Abstract
When speed changes during locomotion, both temporal and spatial parameters of the pattern must adjust. Moreover, at slow speeds the step-to-step pattern becomes increasingly variable. The objectives of the present study were to assess if the spinal locomotor network adjusts both temporal and spatial parameters from slow to moderate stepping speeds and to determine if it contributes to step-to-step variability in left-right symmetry observed at slow speeds. To determine the role of the spinal locomotor network, the spinal cord of 6 adult cats was transected (spinalized) at low thoracic levels and the cats were trained to recover hindlimb locomotion. Cats were implanted with electrodes to chronically record electromyography (EMG) in several hindlimb muscles. Experiments began once a stable hindlimb locomotor pattern emerged. During experiments, EMG and bilateral video recordings were made during treadmill locomotion from 0.1 to 0.4 m/s in 0.05 m/s increments. Cycle and stance durations significantly decreased with increasing speed, whereas swing duration remained unaffected. Extensor burst duration significantly decreased with increasing speed, whereas sartorius burst duration remained unchanged. Stride length, step length, and the relative distance of the paw at stance offset significantly increased with increasing speed, whereas the relative distance at stance onset and both the temporal and spatial phasing between hindlimbs were unaffected. Both temporal and spatial step-to-step left-right asymmetry decreased with increasing speed. Therefore, the spinal cord is capable of adjusting both temporal and spatial parameters during treadmill locomotion, and it is responsible, at least in part, for the step-to-step variability in left-right symmetry observed at slow speeds.
Keywords: locomotion, spinal cord, kinematics, variability, left-right coordination, speed
the ability to modulate locomotor speed is essential for adapting to environmental demands and for goal-oriented behaviors. Under normal circumstances, the locomotor pattern and its adjustment to speed are the result of dynamic sensorimotor interactions between supraspinal inputs, intrinsic spinal mechanisms, and sensory feedback from the periphery (Rossignol et al. 2006). It is well established that the basic pattern of locomotion is produced by a specialized network within the spinal cord, the so-called locomotor central pattern generator (CPG) (reviewed in Frigon 2012; Kiehn 2011; McCrea and Rybak 2008). The presence of a spinal locomotor CPG working in concert with sensory feedback from the periphery allows recovery of hindlimb locomotion following complete spinal transection either spontaneously or with training in many mammalian species, such as mice (Leblond et al. 2003), rats (Slawinska et al. 2012), cats (Barbeau and Rossignol 1987; Lovely et al. 1986), and dogs (Naito et al. 1990). Spinal cord-transected (spinalized) animal models are powerful tools to assess spinal mechanisms of locomotor control and examine how sensory feedback interacts with spinal circuits during real stepping movements.
It is well known that the hindlimb locomotor pattern of chronic spinalized cats can adjust to changes in treadmill speed (Barbeau and Rossignol 1987; de Guzman et al. 1991; Forssberg et al. 1980a, 1980b; Frigon et al. 2013; Lovely et al. 1986, 1990; Smith et al. 1982), indicating that the spinal locomotor CPGs can process sensory feedback from the moving limbs to adjust their output. As speed increases, cycle and stance phase durations are reduced while the swing phase remains relatively invariant in intact and chronic spinalized cats (Gossard et al. 2011). Although temporal and related electromyographic adjustments of the hindlimb locomotor pattern have been well described in chronic spinalized cats, changes in spatial parameters with speed have been largely unexplored. It is possible that spatial adjustments to speed, such as increases in stride length and step length with increasing speed, observed in intact quadrupeds (Afelt et al. 1983; Blaszczyk and Dobrzecka 1989; Frigon et al. 2014; Thibaudier and Frigon 2014) are mainly controlled at a spinal level. The placement of the paw at contact, an important event in the cycle that ensures a stable base of support (Halbertsma 1983; Klishko et al. 2014), could also be controlled primarily at a spinal level.
Understanding the control of locomotion at slow speeds is of prime importance because walking speed can be severely reduced in several pathological states (Pepin et al. 2003; Schniepp et al. 2012; Wuehr et al. 2014), as well as in normal aging (Himann et al. 1988), thus limiting the range of possible locomotor speeds. Slow walking speeds have been associated with increased gait variability, reduced stability, and increased risk of falling (Espy et al. 2010; Schniepp et al. 2012; Wuehr et al. 2014). The mechanisms leading to increased gait variability at slow walking speeds are poorly understood because healthy animals, including humans, do not voluntarily step at slow speeds under normal circumstances (Den Otter et al. 2004). One way to circumvent this limitation and to specifically address the contribution of spinal mechanisms in producing gait variability is to study locomotion at slow speeds in a chronic spinalized model.
During terrestrial quadrupedal locomotion, walking and trotting forms of gait, generally found at speeds <1.0 m/s in cats, are characterized by an out-of-phase alternation between homologous limbs (English and Lennard 1982; Frigon et al. 2014; Hildebrand 1976). For the hindlimbs, contact of the left hindlimb occurs at half of the cycle measured from successive right hindlimb contacts. Thus perfect left-right symmetry is defined as a value of 0.5 in temporal phasing between homologous limb contacts. Moreover, in such gaits, stance and swing durations as well as support periods are approximately equal on the left and right sides (Frigon et al. 2014). However, during split-belt locomotion with one side stepping faster than the other (D'Angelo et al. 2014; Frigon et al. 2013) or during normal locomotion following a spinal lesion that primarily damages one side of the spinal cord (Frigon et al. 2009; Martinez et al. 2013), left-right asymmetries in the pattern can occur. For instance, during split-belt locomotion, the stance phase is longer on the slow side while the swing phase is longer on the fast side (Dietz et al. 1994; Frigon et al. 2013). It is possible that left-right asymmetries increase with slower speeds, leading to a more variable gait pattern.
Therefore, the objective of the present study was to assess the temporal and spatial control of hindlimb locomotion from slow (0.1 m/s) to moderate (0.4 m/s) treadmill speeds in chronic spinalized adult cats. We hypothesized that both temporal and spatial adjustments in the locomotor pattern with speed are primarily determined at a spinal level. We also hypothesized that temporal and spatial gait variability increases at slow speeds in chronic spinalized adult cats, indicating that it is generated, at least in part, at the level of the spinal cord.
MATERIALS AND METHODS
Ethical Approval
Experimental procedures were approved by the Animal Care Committee of the Université de Sherbrooke in accordance with policies and directives of the Canadian Council on Animal Care. Six adult cats (2 males, 4 females) weighing between 3.0 and 4.5 kg were used for the current data set. As part of our effort to maximize the scientific output of each animal, all cats were used in other studies to provide answers to other scientific questions (D'Angelo et al. 2014; Frigon et al. 2013, 2014, 2015; Hurteau et al. 2015).
Surgical Procedures
General.
