Abstract
Corollary discharge (CD) refers to “copies” of motor signals sent to sensory areas, allowing prediction of future sensory states. They enable the putative mechanisms supporting the distinction between self-generated and externally generated sensations. Accordingly, many authors have suggested that disturbed CD engenders psychotic symptoms of schizophrenia, which are characterized by agency distortions. CD also supports perceived visual stability across saccadic eye movements and is used to predict the postsaccadic retinal coordinates of visual stimuli, a process called remapping. We tested whether schizophrenia patients (SZP) show remapping disturbances as evidenced by systematic transsaccadic mislocalizations of visual targets. SZP and healthy controls (HC) performed a task in which a saccadic target disappeared upon saccade initiation and, after a brief delay, reappeared at a horizontally displaced position. HC judged the direction of this displacement accurately, despite spatial errors in saccade landing site, indicating that their comparison of the actual to predicted postsaccadic target location relied on accurate CD. SZP performed worse and relied more on saccade landing site as a proxy for the presaccadic target, consistent with disturbed CD. This remapping failure was strongest in patients with more severe psychotic symptoms, consistent with the theoretical link between disturbed CD and phenomenological experiences in schizophrenia.
Keywords: corollary discharge, efference copy, schizophrenia, saccadic eye movements, remapping
to successfully distinguish between self-generated and externally generated experiences, sensory brain regions must be updated about motor commands in order to predict future sensory states. A match between predicted and actual sensations is theorized to engender a sense of agency over action (Feinberg 1978; Frith 1992). These predictions can be accomplished via corollary discharge (CD), correlates of motor commands that are sent to sensory areas (Crapse and Sommer 2008). CD signals attenuate sensations that match predictions, rendering unpredicted, externally generated sensations more salient. Accordingly, disturbed CD might cause those psychotic symptoms of schizophrenia that are characterized by agency abnormalities (e.g., delusions of being controlled by aliens; Feinberg 1978).
The most rigorous investigations of CD have been performed in the saccadic eye movement system. Saccadic CD signals convey spatial information about the impending eye movement to visual neurons, allowing these neurons to update activity in their retinotopic maps to compensate for the displacement of the retinal image that results from the impending saccade (Hall and Colby 2011; Wurtz 2008). Although saccadic eye movements can provide valuable insight into the integrity of CD, few studies have investigated saccade-related CD in the oculomotor system of schizophrenia patients (Richard et al. 2014; Thakkar et al. 2015).
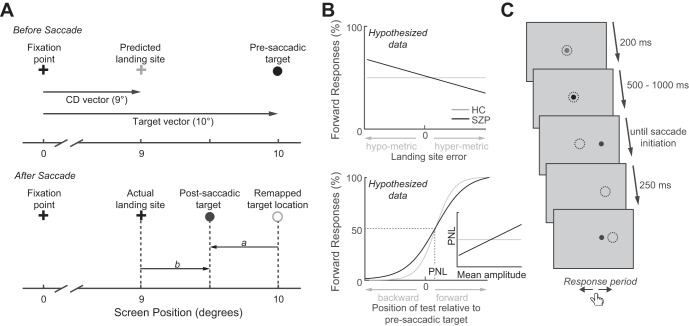
Previous studies indicate that saccadic CD signals contain information about spatial error in the saccade vector (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010). Support for this claim comes from a task in which a saccadic target disappeared upon saccade initiation and reappeared after a brief delay to the right or left of its presaccadic location, after which observers had to indicate their perceived direction of the shift. Results showed that these perceptual judgments were independent of the distance between saccade landing site and presaccadic target, henceforth referred to as landing site error, suggesting that observers did not use postsaccadic eye position as a proxy for the presaccadic target location. Rather, they appeared to use a CD signal associated with the actual (rather than intended) saccade vector to correctly remap the presaccadic target location and accurately localize the postsaccadic target. Accurate performance on this task, therefore, requires an intact CD signal (see Fig. 1A).
Fig. 1.
A: stimulus configuration and basis of perceptual judgment. Upon presaccadic target presentation (top), the corollary discharge (CD) vector can be used to predict the saccadic landing site (gray cross). In this example, the planned saccade will fall short of the presaccadic target. After saccade initiation (bottom), the presaccadic target is extinguished and reappears displaced to the left or right. If the presaccadic target has been appropriately remapped, then the vector a between this remapped location and the postsaccadic target should roughly match the actual target displacement. Because, in this example, the postsaccadic target appears to the left of the presaccadic target, proper remapping would predict that participants will judge the displacement as backward. Alternatively, saccade landing site could serve as a proxy of the presaccadic target location, predicting that the location of the postsaccadic target relative to the saccadic landing site (vector b) drives the judgment (forward in this example). B: predictions in schizophrenia patients (SZP) and healthy controls (HC). If CD is disturbed in schizophrenia, we expect SZP to use the actual landing site as a proxy for the presaccadic target, rather than its remapped location. Consequently, we expect that SZP rely less on the target displacement predicted from remapping (vector a in A) and thus make more perceptual judgment errors. As a result of impaired remapping in SZP, we expect that they will show an increased relationship between the percentage of forward responses and the saccadic landing site error (top): the more hypometric a saccade, the more likely SZP should be to report “forward.” In the relationship between the percentage of forward responses and target displacement (bottom), less reliance on the actual target displacement in SZP should manifest in a larger just noticeable difference (JND). The perceptual null location (PNL) denotes the postsaccadic target location at which participants report an equal proportion of forward and backward jumps. Because we expect patients to rely more on saccade landing site when making a judgment, we expect that SZP with shorter average saccade amplitudes should perceive the postsaccadic target as jumping forward more frequently (as it will more often fall forward of saccade landing site). This increase in forward judgments will result in a smaller PNL. Thus we expect a positive relationship between mean saccade amplitude and PNL in SZP but not in HC (see inset). C: trial procedure. Dotted circles denote eye position. See methods for details.
