = ventilatory equivalent for CO2; bpm = beats per minute; L = liters, min = minute; S6 = Set 6; S18 = Set 18; S21 = S21.
Constant-load resistance exercise test
Lactate kinetics and cardiovascular response: Through repeated measures ANOVA with Greenhouse-Geisser correction significant differences were detected in mean blood lactate concentrations between sets (F = 51.4, p < 0.0001, ES = 0.924). Variables were compared across the different time points (S3, S6, S9, S12, S15, S18 and S21) using a post hoc test with Bonferroni correction. Blood lactate concentrations remained stable from S3 to S21 (3.07 ± 1.0 mmol·L-1 and 3.37 ± 0.7 mmol·L-1 respectively, p = 1.000) (Figure 2). In addition, HR varied significantly between sets (F = 91.6, p < 0.0001, ES = 0.917). Thus, HR recorded in S3 (130.0 ± 17.5 bpm) varied significantly (p < 0.05) from the rates recorded in the last four sets (S12, S15, S18, S21). From S6 to the end of the test (S21), HR remained stable (p > 0.05), although a slight sustained increase was observed (Figure 3).
Figure 2.
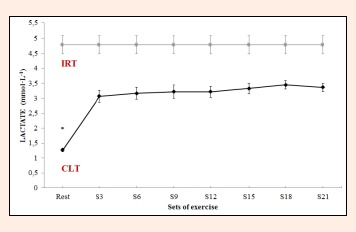
Blood lactate concentrations during constant-load resistance exercise. IRT = incremental resistance test; CLT = constant load test. * Differs significantly versus remaining values (p < 0.05).
Figure 3.
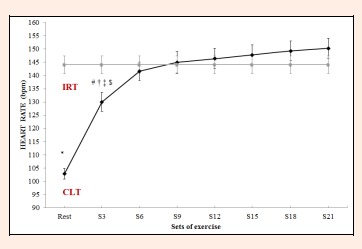
Heart rate (HR) during constant-load resistance exercise. IRT = incremental resistance test; CLT = constant load test. * Differs significantly versus remaining values (p < 0.05). # Differs significantly versus Set 21 (p < 0.05). † = Differs significantly versus Set 18 (p < 0.05). ‡ = Differs significantly versus Set 15 (p < 0.05). $ = Differs significantly versus Set 12 (p < 0.05).
Ventilatory responses
Significant differences also emerged among the different sets for VO2 (F = 84.1, p < 0.0001, ES = 0.955), VCO2 (F = 70.6, p < 0.0001, ES = 0.920) and VE (F = 56.4, p < 0.0001, ES = 0.949). Thus, VO2, VCO2 and VE at rest differed from the values observed following each set. However, the increases produced in VO2 and VCO2 from S3 to S21 (0.168 ± 0.02 L·min-1, 0.184 ± 0.01 L·min-1, respectively) were not significant (p = 0.471, p = 0.136, Figure 4, Figure 5). For VE, significant differences were detected between S3 and S18 or S21 (7.2 ± 0.0 L·min-1 p = 0.009, 8 ± 1.0 L·min-1 p = 0.002, respectively), with no significant increases product in the remaining set Figure 6).
Figure 4.
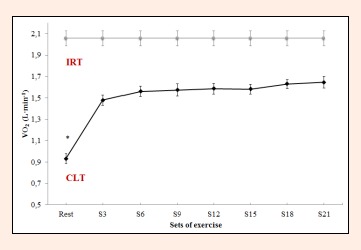
Oxygen consumption (VO2) during constant-load resistance exercise. * Differs significantly versus remaining value (p<0.05).
Figure 5.
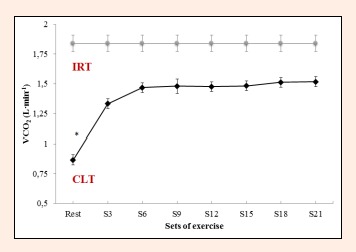
Carbon dioxide production (VCO2) during constant-load resistance exercise. * Differs significantly versus remaining value (p<0.05).
Figure 6.