The implantation and spinal transection (spinalization) surgeries were performed on separate days under aseptic conditions in an operating room with sterilized equipment. Before surgery, cats were sedated with an intramuscular (im) injection of butorphanol (0.4 mg/kg), acepromazine (0.1 mg/kg), and glycopyrrolate (0.01 mg/kg). Induction was done with ketamine-diazepam (0.11 ml/kg im, 1:1 ratio). The cat was then anesthetized with isoflurane (1.5–3%) via a mask for a minimum of 5 min and then intubated with a flexible endotracheal tube. Isoflurane concentration was confirmed and adjusted throughout the surgery by monitoring cardiac and respiratory rates, applying pressure to the paw to detect limb withdrawal, and assessing muscle tone. Body temperature was monitored with a rectal thermometer and kept between 35° and 37°C using a water-filled heating pad placed under the animal and an infrared lamp positioned ∼50 cm above the cat. In cats PB and BL, the implantation surgery was performed before spinalization, whereas in cats BC, SC, PZ, and FX, implantation was performed after cats had recovered hindlimb locomotion following spinalization. During surgery, an antibiotic (Convenia; 0.1 ml/kg) was injected subcutaneously. To provide analgesia, a transdermal fentanyl patch (25 μg/h) was taped to the back of the animal 2–3 cm rostral to the base of the tail, and another analgesic (buprenorphine; 0.01 mg/kg) was administered subcutaneously during surgery and ∼7 h after. Following surgery, cats were placed in an incubator and closely monitored until consciousness was regained. At the end of the experiments, a lethal dose of pentobarbital sodium was injected in the left or right cephalic vein.
Spinal transection.
The spinal cord was completely transected at low thoracic levels. A small laminectomy was performed between the 12th and 13th thoracic vertebrae. After the spinal cord was exposed, lidocaine (Xylocaine; 2%) was topically applied and injected intraspinally. The spinal cord was then transected with surgical scissors. After spinal transection, the two cut ends of the spinal cord retracted, producing a gap >0.5 cm. The gap was then cleaned, and any residual bleeding was completely stopped with cotton tip applicators. The completeness of the section was inspected visually and verified by moving a small metal spatula within the gap. Hemostatic material (Spongostan) was then inserted within the gap, and muscles and skin were sewn back to close the opening in anatomic layers. Following spinalization, the bladder was manually expressed one or two times each day, and the hindlimbs were frequently cleaned by placing the lower half of the body in a warm soapy bath.
Implantation.
Cats were implanted with electrodes to chronically record muscle activity (electromyography, EMG). Pairs of Teflon-insulated multistrain fine wires (AS633; Cooner Wire, Chatsworth, CA) were directed subcutaneously from a head-mounted 34-pin connector (Omnetics Connector, Minneapolis, MN) and sewn into the belly of selected hindlimb muscles for bipolar recordings. During surgery, electrode placement was verified by electrically stimulating each muscle through the appropriate head connector channel.
Locomotor Training
One week after spinalization, cats began a training regimen consisting of five sessions per week (20–30 min per session) to recover involuntary hindlimb locomotion. Early after spinalization, two experimenters moved the hindlimbs to reproduce a locomotor pattern with similar joint kinematics and paw contacts while the forelimbs were positioned on a fixed platform located ∼1 cm above the belt. Weight support and equilibrium were provided by an experimenter gently holding the base of the tail. A Plexiglas separator placed between the hindlimbs prevented them from impeding each other. After a few days, the skin of the perineal region was stimulated to evoke stepping movements. Experimenter-assisted weight support was gradually reduced as extensor tonus recovered. Recording sessions started once the animals attained a stable plateau in their locomotor performance, characterized by full weight bearing and consistent plantar foot placement. Training was continued three to five times per week to maintain a stable level of locomotor performance.
Experimental Protocol
Experiments were performed 88 to 562 days (mean 243.3 ± 193.4 days) after spinalization. All experiments were performed on an animal treadmill with two independently controlled running surfaces 120 cm long and 30 cm wide (Bertec, Columbus, OH). Cats performed tied-belt locomotion (equal speed for left and right belts) from 0.1 to 0.4 m/s in 0.05 m/s increments. A speed of 0.1 m/s was used as the slowest speed because it was determined from pilot studies that it was the slowest speed where the pattern still resembled a stepping behavior with proper out-of-phase alternation between the left and right hindlimbs, as opposed to a series of postural corrections. A speed of 0.4 m/s was chosen as the fastest speed because it falls within the range of preferred treadmill speeds for intact cats (discussed in Frigon 2011). All cats could perform hindlimb stepping up to 1.0 m/s, which was the maximal speed tested (data not shown in this study). During an episode, the treadmill belt attained the desired speed with an acceleration of 0.1 m/s2. Recordings began once the treadmill belt reached the desired speed and the hindlimbs performed a minimum of five consistent consecutive steps. The treadmill belt was stopped after 15–20 consistent cycles were obtained. Approximately 15–30 s of rest were given between episodes. Trials were repeated, if necessary, until 15–20 stable consecutive cycles were obtained at each speed. During experiments, hindlimb locomotion was performed without perineal stimulation. and weight support was not provided, although an experimenter gently held the tail to provide lateral equilibrium.
Data Acquisition and Analysis
Videos of the left and right sides were captured with two cameras (Basler acA640-100 gm) at 60 frames/s with a spatial resolution of 640 × 480 pixels. A custom-made LabVIEW program acquired images and synchronized the cameras with the EMG. Videos were analyzed off-line at 60 frames/s using custom-made software. A reflective marker was placed on the iliac crest as an indicator of hip position.
Kinematic parameters.
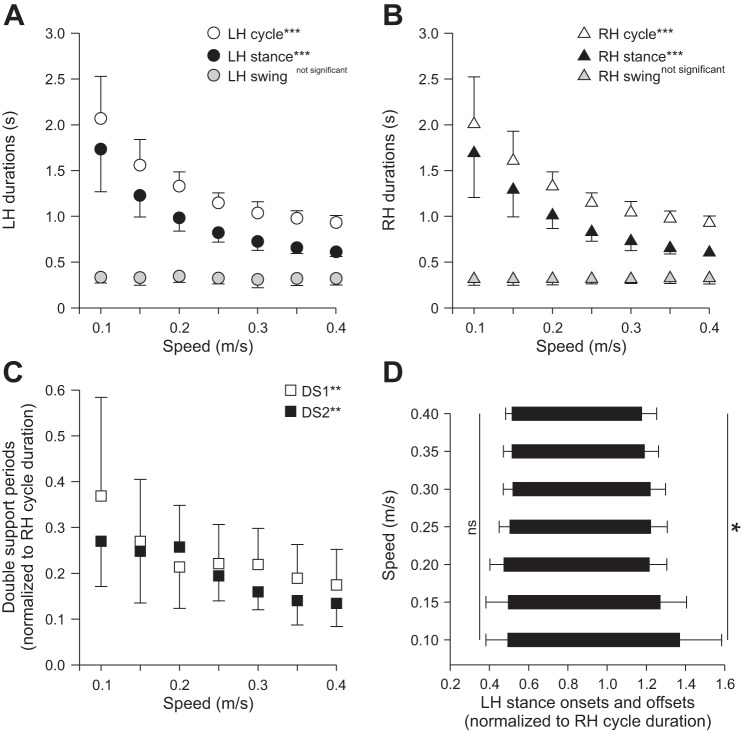
Figure 1 shows the temporal (A) and spatial (B) parameters that were measured from kinematic events. Stance onset and offset were determined from paw contact and the most caudal displacement of the toe, respectively, for the left (LH) and right hindlimbs (RH) by visual inspection. Cycle duration was measured from successive paw contacts, whereas stance duration corresponded to the interval of time from stance onset to offset. Swing duration was measured as cycle duration minus stance duration. Cycle, stance, and swing durations were measured for LH and RH. Double support periods were measured from RH stance onset to LH stance offset (DS1) and from LH stance onset to RH stance offset (DS2). The temporal phase interval (φt) was measured by dividing the interval of time between RH and LH stance onsets by RH cycle duration.