In the present study we used this same paradigm to investigate oculomotor CD signals in schizophrenia. We hypothesized that CD dysfunction would result in a failure to accurately remap the presaccadic target location. Consequently, patients would rely more on saccadic landing site to estimate the direction of the target displacement (Fig. 1B, top). Because saccadic landing site is generally an unreliable proxy of the presaccadic target location, we expect patients to be less precise in judging the direction of the target displacement (Fig. 1B, bottom). Moreover, because CD dysfunction has been purported to give rise to the failure to distinguish internally vs. externally generated sensory experiences, and psychotic symptoms are characterized by such agency disturbances, we further hypothesized that both of these effects, i.e., increased reliance on saccade landing site and reduced precision of perceptual judgments, would correlate with psychotic symptom severity.
METHODS
Participants.
We recruited 21 antipsychotic-medicated schizophrenia patients (SZP) from a longitudinal study (Genetic Risk and Outcome in Psychosis Investigators 2011) and an outpatient psychiatric facility in The Netherlands. Schizophrenia or schizoaffective disorder diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria and verified with the Comprehensive Assessment of Symptoms and History interview (Andreasen et al. 1992) or Schedules for Clinical Assessment for Neuropsychiatry, version 2.1 (Wing et al. 1990). Chlorpromazine (CPZ) equivalent antipsychotic dosages were calculated for each patient (Woods 2003). Clinical symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS; Kay et al. 1987). Twenty healthy controls (HC) without a personal or family history of DSM-IV Axis I diagnosis were recruited via community advertisements. Criteria for participant exclusion were a history of head trauma or neurological illness and recent substance abuse or dependence. One SZP was excluded on the basis of performance (see Data Analysis). The remaining SZP and HC were matched for sex, age, and handedness (Table 1). Although groups were not IQ-matched, there was no significant relationship between IQ and any CD measures in either group. All subjects gave written informed consent and were reimbursed for participation. The study was approved by the Human Ethics Committee of the University Medical Center, Utrecht.
Table 1.
Participant demographics
| HC (n = 20) | SZP (n = 20) | t Value | P Value | |
|---|---|---|---|---|
| Sex, M/F | 13/7 | 15/5 | 1.378 | 0.176 |
| Age, yr | 35.05 (9.14) | 36.40 (7.78) | −0.503 | 0.618 |
| IQ | 105.33 (11.28) | 94.6 (12.63) | 2.602 | 0.014 |
| Education | 7.32 (1.10) | 4.65 (1.84) | 5.437 | <0.0001 |
| Handedness | 0.84 (0.47) | 0.94 (0.19) | −0.917 | 0.365 |
| PANSS positive | 11.35 (5.05) | |||
| PANSS negative | 12.45 (5.84) | |||
| PANSS general | 24.65 (7.49) | |||
| PANSS total | 48.55 (16.19) | |||
| Illness duration, yr | 14.42 (5.35) | |||
| CPZ equivalent dose, mg | 274.4 (242.26) |
Values are means (SD) in healthy controls (HC; n = 20) and schizophrenia patients (SZP; n = 20) with t and P values for comparison between groups. IQ was based on the Nederlandse Leestest voor Volwassenen (NLV). Education was scored from 0 to 8 as follows: 0, <6 yr of primary education; 1, finished 6 yr of primary education; 2, 6 yr of primary education and low-level secondary education; 3, 4 yr of low-level secondary education; 4, 4 yr of average-level secondary education; 5, 5 yr of average-level secondary education; 6, 4 yr of secondary vocational training; 7, 4 yr of high-level professional education; 8, university degree. Handedness was based on the Edinburgh Handedness Inventory; scores range from 0.00, indicating complete left-handedness, to 1.00, indicating complete right handedness.
CPZ, chlorpromazine; PANSS, Positive and Negative Syndrome Scale.
Apparatus and stimuli.
Participants were seated in a dimly lit room with their head stabilized 68 cm away from a computer screen. Stimuli were black and red 0.2°-diameter dots on a gray background presented on a 24-in. monitor (spatial resolution 1,920 × 1,080 pixels, vertical refresh rate 100 Hz). Eye position was recorded using an EyeLink 1000 (SR Research, Mississauga, ON, Canada). Manual responses were recorded with a button box. Stimulus presentation and response collection were controlled with MATLAB (The MathWorks, Natick, MA) using the Psychophysics (Brainard 1997) and EyeLink (Cornelissen et al. 2002) toolboxes.