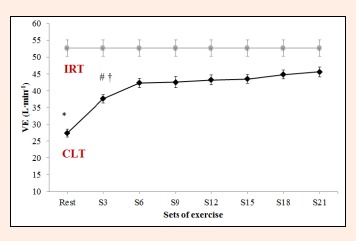
Minute ventilation (VE) during constant-load resistance exercise. IRT = incremental resistance test; CLT = constant load test;* Differs significantly versus remaining values (p < 0.05). # Differs significantly versus Set 21 (p < 0.05). † = Differs significantly versus Set 18 (p < 0.05).
RER failed to vary significantly between S21 and any of the other time points (F = 2.5, p = 0.103, ES = 0.710; Figure 7). For VE·VO2-1 and VE·VCO2-1, significant differences were observed between sets in the constant-load test (F = 9.8, p < 0.0001, ES = 0.666 and F = 9.3, p < 0.0001, ES = 0.666). VE·VO2-1 failed to vary from S6 to S21 (S6 = 27.3 ± 1.9 L·min-1, S21 = 28.0 ± 2.1 L·min-1 p = 1.000, Figure 8). VE·VCO2-1 only showed significant differences between resting values and S15, S18 or S21 (rest = 27.3 ±2 .4 L·min-1 versus S15 = 29.7 ± 1.8 L·min-1, S18 = 29.8 ± 1.9 L·min-1, S21 = 30.0 ± 2.0 L·min-1, p = 0.021, p = 0.012, p = 0.0004, respectively, Figure 9).
Figure 7.
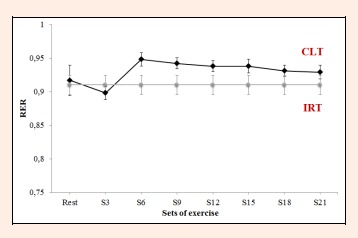
Respiratory exchange ratio (RER) values recorded during the constant-load test. IRT = incremental resistance test; CLT = constant load test;
Figure 8.
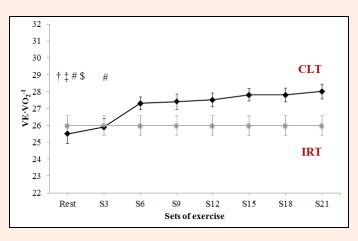
Ventilatory equivalent for O2 (VE·VO2-1) during constant-load resistance exercise. IRT = incremental resistance test; CLT = constant load test; # Differs significantly versus Set 21 (p < 0.05). † = Differs significantly versus Set 18 (P<0.05). ‡ = Differs significantly versus Set 15 (p < 0.05). $ = Differs significantly versus Set 12 (p < 0.05).
Figure 9.
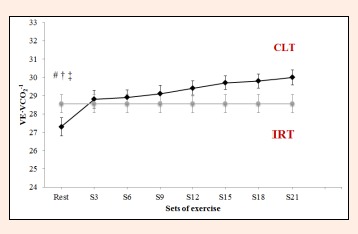
Ventilatory equivalent for CO2 (VE·VCO2-1) during constant-load resistance exercise. IRT = incremental resistance test; CLT = constant load test;# Differs significantly versus Set 21 (p < 0.05). † = Differs significantly versus Set 18 (p < 0.05). ‡ = Differs significantly versus Set 15 (p < 0.05).
The variables determined in each subject during the constant-load test (means and maximum values) are provided in Table 2. This table also compares the IRT and CLT data, indicating significant differences in all mean and maximum values recorded for the two tests with the exceptions of mean HR and RER (mean values) and maximal HR (maximum values) (p > 05).
Discussion
In this study, we were able to readily identify the LT2 during an incremental resistance exercise test (half squat). In other studies in which a RT protocol for the LP was assessed, higher LT2 values than those obtained here were recorded: 28 ± 6.3%-32.2 ± 4.4% 1RM (Barros et al., 2004); 36.6 ± 1.4% 1RM (Oliveira et al., 2006), 31.0 ± 5.3% -36.7 ± 5.6 % 1RM (Moreira et al., 2008), 27.1 ± 3.7% 1RM (de Sousa et al., 2012); 30 ± 6% 1RM (Simões et al., 2010); 29.2 ± 6% 1RM in young men and 28 ± 4% 1RM in older men (Simões et al., 2013); and 27.9 ± 5.0% 1RM (de Sousa et al., 2013). This difference may be attributed to a greater exercise stimulus in HS than LP, whereby a larger number of muscle groups are involved due to the work of synergist and stabilizer muscles needed to maintain posture and hold the barbell.