Fig. 1.
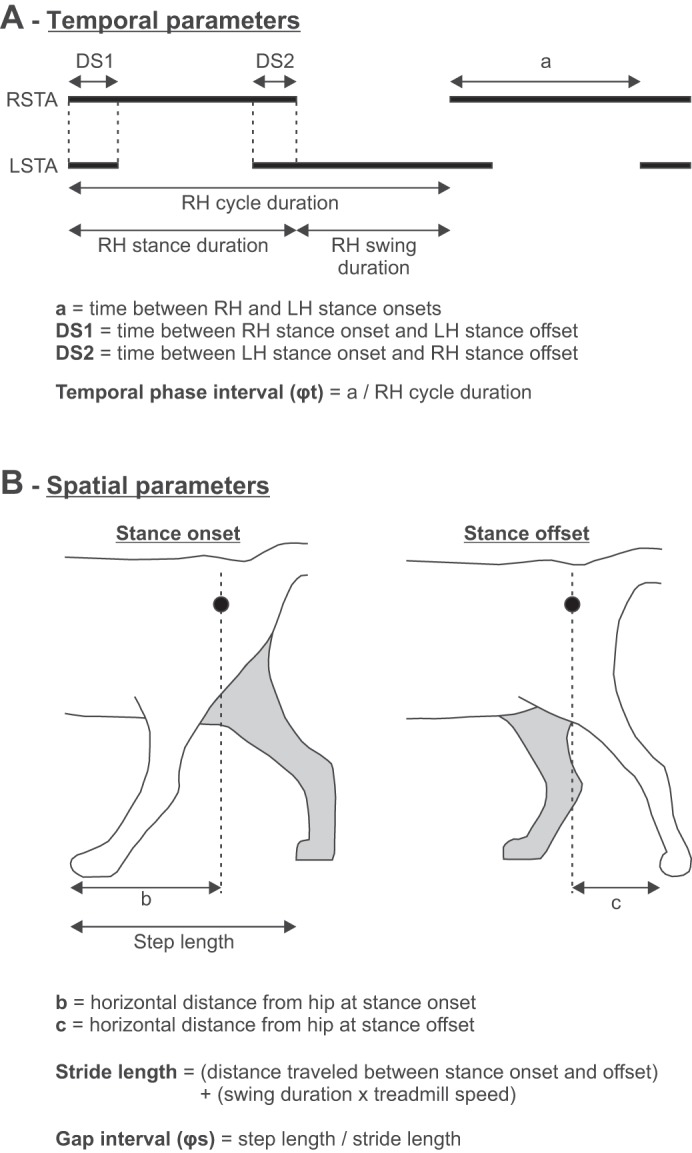
Temporal and spatial parameters measured from kinematic events. In A, thick horizontal lines indicate durations of the right (RSTA) and left (LSTA) stance phases, which were measured from paw contact (stance onset) to the most caudal displacement of the toe (stance offset), as shown in B. LH, left hindlimb; RH, right hindlimb. The calculations for temporal and spatial kinematic parameters are shown in A and B, respectively.
The horizontal distance between the toe and hip marker at stance onset and offset was measured bilaterally. Stride length was measured as the distance between stance onset and offset of a given limb added to the distance traveled by the treadmill during the swing phase, which was calculated by multiplying swing duration by treadmill speed (Courtine et al. 2005; Thibaudier and Frigon 2014). Step length was measured as the distance between the leading and trailing limbs at stance onset of the leading limb (Hoogkamer et al. 2014). LH step length is defined as the distance between LH at contact and RH while RH step length is the distance between RH at contact and LH. The gap interval (φs), the spatial analog of the phase interval, was measured by dividing RH step length by RH stride length (Abourachid et al. 2007; Thibaudier and Frigon 2014). Stride lengths and step lengths were measured bilaterally. Each temporal and spatial parameter was measured from 10–15 cycles and averaged for an episode.
To assess left-right symmetry, an asymmetry index (ASI) was calculated for stance duration, swing duration, double support periods, stride length, step length, distance at stance onset, and distance at stance offset using the following formula (adapted from Martinez et al. 2013):
To determine whether the temporal and spatial control of left-right coordination remains symmetric on a step-to-step basis, we measured the phase (PCI) and gap (GCI) coordination indexes (Thibaudier and Frigon 2014). Perfect left-right symmetry is defined as a value of 0.5 for φt (contact of LH occurs halfway between 2 successive RH contacts) and φs (step length is half of stride length). For PCI (Plotnik et al. 2007), we first measured the mean value of the absolute difference in φ for each cycle from 0.5, denoted as
where φti is the temporal phase interval of the ith cycle. This value was then divided by 0.5 and multiplied by 100, and the result was denoted as Pφ_ABS. The coefficient of variation of the mean of φt multiplied by 100 was then calculated, denoted as φt_CV. PCI is equal to the sum of Pφt_ABS + φt_CV.
For GCI (Thibaudier and Frigon 2014), we first measured the mean value of the absolute difference in φs for each cycle from 0.5, denoted as
where φsi is the gap interval of the ith step. This value was then divided by 0.5 and multiplied by 100, and the result was denoted as Pφs_ABS. The coefficient of variation of the mean of φs multiplied by 100 was then calculated, denoted as φs_CV. GCI is equal to the sum of Pφs_ABS + φs_CV.
Electromyography.
EMG was preamplified (×10; custom-made system), bandpass filtered (30-1,000 Hz), and amplified (×100-5,000) using a 16-channel amplifier (model 3500; AM Systems, Sequim, WA). EMG data were digitized (2,000 Hz) with a National Instruments card (NI 6032E), acquired with custom-made acquisition software, and stored on a computer. The current EMG data set was obtained from the right anterior sartorius (Srt; hip flexor/knee extensor) and the right lateral gastrocnemius (LG; ankle plantar flexor/knee flexor). Burst onsets and offsets were determined by visual inspection by the same experimenter (Dambreville) from the raw EMG waveforms using a custom-made program. Burst duration was measured from onset to offset.