Design and procedure.
Each trial (Fig. 1C) began with participants fixating a red circle appearing at one of six equiprobable locations presented at an x-location of either 1° or −1° relative to center and at a y-location of either 0°, 1°, or −1° relative to center. This was done to reduce anticipation and stereotypical behavior (cf. Collins et al. 2009). Once fixation had been maintained for 200 ms, the target turned black and, after a random delay of 500-1,000 ms, jumped to a new location 10° to the left or right of fixation. Participants were instructed to look at the target as quickly as possible. Saccade initiation caused the target to be extinguished for 250 ms and then reappear at 1 of 13 equiprobable locations from −3° to 3°, in increments of 0.5°, to the left or right of the presaccadic location. Negative displacements indicate backward jumps (toward the initial fixation position), and positive displacements indicate forward jumps (farther away from the initial fixation position). On target displacement, participants reported whether the postsaccadic target appeared to the right or left of the presaccadic target. Each combination of fixation position and postsaccadic target location was tested twice, one for each saccade direction, resulting in 156 total trials. The experiment duration varied across participants and typically ranged from 15 to 30 min.
Online saccade detection was performed using a boundary technique. Saccade initiation was defined by the eyes leaving a 2° window around fixation. Saccades detected before target presentation triggered a warning on the screen, and the trial was restarted.
Data analysis.
Off-line, saccades were detected using the automated EyeLink procedure (velocity > 30°/s, acceleration > 8,000°/s2, motion > 0.1°). Response saccades were defined as the first saccade following presaccadic target presentation that was larger than 1° and brought gaze within 8° of the presaccadic target. If observers made a saccade before presaccadic target presentation or a key press before postsaccadic target presentation, the trial was excluded. There was no difference between the number of excluded trials between HC and SZP [HC: mean 2.4%, SD 2.4%; SZP: mean 2.8%, SD 3.6%; t(38) = 0.53, P = 0.60]. Although observers reported target jumps as left or right, we recoded responses as forward and backward when subjects perceived the postsaccadic target as jumping away from and toward the initial fixation point, respectively.
We used signal detection theory (Green and Swets 1966) to evaluate overall response accuracy to exclude subjects on the basis of performance. We defined hit rate as the proportion of forward responses for forward jumps and false alarm rate as the proportion of forward responses for backward jumps (due to the lack of a correct response alternative, the zero displacement condition was excluded for this analysis). The sensitivity index (d′) was calculated as the z score of the hit rate minus the z score of the false alarm rate. Subjects were excluded from analysis if d′ was smaller than 1, resulting in the exclusion of one SZP.
To evaluate the task performance of each individual, we collapsed trials across initial fixation positions and saccade directions and calculated the percentage of forward responses as a function of target displacement. A four-parameter logistic function of the following form was fit to these data:
where a and d are minimum and maximum values, respectively; b is the slope; and c is the point midway between a and d. Furthermore, we determined the perceptual null location (PNL) for each individual. The PNL is the displacement at which subjects did not perceive a difference between the pre- and postsaccadic target location, i.e., the postsaccadic target location for which observers reported an equal proportion of forward and backward jumps (50% point on the logistic function).1
Moreover, we derived the just noticeable difference (JND) from each participant's logistic function to measure the extent to which participants relied on target displacement to make perceptual judgments. JND was calculated as the difference in target displacements between the points at which the function reached 50% vs. 75% of its full growth. Smaller JND indicates greater precision and, accordingly, greater sensitivity to target displacements.
To investigate whether landing site error was associated with perceptual judgments, we collapsed trials across target displacements for each participant and divided landing site errors into the same bins for each observer. These bins ranged from −8° to 8° from the presaccadic target, in increments of 0.5°. For each individual, we then calculated the slope of the relationship between the mean saccade landing site of each bin and the corresponding proportion of forward responses. Because the number of included trials varied per bin, we used a weighted linear regression to derive the slope parameters, weighting perceptual judgments according to the number of observations in each bin.
Statistical analysis.
To test for group effects and asymmetries in response kinematics, we assessed reaction times (RTs; saccade and perceptual judgment), saccade amplitude, saccade peak velocity, and saccade peak acceleration using separate mixed-design ANOVAs, including group as a between-subjects factor and response direction as a within-subjects factor in the model. For simple group comparisons, we first assessed normality of the dependent measures within diagnostic groups using Shapiro-Wilk tests. If the dependent measure was normally distributed in both groups, we evaluated group performance differences using two-tailed independent-sample t-tests and computed Pearson's product-moment correlation coefficients (r) to evaluate relationships between continuous measures. For non-normally distributed measures, we used independent-samples Mann Whitney U-tests to evaluate group differences and Spearman's rho (signified as rs) to evaluate relationships between continuous measures.