Once the LT2 had been identified, we examined the effects of HS exercise performed for more than 30 min at a constant exercise intensity equivalent to that of the LT2 and noted that using this training protocol, all physiological variables remained stable. When these variables were compared with those obtained at the LT2 in the incremental test, it emerged that with the exception of HR, VE·VO2-1 and VE·VCO2-1, these were significantly higher in the IRT. This difference could perhaps be attributable to the varying duration of exercises, 1 min in the incremental versus 30 seconds in the constant load test, along with 2 min versus 1 min rest periods, respectively. Although the exercise:rest ratio was 1:2 in both tests, the longer exercise time in the incremental test likely determined the higher blood lactate concentrations recorded (IRT = 4.58 ± 1.50 mmol·L-1 vs. CLT = 3.26 ± 0.83 mmol·L-1). This would increase H+ buffering due to bicarbonate, driving an increase in minute ventilation (IRT = 52.72 ± 11.94 L·min-1 vs. CLT = 42.75 ± 6.7 L·min-1) to clear the excess VCO2 (IRT = 1.838 ± 0.34 L·min-1 vs. CLT = 1.465 ± 0.2 L·min-1) (Meyer et al., 2005) and would explain the higher respiratory and metabolic variables observed in the IRT.
In the present study, blood lactate concentrations remained stable during sets 3 and 21 of constant-load exercise, while in the study by de Sousa et al. (2012), blood lactate stabilization occurred from S9 until the end of the test (S15), reaching a steady state after 15 min of exercise. Here, this occurred before 5 min of exercise, with values slightly above 3 mmol·L-1, indicating that this exercise intensity was above LT1 (and below LT2) (Hofmann and Tschakert, 2011). There could be two reasons for this: 1) it could be that the constant load established was at the limit of a predominantly aerobic metabolism such that blood lactate levels did not increase, or 2) a balance may have been reached between the rates of lactate appearance and disappearance in blood (Brooks et al., 1985). Both these possibilities are feasible since in the HS, the work intensity of the LT2 does not exceed 25% of 1RM. As RT intensity increases (% 1RM), intramuscular pressure rises reducing blood flow and causing muscle ischemia and hypoxia (Williams and Cavanagh, 1987). This blockade raises anaerobic glycolytic activity, increasing blood lactate levels.
In the study by de Sousa et al. (2012), lactate levels ranged from ~2.2 mmol·L-1 in S3 to ~3.8 mmol·L-1 in S15, showing greater variability during the 30 min of exercise than in our study. This is because LP exercise affects the lower limbs more, whereas the greater muscle mass involvement in HS is likely to promote lactate clearance due to increased blood flow (Billat et al., 2003).
During our constant-load HS exercise, HR showed an initial increase followed by stabilization from S6 to the end of the exercise (approximately 24 min). Although no significant difference in HR was produced from S6 to S21, a slight and persistent increase was observed across these sets (HR S6 = 141.6 ± 17.0 bpm, HR S9 = 145.0 ± 19.9 bpm, HR S12 = 146.4 ± 18.7 bpm, HR S15 = 147.8 ± 18.5 bpm, HR S18 = 149.3 ± 18.3 bpm, HR S21 = 150.3 ± 18.5 bpm). When these data were compared with HR recorded during the IRT at the LT2 intensity (144.1 ± 16.27 bpm), it was observed that as from S12 (after approximately 17 min of exercise) in the CLT, HR was higher than the values corresponding to LT2 in the IRT. A possible explanation for this is cardiovascular drift due to the duration of resistance exercise and the muscular work involved. Studies examining the effects of long-term endurance exercise have shown a progressive increase in serum catecholamine (noradrenalin and adrenalin) levels (Mora-Rodríguez et al., 1996). Thus, the discrete sustained HR increase detected in our study could be explained by a possible increase in catecholamine levels. Such an increase in HR was also described by de Sousa et al. (2012) from S3 to S15 (approximately 18 min) of LP exercise. Thus, the more cardiovascular stress exerted by constant HS exercise than LP (HS mean S6 to S21=146.7 bpm, vs LP mean S6 to S15=124.5 bpm) could mean that this exercise modality (HS) is an effective way to improve functional capacity and cardiovascular fitness (de Sousa et al., 2012; Garnacho-Castaño et al., 2015b). The greater increase observed in HR during constant-load resistance exercise in our study may be attributed to the greater cardiovascular demands of HS due to the vertical position adopted as opposed to the 45° angle needed for LP, for which the subject lies down reducing the effects of gravity. It is likely that this greater HR response is produced by increased sympathetic activity of the heart (Simões et al., 2010), causing redistribution of blood flow and reduced vein capacitance (Thomas et al., 2004). In turn, this will increase the cardiac cost needed for metabolic regulation avoiding the build-up of high lactate concentrations in the muscles that are active during exercise.