Statistical Analysis
One- or two-factor repeated-measures analyses of variance (ANOVA) were performed with IBM SPSS Statistics 22.0 (IBM, Armonk, NY) to determine the effect of speed and/or side on the various parameters. Group data shown in graphs are means ± SD. Significance level was set at P < 0.05.
RESULTS
Following spinal transection, all cats recovered full weight-bearing hindlimb locomotion with 6–10 wk of treadmill training. The following sections describe spatiotemporal and EMG adjustments during hindlimb locomotion in chronic spinalized adult cats from slow (0.1 m/s) to moderate (0.4 m/s) stepping speeds.
Temporal Adjustments as a Function of Speed
Figure 2 shows the hindlimb locomotor pattern at three speeds in one chronic spinalized adult cat. As expected, extensor burst and stance phase durations decreased with increasing treadmill speed whereas Srt burst and swing phase durations were relatively unchanged. Double support periods were longer at slower speeds with a noticeable left-right asymmetry. For instance, at 0.1 m/s, the period of double support from RH contact to LH liftoff (DS1) was longer than from LH contact to RH liftoff (DS2) (Fig. 2A). This was due to differences in stance phase durations between LH and RH.
Fig. 2.
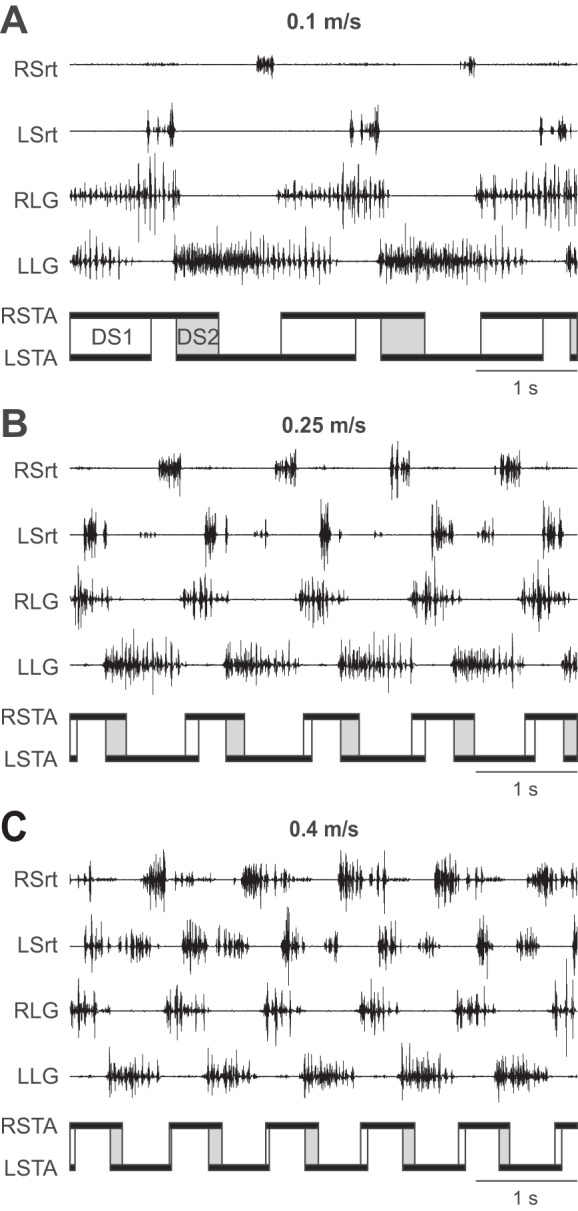
Speed-dependent adjustments in the hindlimb locomotor pattern of one chronic spinalized adult cat. The pattern is shown at speeds of 0.1 (A), 0.25 (B), and 0.4 m/s (C) with electromyography (EMG) from the right (RSrt) and left sartorius (LSrt) and from the right (RLG) and left lateral gastrocnemius (LLG). The RSTA and LSTA phases are shown below the EMGs. Periods of double support from right stance onset to left stance offset (DS1; open rectangles) and from left stance onset to right stance offset (DS2; shaded rectangles) are also shown. Data are from cat PZ.
Figure 3, A and B, shows cycle and phase durations measured from LH and RH contacts and liftoffs across speeds for the group. Cycle and stance durations significantly decreased (P < 0.001), whereas swing duration was unaffected (P = 0.70). There was no effect of side (LH vs. RH) on cycle (P = 0.89), stance (P = 0.80), and swing (P = 0.42) durations. The two periods of double support significantly decreased with increasing speed (P < 0.01; Fig. 3C), with no difference between the two periods (P = 0.42). The reduction in double support periods with increasing speed was due to a relatively earlier swing onset (P < 0.05), because there was no change in stance onset with increasing speed (P = 0.52; shown for LH in Fig. 3D). The normalized stance onset, which is equal to the temporal phase interval between hindlimb contacts, was maintained around 0.5 across speeds, indicating a strict out-of-phase temporal alternation.
Fig. 3.
Temporal parameters during hindlimb locomotion across speeds for the group. Cycle and phase durations are shown for LH (A) and RH (B). C: DS1 and DS2 across speeds. D: stance onset and offset of LH normalized to RH cycle duration. At each speed, 10–15 cycles were averaged for each cat. Individual cat averages were then averaged for the group (n = 6). Each data point or bar indicates mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 indicate significant effect of speed (repeated-measures ANOVA); ns, not significant.
The LG (extensor muscle) burst duration significantly decreased with increasing speed (P < 0.001; Fig. 4A), whereas Srt (flexor muscle) burst duration was not affected (P = 0.31). To determine if burst durations in extensor and flexor muscles displayed a pattern of change similar to that of stance and swing phases, respectively, we plotted EMG burst durations as a function of kinematic phase durations and measured Pearson's correlation coefficient (r). There was a strong significant relationship between extensor burst and stance duration and a moderately strong significant relationship between flexor burst and swing duration (Fig. 4B).
Fig. 4.