With respect to correlations between clinical measures and performance measures, our only two a priori correlations of interest were between PANSS positive scores and both the JND of the psychometric function and the slope of the function plotting perceptual judgments against landing site error. Thus we evaluated these correlations at an uncorrected α level of 0.05. Six additional exploratory analyses were performed. Correlations between both JND and the slope of the forward response-landing site error function and both negative symptom severity and standardized medication dose were computed. In addition, mean saccade amplitude was correlated with both positive and negative symptom severity. These exploratory correlation analyses were assessed at a Bonferroni-corrected α level of 0.008 (0.05/6), although uncorrected P values are still reported. All other statistical tests were evaluated at an α level of 0.05.
RESULTS
Saccade and manual response metrics.
As a first step, we report response kinematics separated by group and saccade direction (Table 2). Saccade RTs did not differ by saccade direction [F(1,38) = 2.79, P = 0.10] or group [F(1,38) = 0.23, P = 0.64], and there was no group-by-direction interaction effect [F(1,38) = 0.001, P = 0.97]. On the other hand, there was an effect of both group [F(1,38) = 8.28, P = 0.007] and direction [F(1,38) = 14.80, P = 0.0004] on saccade amplitude. Rightward saccades were larger than leftward saccades, and SZP made shorter saccades than HC. There was no significant group-by direction interaction [F(1,38) = 0.07, P = 0.79]. Consistent with directional effects on amplitude, there was also a significant effect of direction on saccade peak velocity [F(1,38) = 7.24, P = 0.01]. Rightward saccades were faster than leftward saccades. However, there was no significant effect of group [F(1,38) = 0.08, P = 0.78] on peak velocity and no group-by-direction interaction effect [F(1,38) = 0.64, P = 0.43]. Saccade peak acceleration did not differ as a function of either group [F(1,38) = 0.10, P = 0.76] or direction [F(1,38) = 0.002, P = 0.96], and there was no group-by-direction interaction effect [F(1,38) = 0.48, P = 0.49]. Finally, although saccade RTs did not differ between groups, RTs of perceptual judgments were significantly longer in SZP than in HC [F(1,38) = 11.2, P = 0.002]. There was, however, no effect of response direction on RTs [F(1,38) = 0.60, P = 0.44] and no group-by-direction interaction effect [F(1,38) = 0.03, P = 0.87].
Table 2.
Response kinematics separated by group and saccade direction
| Left |
Right |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Saccade RT, ms | ||||
| HC | 207 | 42 | 219 | 56 |
| SZP | 201 | 42 | 212 | 47 |
| Saccade amplitude, deg | ||||
| HC | 9.4 | 0.7 | 9.8 | 0.8 |
| SZP | 8.7 | 0.7 | 9.2 | 1.0 |
| Saccade peak velocity, deg/s | ||||
| HC | 393.3 | 64.0 | 408.9 | 71.5 |
| SZP | 381.1 | 68.2 | 409.9 | 73.0 |
| Saccade peak acceleration, deg/s2 × 10−4 | ||||
| HC | 6.27 | 2.29 | 6.04 | 1.53 |
| SZP | 6.20 | 1.38 | 6.40 | 1.68 |
| Perceptual decision RT, ms | ||||
| HC | 532 | 112 | 558 | 169 |
| SZP | 739 | 239 | 756 | 281 |
RT, reaction time.
Thus, although there were some effects of saccade direction on movement kinematics, these parameters were not differentially affected by saccade direction in controls and patients. That is, we did not observe any significant group-by-saccade direction interaction effects. Moreover, we had no a priori hypotheses regarding saccade direction. Therefore, unless otherwise specified, we collapsed across saccade directions for subsequent analyses.
Perceptual judgments as a function of target displacement.
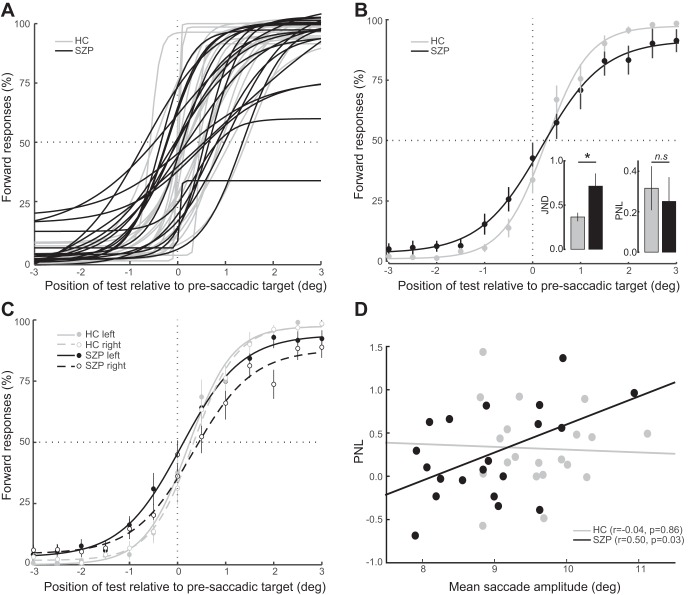
Figure 2A shows the fit of the four-parameter logistic function plotting the percentage of forward reports as a function of target displacement for each participant (group-averaged psychometric functions are shown in Fig. 2B). These functions provided very good fits to the data, as measured by R2 values, in both HC (mean 0.98, SD 0.02) and SZP (mean 0.94, SD 0.07).