The respiratory parameters VO2, VCO2, and VE·VCO2-1 showed an initial increase followed by stabilization from S3 to the end of the constant-load HS test, while VE and VE·VO2-1 stabilized from S6. The stable behavior of ventilatory variables is characteristic from minutes 10 to 30 of resistance exercises performed using a constant load corresponding to the LT2 (de Sousa et al., 2012). Moreover, if RER remains constant throughout the test, it could be that this intensity of exercise is consistent with a steady respiratory state, in line with the results of other studies involving aerobic exercises (Baron et al., 2003, Ribeiro et al., 1986). In the present study, RER failed to vary during the course of the test remaining at 0.90 to 0.95. Thus, we may assume a predominant aerobic metabolism in this CLT, despite varying contributions of carbohydrates and fats during the 30 min of exercise. RER values diminished slightly from S6 (0.95) until the end of exercise (RER S9 = 0.94, RER S12 = 0.94, RER S15 = 0.94, RER S18 = 0.93, RER S21 = 0.93). Thus, according to the table given by De Zuntz (1901), the contribution of carbohydrates will have varied from S6 (84%) to S21 (77%), while the participation of fats as an energy source will increase with the duration of exercise (S6 = 16%, S21 = 23%). In metabolic terms, in HS exercise loaded at around 25% of 1RM, the role played by anaerobic pathways is minimal, while RT using loads of 40%-70% 1RM is highly dependent on anaerobic metabolism (Collins et al., 1989). Accordingly, resistance training at an intensity corresponding to the LT2 could serve to reduce the RER, improving the contribution of fats to energy metabolism. This would have implications for running economy, as one of the main determinants for improving performance in endurance sports (Jung et al., 2003, Støren et al., 2008). The reduced use of glycogen stores in the muscles involved in exercise, improves the function and morphology of mitochondria (Holloszy et al., 1977). This means that as exercise continues, more fats can be oxidized to satisfy energy demands, reserving muscle glycogen for other uses such as sprints or rhythm changes in resistance sports. Further, the greater use of the aerobic pathway has been related to a greater availability of type I or slow–twitch muscle fibers, a high proportion of these fibers being linked to running economy (Williams et al., 1987). Further studies would have to confirm these issues.
There are methodological differences between our constant-load HS exercise and the resistance LP protocol described by de Sousa et al. (2012) (30 min divided into 15 sets of 1 min each; subjects performed 20 repetitions, each lasting about 3 s; rest between sets was 1 min). Our objective was to design a constant load HS exercise protocol that could be continued for at least 30 min. This was achieved by establishing repetitions of 1 s of eccentric phase plus 1 s of concentric phase exercise, since performing the squat in 3 s would require high levels of muscular activity for the maintenance and stabilization of posture. In effect this occurs in exercises performed under conditions of instability (Behm et al., 2002) and causes intramuscular pressure increases and blood capillary blockade (Williams et al., 2007). Such capillary blockade would produce an increase in anaerobic glycolysis and possibly a gradual rise in blood lactate. Therefore, to achieve the high volume of exercise (30 min) it was decided to perform 15 repetitions with 1 min recovery periods as 21 sets. In preliminary tests, we noted that a greater number of repetitions or longer repetition time led to a large increase in blood lactate concentrations. The differences observed between the findings of our study and the results of other investigations are likely due to the different methods used in terms of the exercise protocol and parts of the body involved. For example, in the study by de Sousa et al. (2012), the ratio between workload and rest time in the constant LP load test was 1:1, while this ratio was 1:2 in our study. Perhaps the different exercise modalities (LP vs. HS) could justify the different exercise protocols, as the exercise stimulus for HS is greater than for LP, given the larger number of muscle groups involved due to the work of synergistic and stabilizing muscles and the HS is performed standing compromising the cardiovascular system more. Our results provide direction for future studies designed to determine the best protocol able to maintain stable metabolic and cardiorespiratory variables by testing different combinations of numbers of sets, repetitions, recovery times and execution velocities.