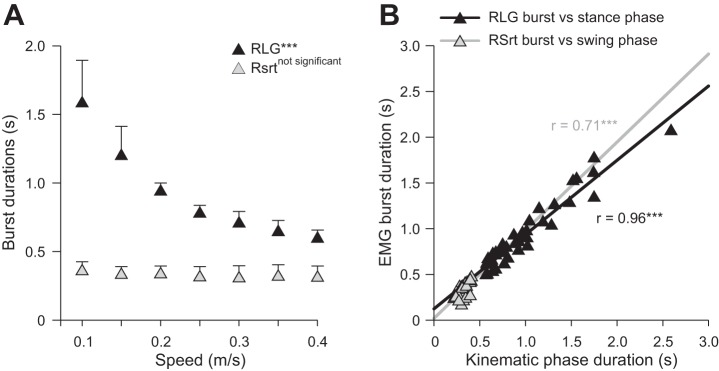
Extensor and flexor burst durations across speeds for the group. A: burst durations for RLG and RSrt across speeds. At each speed, 10–15 cycles were averaged for each cat. Individual cat averages were then averaged for the group (n = 6). Each data point indicates mean ± SD. B: burst durations for RLG and RSrt plotted as a function of right stance phase and right swing phase durations, respectively. Pearson's correlation coefficient (r) was measured; asterisks indicate a significant correlation.
Spatial Adjustments as a Function of Speed
Stride length (P < 0.001; Fig. 5A) and step length (P < 0.001; Fig. 5B) significantly increased from slow to moderate stepping speeds in LH and RH, with no significant difference between sides (P > 0.05). The gap interval, the spatial analog to the temporal phase interval between hindlimb contacts (see Fig. 3D), was not affected by increasing speed (P = 0. 61; Fig. 5C), indicating a strict out-of-phase spatial alternation. The distance at liftoff (negative values) between the toe and the hip significantly increased with increasing speed (P < 0.001), whereas the distance at contact (positive values) was not affected (P > 0.70) for both LH (Fig. 6A) and RH (Fig. 6B), with no difference between sides for distance at liftoff (P = 0.27) and contact (P = 0.95). In other words, the relative position of the paw was more caudal at liftoff with increasing speed, whereas placement of the paw at contact was consistently made at the same relative place.
Fig. 5.
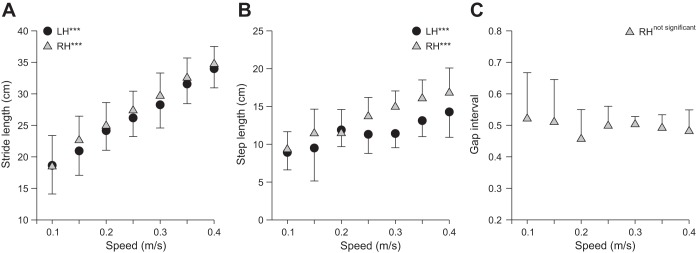
Spatial parameters during hindlimb locomotion across speeds for the group. Stride lengths (A) and step lengths (B) are shown for LH and RH across speeds along with the gap interval (C). At each speed, 10–15 cycles were averaged for each cat. Individual cat averages were then averaged for the group (n = 6). Each data point indicates mean ± SD. ***P < 0.001 indicates a significant effect of speed (repeated-measures ANOVA).
Fig. 6.
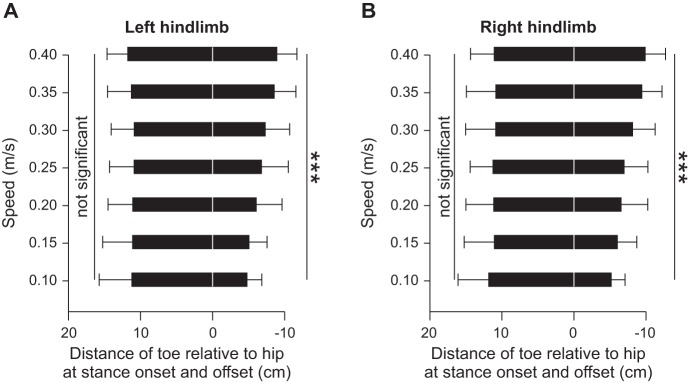
Distance at stance onset and offset across speeds for the group. The horizontal distance from the toe to the hip marker was measured for LH (A) and RH (B). The distances at stance onset and offset are shown as positive and negative values, respectively. At each speed, 10–15 cycles were averaged for each cat. Individual cat averages were then averaged for the group (n = 6). ***P < 0.001 indicates a significant effect of speed (repeated-measures ANOVA).
Left-Right Symmetry as a Function of Speed
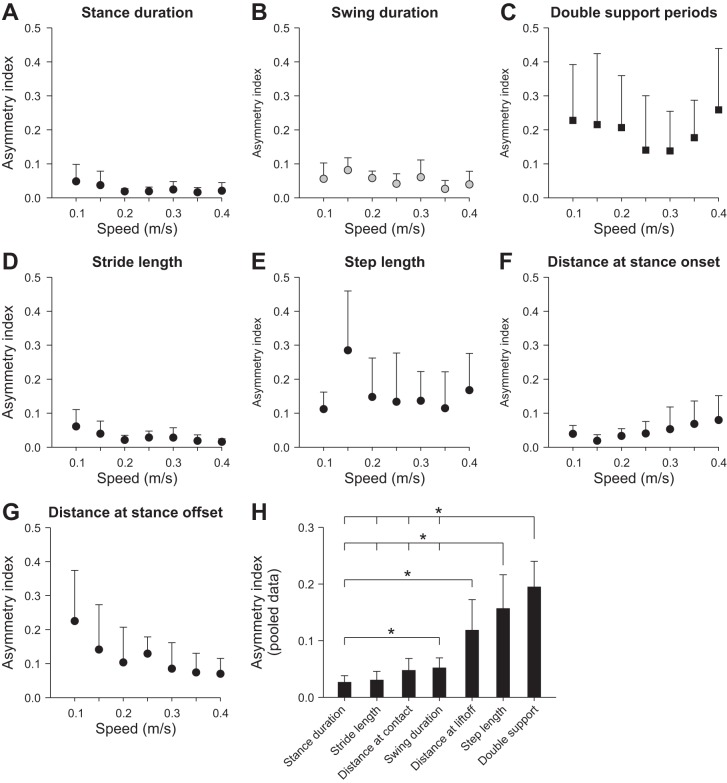
For all temporal and spatial parameters there were no significant differences between LH and RH, indicating that there is no bias for left or right dominance at the level of the spinal locomotor networks across cats. However, this does not indicate that there is no left-right asymmetry at the individual level, because some cats might have longer temporal and spatial parameters on the left or right side. To answer this question, an asymmetry index (ASI; see materials and methods) was measured for several parameters across speeds. Across parameters, speed did not significantly affect ASIs (P = 0.30; Fig. 7, A–G). However, the ASI for some parameters was significantly different from that of others (P < 0.01). As such, data across speeds were pooled for each parameter, and significant differences were assessed with pairwise comparisons (Fig. 7H). Parameters were arranged from smallest (stance duration) to largest ASI (double support period). The ASIs for step length and double support period were significantly greater than for stance duration, stride length, distance at contact, and swing duration. Thus the highest ASIs were found for interlimb parameters.
Fig. 7.