Fig. 2.
A: individual fits of the mean percentage of forward responses as a function of target displacement. On the x-axis, negative and positive values indicate postsaccadic targets that are presented toward or away from the fixation point, respectively, relative to the presaccadic target location. B: functions shown were fitted to group-averaged responses for visualization purposes only and thus differ from the analyses of individual observers' data described in results. Insets show median JND and mean PNL values from the individually fitted psychometric functions. C: mean percentage of forward responses as a function of target displacement fitted to group-averaged responses, separated by saccadic directions. D: relationship between PNL and mean saccade amplitude. Each circle corresponds to one participant.
One-sample t-tests revealed that the PNL was significantly greater than zero in both HC [mean 0.32°, SD 0.48°, t(19) = 2.9, P = 0.008] and SZP [mean 0.25°, SD 0.53°, t(19) = 2.1, P = 0.048], indicating that when there was no displacement, the postsaccadic target was perceived as moving slightly toward fixation. There were no group differences in PNL [t(38) = 0.4, P = 0.69].
We also examined the correlation between mean saccade amplitude and PNL across subjects. We expected that if saccade landing site was being used as a proxy for the presaccadic target location due to failed remapping, then participants with smaller saccade amplitudes would have reduced PNL (Fig. 1B, bottom, inset). That is, if a subject's saccades were more hypometric, the postsaccadic target would appear forward of the saccade landing site more frequently. This would manifest in a psychometric function that was shifted to the left and, accordingly, a smaller PNL. Consistent with disturbed CD-based remapping, mean saccade amplitude was correlated with PNL in SZP (r = 0.50, P = 0.03) but not in HC (r = −0.04, P = 0.86; Fig. 2D). We used a Fisher r-to-Z transformation to test our a priori hypothesis that this correlation was greater in SZP and evaluated the significance using a one-tailed test. Indeed, we found that the correlation between mean saccade amplitude and PNL was significantly greater in SZP than in HC (Z = 1.7, P = 0.04). Despite this correlation between mean saccade amplitude and PNL in SZP and group differences in mean saccade amplitude, there was still no group difference in PNL when an ANCOVA was conducted and controlled for mean saccade amplitude [F(1,37) = 0.13, P = 0.73].
To investigate the extent to which participants relied on target displacement for their perceptual reports, we compared JND values of the observers' logistic functions. SZP had significantly larger JND than HC (U = 298, P = 0.007; Fig. 2B, inset), indicating reduced precision of perceptual reports in SZP. There was no correlation between mean saccade amplitude and precision in either HC (rs = 0.19, P = 0.44) or SZP (rs = −0.01, P = 0.97). Furthermore, we observed a positive relationship between PNL and JND in HC (rs = 0.60, P = 0.006), indicating that HC who perceived the target displacement more accurately were also more precise in their perceptual judgments. In SZP, we did not see a correlation between JND and PNL (rs = 0.08, P = 0.75), suggesting either a dissociation of the precision (JND) and accuracy (PNL) of the response in SZP or a reduced reliability of these measures in SZP.
We found no correlation between symptom severity and either PNL or JND (all rs < 0.34, P > 0.14). At an uncorrected significance level, there was a trend toward a relationship between CPZ dose and the JND (rs = −0.40, P = 0.10). SZP with higher antipsychotic dosages tended to respond with higher precision, suggesting that medication may partially normalize performance.
Finally, we examined potential asymmetries in performance by testing whether PNL and JND were differentially affected by saccade direction in controls and patients. To this end, we fitted separate logistic functions for leftward and rightward saccades in each participant and derived the JND and PNL for each saccade direction (Fig. 2C). We submitted PNL and JND values to separate mixed-design ANOVAs, including diagnostic group as a between-subjects factor and saccade direction as a within-subjects factor. For the JND, there was a trend effect of group [F(1,38) = 3.0, P = 0.09], with SZP having a larger JND than HC. Note that when we fitted psychometric functions on the basis of all trials (i.e., collapsed across leftward and rightward saccades), the group difference in JND was much more robust. We attribute this to more reliable curve fits when more trials were used for the estimation. There was no significant effect of saccade direction on JND [F(1,38) = 0.03, P = 0.85] and no group-by-saccade direction interaction effect. For the PNL, there was no significant effect of group [F(1,38) = 0.03, P = 0.87], which we also observed when we collapsed data across leftward and rightward saccades. However, there was a trend-level main effect of saccade direction [F(1,38) = 3.1, P = 0.09] and a trend-level group-by-saccade direction interaction effect [F(1,38) = 4.0, P = 0.054]. Post hoc paired t-tests indicated that the PNL was smaller for leftward saccades than rightward saccades in SZP [t(19) = 2.3, P = 0.04] but not in HC [t(19) = 0.2, P = 0.84]. Nevertheless, the PNL did not differ significantly between HC and SZP for either leftward [t(38) = 1.4, P = 0.18] or rightward [t(38) = 0.84, P = 0.40] saccades.