RT programs based on light loads (<50% 1RM) and large numbers of sets and repetitions with short rest periods pursue the improvement of local muscle resistance (Garber et al., 2011; Ratamess et al., 2009). Campos et al. (2002) noted improved local muscle resistance (more repetitions in a 60% 1RM test) in a group of untrained individuals following 8-weeks of lower limb RT with a high number of repetitions (as 2 sets of 20-28 reps, rest 1 min) compared to an intermediate repetition group (3 sets of 9-11 reps, rest 2 min) and a low repetition group (4 sets of 3-5 reps, rest 3 min). In addition, the subjects in the high repetition group showed improved cycle ergometry performance (improved maximal aerobic power and time to exhaustion) with no change in the VO2max. The predicted improvements that could be obtained in response to a half-squat RT program executed at the intensity of LT2 (25% 1RM) involving a large volume of sets and repetitions (21x15) and 1 min interset rest periods would make such a program ideal for older adults, subjects with a lower functional capacity, sedentary individuals starting an RT program, patients with heart disease or DM2 and even for resistance sports athletes.
The main limitation of our study was related to the metabolic characteristics of RT. To date, the different studies that have examined the LT2 in RT have not been able to use a continuous exercise protocol as opposed to other forms of exercise (e.g. treadmill or ergometry exercises). The predominant anaerobic metabolism involved in RT determines a need for recovery periods, which evidently conditions cardiorespiratory responses. Performing a single exercise (HS) in a RT protocol at the intensity of LT2 will perhaps not avoid the need for rest periods. Thus, although HS is conducted at a low work intensity and involves numerous muscle groups, its nature still differs from that of a more endurance-type exercise and rest periods are necessary. What could be done to avoid rest intervals is to alternate different exercises in which different muscle groups are trained (e.g., HS and bench press) in a single exercise protocol. This will mean that when one muscle group is resting the other one is working allowing for a predominantly aerobic metabolism without having to interrupt the exercise.
However, according to the present findings it seems that once the LT2 has been established in an IRT such as HS, working at this intensity will enable the subject to execute a large volume of exercise while maintaining stable cardiorespiratory and metabolic variables, provided exercise and rest periods are alternated. With regards to the most suitable protocol for a RT program at the work intensity of the LT2, the type of exercise (HS or LP) would first have to be considered. The duration of interset rest could be standardized at 1 min. However, recommended exercise volume would be 15 to 20 repetitions per set, with repetition duration between 2 and 3 s respectively for HS and LP. This means an exercise/rest ratio of 1:2 for HS and one of 1:1 for LP (de Sousa et al., 2012). In individuals with poor functional capacity for whatever reason, we would recommend starting with 2 or 3 sets, and gradually increasing the exercise volume until at least 15 min of exercise at a frequency of 2 to 3 days per week (Garber et al., 2011).
Future research is needed to: 1) address the adaptations produced in response to the RT program proposed here in the short- and long term, 2) confirm the data of this study using another exercise protocol, 3) determine the effects of a continuous RT program by monitoring LT2, 4) transfer this methodology to other exercises like the bench press, squat or shoulder press.
Conclusion
Our findings indicate that:
The LT2 can be identified during an incremental HS test.
All cardiorespiratory and metabolic variables stabilize at a constant load corresponding to the LT2 for the HS during a little over 30 min of discontinuous exercise (21 sets, 15 repetitions, 1 min rest) such that our protocol is suitable for controlling work intensity.