Left-right symmetry for temporal and spatial parameters during hindlimb locomotion across speeds for the group. An asymmetry index was measured for stance duration (A), swing duration (B), double support periods (C), stride length (D), step length (E), distance of the toe relative to the hip at stance onset (F), and distance of the toe relative to the hip at stance offset (G). At each speed, 10–15 cycles were averaged for each cat. Individual cat averages were then averaged for the group (n = 6). Each data point indicates mean ± SD. H: data were pooled and averaged for each parameter across speeds and compared with one another. *P < 0.05 indicates a significant difference between parameters (pairwise comparisons). Note that for step length and double support, the longer vertical line indicates a comparison with multiple parameters.
The Step-to-Step Spinal Control of Left-Right Symmetry as a Function of Speed
To assess if the step-to-step temporal and spatial control of left-right symmetry was influenced by speed, the phase (PCI) and gap (GCI) coordination indexes were measured (see materials and methods). Low values of PCI and GCI indicate small variations in temporal and spatial phasing symmetry, respectively, on a step-to-step basis. The PCI (P < 0.01; Fig. 8A) and GCI (P < 0.05; Fig. 8B) significantly decreased with increasing speed. Pairwise comparisons showed significant differences in PCI and GCI between the two slowest speeds (0.1 and 0.15 m/s) and faster speeds. Therefore, the slowest locomotor speeds show a more variable regulation of temporal and spatial left-right coordination. Whereas PCI continued to decrease up to 0.4 m/s (Fig. 8A), GCI flattened out at 0.2 or 0.25 m/s (Fig. 8B).
Fig. 8.
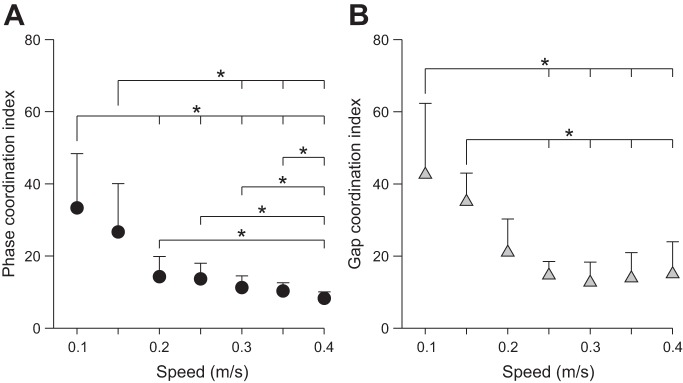
Step-to-step temporal and spatial phasing across speeds for the group. For each cat, the phase (A) and gap coordination indexes (B) were measured and then averaged for the group (n = 6) at each speed. Each data point indicates mean ± SD. *P < 0.05 indicates a significant difference between speeds (pairwise comparisons).
DISCUSSION
The present study is the first to characterize both temporal and spatial adjustments in the hindlimb locomotor pattern from slow to moderate stepping speeds in a chronic spinalized animal model. Consistent with our hypotheses, we showed that the hindlimb locomotor pattern of chronic spinalized adult cats 1) concomitantly adjusts both temporal and spatial kinematic parameters from slow to moderate stepping speeds and 2) displays increased step-to-step temporal and spatial variability in left-right symmetry at slow speeds.
Spinal Control of Temporal and Spatial Adjustments with Speed
As stated in the Introduction, studies investigating hindlimb locomotor control in chronic spinalized animals across speeds have mainly described temporal, electromyographic, and joint angle changes at a few speeds (Barbeau and Rossignol 1987; de Guzman et al. 1991; Forssberg et al. 1980a, 1980b; Frigon et al. 2013; Lovely et al. 1986, 1990; Smith et al. 1982). Here we showed kinematic spatial adjustments during hindlimb locomotion in chronic spinalized adult cats that closely resembled those found in intact animals, indicating that the spinal cord plays a major role in controlling these parameters. For instance, animals, including humans, adjust to an increase in speed over a certain range by increasing step frequency (or reducing cycle duration), stride length, and step length (Blaszczyk and Dobrzecka 1989). Increases in stride length and step length observed with increasing treadmill speed in chronic spinalized adult cats (Fig. 5) were strikingly similar to those found in intact quadrupeds (Blaszczyk and Dobrzecka 1989; Thibaudier and Frigon 2014).
We also found that double support periods at the slowest speeds were markedly longer than at faster speeds (Fig. 3C). Prolonged double support periods at slow speeds were proposed as a compensatory mechanism to improve dynamic stability (Wuehr et al. 2014). Here we show that the increase in double support periods at slower speeds is a consequence of proportionally longer stance durations bilaterally coupled to swing phase durations on the left and right sides that remain relatively invariant as a function of speed (see Fig. 3). Thus the increase in double support periods with a reduction in speed is a built-in property of the spinal control system, possibly to ensure greater stability at slower speeds.
The temporal (Fig. 3D) and spatial (Fig. 5C) phasing between hindlimbs was not affected by speed and was maintained at around 0.5, as shown previously in intact cats and dogs during symmetric gaits, such as walking and trotting (Afelt et al. 1983; Blaszczyk and Dobrzecka 1989; Frigon et al. 2014). In other words, the time between contacts of the two hindlimbs is approximately half of the cycle, whereas the step length is approximately half the stride length. Thus the ability to maintain an out-of-phase temporal and spatial alternation between the left and right hindlimbs is mediated at the level of the spinal cord, most likely by using sensory cues from the periphery. A precise spatiotemporal control of left-right coordination is critical to maintain dynamic stability.
The position of the paw at contact is arguably the most important event in the step cycle because it is critical to maintain dynamic stability (Halbertsma 1983; Klishko et al. 2014). Here we show that placement of the hind paw relative to the hip at contact in chronic spinalized adult cats is invariant across speeds for both the left and right sides (Fig. 6). As others have proposed (Hollands and Marple-Horvat 1996; Klishko et al. 2014; Patla and Vickers 2003) and based on our results, it is likely that paw placement at contact is planned before swing onset. Initial position of the paw at swing onset does not appear to be a factor in determining paw placement at contact because the transition from stance to swing occurred with the paw positioned more caudally with increasing speed (Fig. 6). Despite a more caudal position at swing onset, the paw was consistently placed at the same relative position at contact. We propose that foot placement is planned at the spinal level and is independent of initial position at swing onset, although it can certainly be modified by supraspinal inputs and sensory feedback from the periphery during the swing phase to adjust to environmental constraints.
The results of the present study indicate that the spinal cord has the ability to control both temporal and spatial kinematic aspects of locomotion when adjusting to changes in speed. Poppele and Bosco (2003) argued that the spinal cord has much of the required circuitry to produce the desired kinematic outcome. For instance, spinalized frogs and turtles can perform a site-appropriate movement to remove an irritant from the surface of the body, an action that requires transformation of sensory information into appropriate spatial coordinates before activation of motor pools (Giszter et al. 1989; Mortin and Stein 1989). The fact that adjustments in stride length and step length as a function of speed, as well as consistent placement of the hind paw at contact, are largely determined at a spinal level via interactions with sensory feedback from the periphery greatly simplifies the control required from supraspinal structures.