Given asymmetries in the PNL, we calculated the correlation between PNL and mean saccade amplitude separately for leftward and rightward saccades within each group and used Raghunathan et al.'s (1996) test to evaluate the difference between two dependent correlations from a single sample. The correlation between PNL and mean saccade amplitude was not different for leftward vs. rightward saccades in either HC (Z = 0.11, P = 0.91) or SZP (Z = 0.38, P = 0.68), thus justifying our decision to collapse across saccade directions for this analysis.
Relationship between saccade landing site and perceptual judgments.
To examine whether subjects used saccade landing sites to make perceptual judgments about postsaccadic target locations, we evaluated the relationship between landing site error and the proportion of forward responses. If participants had impaired CD and were relying on saccade landing site rather than the remapped presaccadic target location, they would show an increased proportion of forward judgments as the saccade fell increasingly short of the target. Figure 3A shows the mean percentage of forward responses as a function of landing site error. In neither HC (W = 66, P = 0.15) nor SZP (W = 85, P = 0.46) did the slope of this function differ from zero. On an individual level, however, saccade landing site was a significant predictor (α level: 0.05) of perceptual judgments in one HC and three SZP such that more hypometric saccades were associated with a greater proportion of forward responses. In a fourth SZP, this relationship was significant in the opposite direction.
Fig. 3.
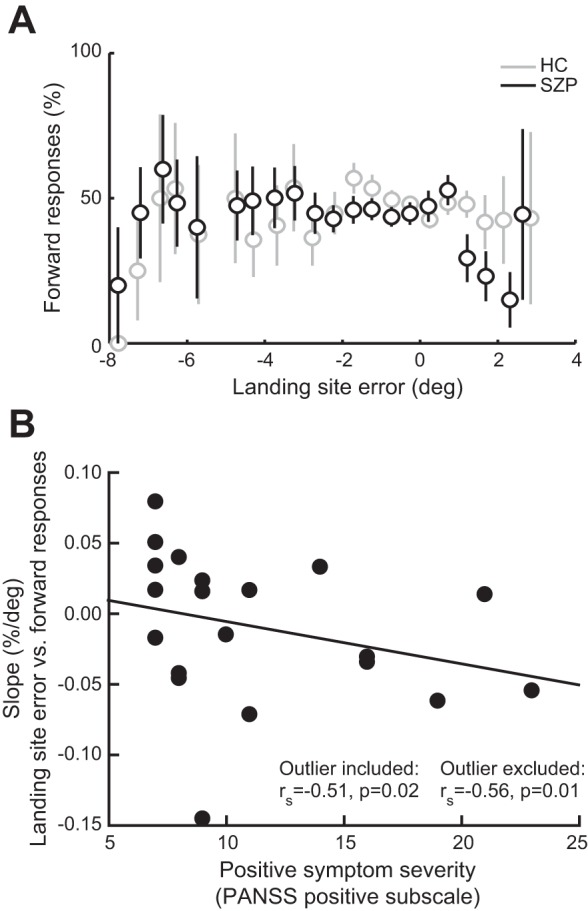
A: relationship between landing site error and perceptual judgments. Mean percentage of forward responses in each group is shown as a function of landing site error (distance between saccadic landing site and presaccadic target). For each observer, we divided the distribution of landing site errors into the same bins, ranging from −8° to 8° of the presaccadic target in increments of 0.5°, and calculated the percentage of forward reports for each bin. We subsequently fitted a weighted linear regression model to these data for each subject, weighting data points by the number of observations in each bin. For visualization purposes, we averaged the proportion of forward responses in each bin per group. Bins in which fewer than 2 participants per group contributed are not depicted. Error bars are SE. B: correlation between positive symptom severity and individual slopes of the function shown in A. Each circle corresponds to one SZP. PANSS, Positive and Negative Syndrome Scale; rs, Spearman's rho.
Because in SZP, but not HC, mean saccade amplitude was significantly correlated with slopes of the linear function plotting forward responses against landing site error (SZP: rs = −0.49, P = 0.03; HC: rs = −0.31, P = 0.18), we performed group comparisons of the slopes using ANCOVAs, including mean saccade amplitude as a covariate. Even when controlling for average saccade amplitude, we found no significant difference in the slope between SZP and HC [F(1,37) = 0.33, P = 0.56].
Finally, given the hypothesized link between disturbed CD and positive symptoms of schizophrenia (e.g., delusions, hallucinations), we tested whether patients who had more severe positive symptoms (greater PANSS positive subscale scores) relied less on the remapped location of the presaccadic target and more on landing site error when making their perceptual judgment. Indeed, positive symptom severity related to a more negative slope of the function plotting the proportion of forward responses against landing site error (rs = −0.51, P = 0.02; Fig. 3B). This relationship became even more robust after one bivariate outlier was removed (rs = −0.56, P = 0.01). There was no significant relationship between these slopes and the PANSS negative subscale (rs = −0.17, P = 0.47). Importantly, because there was no relationship between saccade amplitude and either positive (rs = 0.31, P = 0.18) or negative (rs = 0.35, P = 0.13) symptom severity, saccade metrics cannot explain the relationship between clinical symptoms and greater influence on eye position on perceptual judgments in SZP. No correlation between the slope and CPZ equivalent dose was observed (rs = 0.31, P = 0.22), suggesting that medication cannot account for the greater reliance on saccade landing site in SZP with more severe symptoms.