The exercise intensity proposed for this type of exercise corresponds to the limit of a predominantly aerobic metabolism in which a balance occurs between the appearance and disappearance of lactate in the blood.
Acknowledgements
This work was supported by grants from the Alfonso X El Sabio University Foundation.
Biographies

José Luis MATÉ-MUÑOZ
Employment
Professor
Degree
PhD
Research interests
Exercise physiology, resistance training, endurance.
E-mail: jmatmuo@uax.es

Raúl DOMÍNGUEZ
Employment
Professor
Degree
PhD
Research interests
Exercise physiology, sports nutrition, iron metabolism, hepcidine, endurance
E-mail: rdomiher@uax.es

Manuel BARBA
Employment
Professor
Degree
MSc
Research interests
Exercise physiology, sports training and team sports.
E-mail: mruizbar@uax.es

Antonio J. MONROY
Employment
Professor
Degree
PhD
Research interests
Training, Exercise Physiology, Sport Management.
E-mail: antonio.monroy@unir.net
Bárbara RODRÍGUEZ
Employment
Professor
Degree
PhD
Research interests
Physical activity and health, sport management.
E-mail: barbara.rodriguezrodriguez@gmail.com

Pedro RUIZ-SOLANO
Employment
Professor
Degree
PhD
Research interests
Performance & Sport
E-mail: pruizsol@myuax.com

Manuel V. GARNACHO-CASTAÑO
Employment
Professor
Degree
PhD, MSc
Research interests
Physiology, resistance training, genetics.
E-mail: mavigarcas@gmail.com
References
- Baechle T.R., Eaerle R.W., Wathen D. (2000) Resistance Training. Essential of Strength Training and Conditioning (NSCA). Eds: Baechle T.R., Earle R.W. Champaign IL: Human Kinetics; 395-425. [Google Scholar]
- Baron B., Dekerle J., Robin S., Neviere R., Dupont L., Matran R., Vanvelcenaher J., Robin H., Pelayo P. (2003) Maximal lactate steady state does not correspond to a complete physiological steady state. International Journal of Sports Medicine 24, 582-587. [DOI] [PubMed] [Google Scholar]
- Barros C.L.M., Agostini G.G., García E.S., Baldissera V. (2004) Lactate threshold in resistance exercise. Motriz 10(1), 31-36. [Google Scholar]
- Behm D. G., Anderson K., Curnew R. S. (2002) Muscle force and activation under stable and unstable conditions. Journal of Strength and Conditioning Research 16, 416-422. [PubMed] [Google Scholar]
- Billat. V.L., Sirvent P., Py G., Koralsztein J.P., Mercier J. (2003) The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Medicine 33, 407-426. [DOI] [PubMed] [Google Scholar]
- Bosquet L., Léger L., Legros P. (2002) Methods to determine aerobic endurance. Sports Medicine 32, 675-700. [DOI] [PubMed] [Google Scholar]
- Brooks G.A. (1985) Anaerobic threshold: review of the concept and directions for future research. Medicine & Science in Sports & Exercise 17, 22-34. [PubMed] [Google Scholar]
- Campos G.E., Luecke T.J., Wendeln H.K., Toma K., Hagerman F.C., Murray T.F., Ragg K. E., Ratamess N.A., Kraemer W.J., Staron R.S. (2002) Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. European Journal of Applied Physiology 88(1-2), 50-60. [DOI] [PubMed] [Google Scholar]
- Collins M.A, Cureton K.J., Hill D.W., Ray C.A. (1989) Relation of plasma volume change to intensity of weight lifting. Medicine & Science in Sports & Exercise 21, 178-185. [PubMed] [Google Scholar]
- de Sousa N.F., Souza M.C., Pereira G. B., Bertucci D.R., Magosso R.F., Baldissera V., Andrade S.P. (2013) Lactate threshold in resistance exercise in elderly. Motricidade 9(1), 87-94. [Google Scholar]
- de Sousa N.M.F., Magosso R.F., Pereira G.B., Leite R.D., Montagnolli A.N., Pérez S.A., Baldissera V. (2011) The measurement of lactate threshold in resistance exercise: a comparison of methods. Clinical Physiology and Functional Imaging 31, 376-381. [DOI] [PubMed] [Google Scholar]