Possible Mechanisms Mediating Step-to-Step Variability at Slow to Moderate Locomotor Speeds
Previous studies have shown that step-to-step variability is increased at slow speeds during human walking (Schniepp et al. 2012; Wuehr et al. 2014). Here we show that the increased step-to-step variability with slower speeds is also present in chronic spinalized adult cats, indicating that it is generated, at least in part, at the spinal cord level, either via intrinsic spinal mechanisms or by interactions with peripheral sensory inputs. During spontaneous fictive locomotion in decerebrate chronic spinalized cats, where supraspinal inputs and phasic sensory feedback from the periphery are absent, cycle and burst durations can considerably vary from one cycle to the next, indicating that rhythm-generating mechanisms within the spinal locomotor CPGs have an important intrinsic variability (Frigon and Gossard 2009, 2010). The cycle-to-cycle variability is also higher at slower frequencies (Frigon and Gossard 2009). Thus greater gait variability at slower stepping speeds could in large part be due to the intrinsic variability within the left and right spinal locomotor CPGs, particularly when the rhythm-generating networks oscillate at slow frequencies.
Step-to-step variability increases, particularly at slow speeds, when visual feedback is removed in healthy subjects (Wuehr et al. 2013) and in pathologies that disrupt supraspinal inputs (Brandt et al. 1999; Schniepp et al. 2012) or sensory feedback (Wuehr et al. 2014). In a similar vein, enhancing sensory feedback from the foot reduces gait variability in elderly subjects (Lipsitz et al. 2015). We propose that under normal circumstances, supraspinal inputs and peripheral sensory feedback help reduce the intrinsic variability of spinal locomotor CPGs. As such, in cases where supraspinal or sensory inputs are diminished, it is better to walk at faster speeds to reduce the intrinsic variability of spinal locomotor CPGs. Increased sensory feedback from peripheral mechanoreceptors with speed (Den Otter et al. 2004) due to increased force generation, loading, and muscle stretch might help reduce the intrinsic variability of spinal locomotor CPGs, thus reducing step-to-step variability.
With the advent of genetic manipulations, studies have begun to elucidate the spinal neuronal circuitry involved in controlling the frequency of locomotor-like rhythmic bursts in terrestrial mammals, primarily by using in vitro neonatal mice preparations (Crone et al. 2009; Gosgnach et al. 2006; Talpalar and Kiehn 2010; Talpalar et al. 2013). An emerging consensus is that the spinal mechanisms at slow frequencies are different from those at higher ones (Shevtsova et al. 2015; Talpalar et al. 2013). Although we can only speculate, it is also possible that an increase in speed activates neuronal mechanisms that help reduce step-to-step variability.
Left-Right Symmetry
Previous studies showed that adjustments to split-belt locomotion were similar if it was the left or right side that was stepping faster (D'Angelo et al. 2014; Zijlstra and Dietz 1995). In the present study, there was no difference in any parameter between the left and right sides with a change in speed, indicating that there is no overall group bias for left or right dominance at the level of spinal locomotor CPGs. However, an asymmetry might have been present at a given speed at the individual level where some cats could have adopted a bias for left or right dominance. Thus we measured the absolute difference between the left and the right sides for several parameters across speeds (Fig. 7). Left-right symmetry was not affected by speed. Interestingly, some parameters displayed more left-right asymmetry than others. Stance duration, swing duration, and stride length had a low ASI, undoubtedly because these parameters are largely determined by belt speed. The distance of the foot at contact also had a low ASI, probably because it is so tightly regulated across speeds. On the other hand, interlimb parameters, such as double support periods and step length had a significantly higher ASI. This could be due to asymmetric influences from peripheral sensory feedback onto the left and right spinal locomotor CPGs. Slight postural shifts can produce asymmetric sensory feedback (e.g., more loading on one side) that could alter interactions between the left and right sides, thus increasing left-right asymmetry for interlimb parameters but not for intralimb parameters that are largely driven by belt speed.
Concluding Remarks
The present study showed that the spinal cord can adjust both temporal and spatial parameters to accommodate a change in locomotor speed due to sensory cues from the periphery. Some parameters, such as the temporal and spatial phasing between hindlimbs as well as the distance of the paw at contact in the sagittal plane, were invariant to changes in speed. The ability of the spinal cord using sensory cues from the periphery in controlling both temporal and spatial gait parameters undoubtedly simplifies the role of supraspinal structures in adapting to speed. The marked increased in step-to-step variability in left-right symmetry at slower speeds in chronic spinalized cats indicates that it is mediated at the level of the spinal cord, either by intrinsic spinal mechanisms or by interactions between peripheral sensory feedback and spinal circuits. Increased step-to-step variability observed in various pathological states at slower speeds might be due to insufficient or altered inputs from other sources (e.g., supraspinal, peripheral) that normally stabilize the intrinsic variability of spinal locomotor CPGs when they oscillate at slow frequencies. A better understanding of the spinal mechanisms that give rise to this variability could lead to better strategies to maximize stability, particularly at slow walking speeds.
GRANTS
The present research was funded by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds de Recherche du Québec–Natures et Technologies (FQRNT) to A. Frigon. M.-F. Hurteau was supported by a master's scholarship from the Fonds de Recherche du Québec–Santé (FRQS), and Y. Thibaudier was supported by a doctoral scholarship from the FQRNT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D. and A.F. conception and design of research; C.D., Y.T., M.-F.H., and A.F. performed experiments; C.D., A.L., and A.F. analyzed data; C.D., Y.T., M.-F.H., and A.F. interpreted results of experiments; C.D. and A.F. prepared figures; C.D., A.L., Y.T., M.-F.H., and A.F. edited and revised manuscript; C.D., A.L., Y.T., M.-F.H., and A.F. approved final version of manuscript; A.F. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Alessandro Telonio for technical assistance and Victoria Kuczynski and Emily Rhodes for helping with some of the experiments.