DISCUSSION
In the present study, we provide evidence that SZP have symptom-related disturbances in the ability to remap visual targets following saccades, a function that crucially relies on intact CD. SZP judged the direction of a postsaccadic target displacement less accurately than HC, potentially relying more on saccade landing site rather than a remapped representation of the presaccadic target.
Across SZP, we found that those individuals that made shorter saccades were more likely to report the postsaccadic target as jumping forward. This relationship was not observed in HC and suggests that SZP are more likely to judge the postsaccadic target location relative to saccade landing site, consistent with disturbed CD-based remapping.
In line with previous studies investigating target localization across saccadic eye movements (Collins et al. 2009; Joiner et al. 2013; Ostendorf et al. 2010), we did not observe a relationship between saccade landing site and perceptual judgments at the single-trial level in HC. However, contrary to our expectations, we also did not observe evidence for such a dependency across the entire sample of SZP, and the trial-by-trial relationship between landing site error and perceptual judgments did not differ between groups. Despite the absence of a group difference in this dependency, its strength correlated with symptom severity within SZP. SZP with more severe positive, but not negative, symptoms showed a greater influence of saccade landing site on perceptual judgments across single trials.
Because a CD vector allows healthy individuals to generate a correct spatial representation of the presaccadic target following the saccade (Collins et al. 2009), CD disturbances could potentially cause the reduced sensitivity to target displacement observed in SZP and increased reliance of perceptual judgments on saccade landing site. Thus these findings support mechanistic theories positing a link between psychotic experiences and disturbed CD (Feinberg 1978; Frith 1992).
The current results are consistent with emerging evidence for disturbed oculomotor CD in SZP and extend these findings in important ways. Recent studies have suggested that failures in continuous CD might underlie the robust smooth pursuit impairments (Hong et al. 2003, 2005; Lindner et al. 2005; Nkam et al. 2010; Spering et al. 2013; Thaker et al. 1996) and larger shifts in perisaccadic localization of visual stimuli in SZP (Richard et al. 2014). Our findings suggest that SZP are also impaired in generating and/or using the transient CD signals that accompany saccadic eye movements (Sommer and Wurtz 2004). These findings are consistent with the study reported by Thakkar et al. (2015), in which participants executed two saccades in rapid succession. SZP showed evidence for failing to appropriately use CD to anticipate the change in eye position brought about by the first saccade when executing the second saccade. In the current study, we now show evidence for putatively disturbed CD having a systematic effect on visual perception, particularly in SZP with more severe psychotic symptoms.
We must consider, however, why we did not see group differences in the dependency between saccade landing site and perceptual judgments at the single-trial level. One potential explanation is that there is more noise in the response and/or visual system in SZP (Loh et al. 2007). SZP might also fail to appropriately encode or maintain the presaccadic target location during the 250-ms blanking period, consistent with spatial working memory deficits (Lee and Park 2005). Increased noise and spatial working memory failures would likely result in an imprecise representation of the presaccadic target and/or motor errors during perceptual reports. We argue that both of these factors would attenuate a relationship between saccade landing site and perceptual judgments. Accordingly, such a dependency between saccade landing site and perceptual judgments may only be observable at the group level, which is indeed what we found. Furthermore, consistent with the observed correlation with positive symptoms, it is possible that as SZP become increasingly psychotic, CD-based remapping disturbances become more severe. Only for these patients do relationships between saccade landing site and perceptual judgments seem to emerge above visual and response system noise. On a related note, SZP in this study were only very mildly ill at the time of testing, based on symptom ratings (Leucht et al. 2005). A sample of more symptomatic patients would potentially result in a significant group difference in the relationship between saccade landing site and perceptual judgments. Alternatively, the relationship between clinical symptoms and the dependency between landing site and perceptual judgments could cause greater variability of this dependency in SZP vs. HC and, accordingly, result in a nonsignificant overall group difference between these slopes. Finally, we must acknowledge that our study comprises a relatively small number of trials. Although a larger number of trials would have certainly rendered our statistical analyses more powerful, increasing the experiment duration would also have led to a decline in subject motivation, particularly in patients. Nevertheless, a significant group difference in the relationship between saccade landing site and perceptual judgments at the single-trial level might have been discernible had we collected more data per subject.
Despite the above limitations, our findings provide support for CD dysfunction in schizophrenia that is related to psychosis. Since for most patients saccade landing site error was not a significant predictor of perceptual judgments (although in 3 patients it was), the current results are limited in terms of their diagnostic utility. Still, the negative relationship between positive symptom severity and the reliance on saccadic landing site to inform perceptual judgments supports an association between psychotic symptoms and disrupted CD signals. That is, the main clinical implication of these findings is that they inform mechanisms of psychosis.