- de Sousa N.M.F., Magosso R.F., Pereira G.B., Souza M.V., Vieira A., Marine D.A., Perez S.E., Baldissera V. (2012) Acute Cardiorespiratory and metabolic responses during resistance exercise in the lactate threshold intensity. International Journal of Sports Medicine 33, 108-113. [DOI] [PubMed] [Google Scholar]
- De Zuntz N. (1901) About the importance of various nutrients as a producer of muscle strength. Pflügers Archives Physiology 83, 557-571. [Google Scholar]
- Dudley G.A. (1988) Metabolic consequences of resistive-type exercise. Medicine & Science in Sports & Exercise 20(Suppl 1), S158-161. [DOI] [PubMed] [Google Scholar]
- Escamilla R.F., Fleisig G.S., Zheng N., Lander J.E., Barrentine S.W., Andrews J.R., Bergemann B.W., Moorman C.T. (2001) Effects of technique variations on knee biomechanics during the squat and leg press. Medicine & Science in Sports & Exercise 33, 1552-1566. [DOI] [PubMed] [Google Scholar]
- Faude O., Kindermann W., Meyer T. (2009) Lactate threshold concepts. How valid are they? Sports Medicine 39, 469-490. [DOI] [PubMed] [Google Scholar]
- Fry A.C. (2004) The role of resistance exercise intensity on muscle fibre adaptations. Sports Medicine 34, 663-679. [DOI] [PubMed] [Google Scholar]
- Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M., Nieman D.C., Swain D.P. (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise 43(7), 1334-1359. [DOI] [PubMed] [Google Scholar]
- Garhammer J. (1981) Free weight equipment for the development of athletic strength and power. National Strength & Conditioning Association Journal 3, 24-26. [Google Scholar]
- Garnacho-Castaño M.V., Domínguez R., Ruiz-Solano P., Maté-Muñoz J.L. (2015a) Acute physiological and mechanical responses during resistance exercise executed at the lactate threshold workload. Journal of Strength and Conditioning Research. (In press). [DOI] [PubMed] [Google Scholar]
- Garnacho-Castaño M. V., Domínguez R., Maté-Muñoz J. L. (2015b) Understanding the meaning of the lactate threshold in resistance exercises. International Journal of Sports Medicine, 36, 371-377. [DOI] [PubMed] [Google Scholar]
- González-Badillo J.J., Sánchez-Medina L. (2010) Movement velocity as a measure of loading intensity in resistance training. International Journal of Sports Medicine 31, 347-355. [DOI] [PubMed] [Google Scholar]
- Hofmann P., Tschakert G. (2011) Special needs to prescribe exercise intensity for scientific studies. Cardiology Research and Practice 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J.O., Rennie M.J., Hickson R.C., Conlee R.K., Hagberg J.M. (1977) Physiological consequences of the biochemical adaptations to endurance exercise. Annals of the New York Academy of Sciences 301, 440-450. [DOI] [PubMed] [Google Scholar]
- Jung A.P. (2003) The impact of resistance training on distance running performance. Sports Medicine 33, 539-552. [DOI] [PubMed] [Google Scholar]
- Kraemer W.J., Deschenes M.R., Fleck S.J. (1988) Physiological adaptations to resistance exercise: implications for athletic conditioning. Sports Medicine 6, 246-256. [DOI] [PubMed] [Google Scholar]
- Lucía A., Hoyos J., Chicharro J.L. (2001) Physiology of professional road cycling. Sports Medicine 31, 325-337. [DOI] [PubMed] [Google Scholar]
- Mclean S.R., Norris S.R., Smith D.J. (2004) Comparison of the Lactate Pro and the YSI 1500 Sport Blood Lactate Analyzers. International Journal of Applied Sports Sciences 16, 22-30. [Google Scholar]
- McNaughton L. R., Thompson D., Philips G., Backx K., Crickmore L. (2002) A comparison of the lactate Pro, Accusport, Analox GM7 and Kodak Ektachem lactate analysers in normal, hot and humid conditions. International Journal of Sports Medicine 23, 130-135. [DOI] [PubMed] [Google Scholar]