REFERENCES
- Abourachid A, Herbin M, Hackert R, Maes L, Martin V. Experimental study of coordination patterns during unsteady locomotion in mammals. J Exp Biol 210: 366–372, 2007. [DOI] [PubMed] [Google Scholar]
- Afelt Z, Blaszczyk J, Dobrzecka C. Speed control in animal locomotion: transitions between symmetrical and nonsymmetrical gaits in the dog. Acta Neurobiol Exp (Wars) 43: 235–250, 1983. [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Dobrzecka C. Speed control in quadrupedal locomotion: principles of limb coordination in the dog. Acta Neurobiol Exp (Wars) 49: 105–124, 1989. [PubMed] [Google Scholar]
- Brandt T, Strupp M, Benson J. You are better off running than walking with acute vestibulopathy. Lancet 354: 746, 1999. [DOI] [PubMed] [Google Scholar]
- Courtine G, Roy RR, Hodgson J, McKay H, Raven J, Zhong H, Yang H, Tuszynski MH, Edgerton VR. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus). J Neurophysiol 93: 3127–3145, 2005. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 29: 7098–7109, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G, Thibaudier Y, Telonio A, Hurteau MF, Kuczynski V, Dambreville C, Frigon A. Modulation of phase durations, phase variations and temporal coordination of the four limbs during quadrupedal split-belt locomotion in intact adult cats. J Neurophysiol 112: 1825–1837, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman CP, Roy RR, Hodgson JA, Edgerton VR. Coordination of motor pools controlling the ankle musculature in adult spinal cats during treadmill walking. Brain Res 555: 202–214, 1991. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait Posture 19: 270–278, 2004. [DOI] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- English AW, Lennard PR. Interlimb coordination during stepping in the cat: in-phase stepping and gait transitions. Brain Res 245: 353–364, 1982. [DOI] [PubMed] [Google Scholar]
- Espy DD, Yang F, Bhatt T, Pai YC. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 32: 378–382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand 108: 269–281, 1980a. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108: 283–295, 1980b. [DOI] [PubMed] [Google Scholar]
- Frigon A. Chapter 7–interindividual variability and its implications for locomotor adaptation following peripheral nerve and/or spinal cord injury. Prog Brain Res 188: 101–118, 2011. [DOI] [PubMed] [Google Scholar]
- Frigon A. Central pattern generators of the mammalian spinal cord. Neuroscientist 18: 56–69, 2012. [DOI] [PubMed] [Google Scholar]
- Frigon A, Barriere G, Leblond H, Rossignol S. Asymmetric changes in cutaneous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat. J Neurophysiol 102: 2667–2680, 2009. [DOI] [PubMed] [Google Scholar]
- Frigon A, D'Angelo G, Thibaudier Y, Hurteau MF, Telonio A, Kuczynski V, Dambreville C. Speed-dependent modulation of phase variations on a step-by-step basis and its impact on the consistency of interlimb coordination during quadrupedal locomotion in intact adult cats. J Neurophysiol 111: 1885–1902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat. J Physiol 587: 4617–4628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J Neurosci 30: 7061–7071, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Hurteau MF, Thibaudier Y, Leblond H, Telonio A, D'Angelo G. Split-belt walking alters the relationship between locomotor phases and cycle duration across speeds in intact and chronic spinalized adult cats. J Neurosci 33: 8559–8566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Thibaudier Y, Hurteau MF. Modulation of forelimb and hindlimb muscle activity during quadrupedal tied-belt and split-belt locomotion in intact cats. Neuroscience 290: 266–278, 2015. [DOI] [PubMed] [Google Scholar]
- Giszter SF, McIntyre J, Bizzi E. Kinematic strategies and sensorimotor transformations in the wiping movements of frogs. J Neurophysiol 62: 750–767, 1989. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H, Frigon A. Chapter 2–the spinal generation of phases and cycle duration. Prog Brain Res 188: 15–29, 2011. [DOI] [PubMed] [Google Scholar]
- Halbertsma JM. The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiol Scand Suppl 521: 1–75, 1983. [PubMed] [Google Scholar]
- Hildebrand M. Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Neural Control of Locomotion, edited by Herman RM, Grillner S, Stein PS, and Stuart DG. New York: Plenum, 1976, p. 203–236. [Google Scholar]
- Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc 20: 161–166, 1988. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE. Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res 109: 343–356, 1996. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Duysens J. Stride length asymmetry in split-belt locomotion. Gait Posture 39: 652–654, 2014. [DOI] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Desaulniers C, Frigon A. Effect of stimulating the lumbar skin caudal to a complete spinal cord injury on hindlimb locomotion. J Neurophysiol 113: 669–676, 2015. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21: 100–109, 2011. [DOI] [PubMed] [Google Scholar]
- Klishko AN, Farrell BJ, Beloozerova IN, Latash ML, Prilutsky BI. Stabilization of cat paw trajectory during locomotion. J Neurophysiol 112: 1376–1391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond H, L'Esperance M, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci 23: 11411–11419, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehabil 96: 432–439, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol 92: 421–435, 1986. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res 514: 206–218, 1990. [DOI] [PubMed] [Google Scholar]
- Martinez M, Delivet-Mongrain H, Rossignol S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J Neurophysiol 109: 2909–2922, 2013. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortin LI, Stein PS. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J Neurosci 9: 2285–2296, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito A, Shimizu Y, Handa Y. Analyses of treadmill locomotion in adult spinal dogs. Neurosci Res 8: 281–290, 1990. [DOI] [PubMed] [Google Scholar]
- Patla AE, Vickers JN. How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp Brain Res 148: 133–138, 2003. [DOI] [PubMed] [Google Scholar]
- Pepin A, Ladouceur M, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 2. Factors limiting the maximal speed. Spinal Cord 41: 271–279, 2003. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson's disease. Exp Brain Res 181: 561–570, 2007. [DOI] [PubMed] [Google Scholar]
- Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends Neurosci 26: 269–276, 2003. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- Schniepp R, Wuehr M, Neuhaeusser M, Kamenova M, Dimitriadis K, Klopstock T, Strupp M, Brandt T, Jahn K. Locomotion speed determines gait variability in cerebellar ataxia and vestibular failure. Mov Disord 27: 125–131, 2012. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Talpalar AE, Markin SN, Harris-Warrick RM, Kiehn O, Rybak IA. Organization of left-right coordination of neuronal activity in the mammalian spinal cord: insights from computational modelling. J Physiol 593: 2403–2426, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Majczynski H, Dai Y, Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol 590: 1721–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Smith LA, Zernicke RF, Hoy M. Locomotion in exercised and nonexercised cats cordotomized at two or twelve weeks of age. Exp Neurol 76: 393–413, 1982. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits 4: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudier Y, Frigon A. Spatiotemporal control of interlimb coordination during transverse split-belt locomotion with 1:1 or 2:1 coupling patterns in intact adult cats. J Neurophysiol 112: 2006–2018, 2014. [DOI] [PubMed] [Google Scholar]
- Wuehr M, Schniepp R, Pradhan C, Ilmberger J, Strupp M, Brandt T, Jahn K. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp Brain Res 224: 287–294, 2013. [DOI] [PubMed] [Google Scholar]
- Wuehr M, Schniepp R, Schlick C, Huth S, Pradhan C, Dieterich M, Brandt T, Jahn K. Sensory loss and walking speed related factors for gait alterations in patients with peripheral neuropathy. Gait Posture 39: 852–858, 2014. [DOI] [PubMed] [Google Scholar]
- Zijlstra W, Dietz V. Adaptability of the human stride cycle during split-belt walking. Gait Posture 3: 250–257, 1995. [Google Scholar]