- Meyer T., Görge G., Schwaab B., Hildebrandt K., Walldorf J., Schäfer C., Kindermann I., Scharhag J., Kindermann W. (2005) An alternative approach for exercise prescription and efficacy testing in patients with chronic heart failure: a randomized controlled training study. American Heart Journal 149, e1–7. [DOI] [PubMed] [Google Scholar]
- Meyer T., Lucia A., Earnest C.P., Kindermann W. (2005) A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters – theory and application. International Journal of Sports Medicine 26 (Suppl 1), S38-S48. [DOI] [PubMed] [Google Scholar]
- Mora-Rodríguez R., González-Alonso J., Below P.R., Coyle E.F. (1996) Plasma catecholamines and hyperglycaemia influence thermoregulation in man during prolonged exercise in the heat. Journal of Physiology 491(2), 529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S.R., Arsa G., Oliveira H.B., Lima L.C.J., Campbell C.S. G., Simões H.G. (2008) Methods to identify the lactate and glucose thresholds during resistance exercise for individuals with type 2 diabetes. Journal of Strength and Conditioning Research 22, 1108-1115. [DOI] [PubMed] [Google Scholar]
- Oliveira J.C., Baldissera V., Simões H.G., Aguiar A.P., Azevedo P.H.M., Poian P.A.F.O., Pérez S.E.A. (2006) Lactate and glucose threshold in resistance exercise. Revista Brasileira de Medicina do Esporte 12, 333-338. [Google Scholar]
- Ratamess N.A., Albar B.A., Evetoch T.K., Housh T.J., Kibler W.B., Kraemer W.J., Triplett M.T. (2009) Special Communication. American College of Sports Medicine Position Stand: Progression models in resistance training for healthy adults. Medicine & Science in Sports & Exercise 41(3), 687-708. [DOI] [PubMed] [Google Scholar]
- Ribeiro J.P., Hughes V., Fielding R.A., Holden W., Evans W., Knuttgen H.G. (1986) Metabolic and ventilatory responses to steady state exercise relative to lactate thresholds. European Journal of Applied Physiology and Occupational Physiology 55, 215-221. [DOI] [PubMed] [Google Scholar]
- Simões R.P., Castello-Simões V., Mendes R.G., Archiza B., Santos D.A., Machado H.G., Bonjorno J.C., Jr., Oliveira C.R., Reis M.S., Catai A.M., Arena R., Borghi-Silva A. (2013) Lactate and heart rate variability threshold during resistance exercise in the young and elderly. International Journal of Sports Medicine 34(11), 991-996. [DOI] [PubMed] [Google Scholar]
- Simões R.P., Mendes R.G., Castello V., Machado H.G., Almeida L.B., Baldissera V., Catai A.M., Arena R., Borghi-Silva A. (2010) Heart rate variability and blood-lactate threshold interaction during progressive resistance exercise in healthy older men. Journal of Strength and Conditioning Research 24(5), 1313-1320. [DOI] [PubMed] [Google Scholar]
- Skinner J.S., McLellan T.H. (1980) The transition from aerobic to anaerobic metabolism. Research Quarterly for Exercise & Sport 51, 234-248. [DOI] [PubMed] [Google Scholar]
- Støren Ø., Helgerud J., Støa E.M., Hoff J. (2008) Maximal Strength Training Improves Running Economy in Distance Runners. Medicine & Science in Sports & Exercise 40(6), 1087-1092. [DOI] [PubMed] [Google Scholar]
- Thomas G.D., Segal S.S. (2004) Neural control of muscle blood flow during exercise. Journal of Applied Physiology 97, 731-738. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Mcllroy M.B. (1964) Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. American Journal of Cardiology 14, 844-852. [DOI] [PubMed] [Google Scholar]
- Williams K.R., Cavanagh P.R. (1987) Relationship between distance running mechanics, running economy, and performance. Journal of Applied Physiology 63, 1236-1245. [DOI] [PubMed] [Google Scholar]
- Williams M.A., Haskell W.L., Ades P.A., Amsterdam E.A., Bittner V., Franklin B.A., Gulanick M., Laing S.T., Stewart K.J. (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 31, 116(5), 572–584. [DOI] [PubMed] [Google Scholar]