Neurophysiological studies can guide mechanistic interpretations of the current findings. Remapping properties have been observed in cortical and subcortical visual neurons in nonhuman primates (reviewed in Hall and Colby 2011). Remapping, at least in frontal eye fields (FEF), is accomplished via CD signals sent from superior colliculus (SC) via the medial dorsal nucleus of the thalamus (MD; Sommer and Wurtz 2002, 2004, 2008). Additionally, lesions to MD in humans results in a dependency between landing site error and perceptual judgment in this task (Ostendorf et al. 2010). We can thus speculate that the current findings in SZP are related to disturbances in this SC-MD-FEF pathway, resulting in abnormalities in the generation, timing, or precision of CD signals. Indeed, recent studies have shown altered MD-cortical connectivity in SZP that is related to psychotic symptoms (Anticevic et al. 2014; Shinn et al. 2013; Woodward et al. 2012). Interestingly, it was recently suggested that receptive field shifts in FEF neurons during saccade planning do not reflect the prediction of retinal changes following the saccade (Zirnsak et al. 2014), but rather a convergence toward the saccade target. This new interpretation of receptive field shifts, however, remains compatible with our hypotheses and findings. CD likely contributes to the acquisition of information about the location of the target space during saccade planning, and impaired CD might accordingly result in flawed compression of visual space, not focused on the actual saccade endpoint.
To conclude, we observed evidence for impairments in remapping of visual targets following saccadic eye movements in schizophrenia, suggesting disturbances in CD. Remapping disturbances were greater in patients with more severe psychotic symptoms, supporting a link between CD disturbances and those symptoms of the disease that manifest in profound agency distortions.
GRANTS
This work was supported by a Netherlands Organization for Scientific Research Rubicon grant (to K. N. Thakkar) and Deutsche Forschungsgemeinschaft Emmy Noether Grant RO 3579/2-1 (to M. Rolfs). The infrastructure for the Genetic Risk and Outcome of Psychosis (GROUP) study was funded through the Geestkracht program of the Dutch Health Research Council (ZON-MW, grant 10-000-1001) and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and Mental Healthcare Organizations [Amsterdam: Academic Psychiatric Center of the Academic Medical Center and the mental health institutions GGZ inGeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Center, and GGZ Noord Holland Noord; Maastricht: Maastricht University Medical Center and the mental health institutions GGZ Eindhoven en de Kempen, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, and OPZ Rekem; Groningen: University Medical Center Groningen and the mental health institutions Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht, and Parnassia Psycho-Medical Center (The Hague); Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, RIAGG Amersfoort, and Delta].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.R. performed experiments; L.R. and K.N.T. analyzed data; L.R., M.R., S.v.d.S., S.F.W.N., and K.N.T. interpreted results of experiments; L.R. and K.N.T. drafted manuscript; L.R., M.R., S.v.d.S., S.F.W.N., W.C., R.S.K., and K.N.T. approved final version of manuscript; M.R., S.v.d.S., S.F.W.N., W.C., R.S.K., and K.N.T. conception and design of research; M.R., S.v.d.S., S.F.W.N., W.C., R.S.K., and K.N.T. edited and revised manuscript; K.N.T. prepared figures.
ACKNOWLEDGMENTS
We thank Ilse Thompson and Helene Hopman for contributions to subject recruitment and clinical interviews.
Footnotes
Note that the PNL equates the parameter c of the above function only if the function is centered on 50% forward reports, that is, if d = 1 − a.
REFERENCES
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 49: 615–623, 1992. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, Pearlson GD, Glahn DC. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull 40: 1227–1243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 21–29, 2009. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The EyeLink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav Res Methods Instrum Comput 34: 613–617, 2002. [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci 9: 587–600, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull 4: 636–640, 1978. [DOI] [PubMed] [Google Scholar]
- Frith C. The Cognitive Neuropsychology of Schizophrenia. Hove, UK: Lawrence Erlbaum, 1992. [Google Scholar]
- Genetic Risk Outcome in Psychosis (GROUP) Investigators. Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry 68: 138–147, 2011. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966. [Google Scholar]
- Hall NJ, Colby CL. Remapping for visual stability. Philos Trans R Soc Lond B Biol Sci 366: 528–539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Avila MT, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res 63: 39–48, 2003. [DOI] [PubMed] [Google Scholar]
- Hong LE, Avila MT, Thaker GK. Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Exp Brain Res 165: 125–131, 2005. [DOI] [PubMed] [Google Scholar]
- Joiner WM, Cavanaugh J, FitzGibbon EJ, Wurtz RH. Corollary discharge contributes to perceived eye location in monkeys. J Neurophysiol 110: 2402–2413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276, 1987. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114: 599–611, 2005. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res 79: 231–238. [DOI] [PubMed] [Google Scholar]
- Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol 15: 1119–1124, 2005. [DOI] [PubMed] [Google Scholar]
- Loh M, Rolls ET, Deco G. A dynamical systems hypothesis of schizophrenia. PLoS Comput Biol 3: e228, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkam I, Bocca ML, Denise P, Paoletti X, Dollfus S, Levillain D, Thibaut F. Impaired smooth pursuit in schizophrenia results from prediction impairment only. Biol Psychiatry 67: 992–997, 2010. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Liebermann D, Ploner CJ. Human thalamus contributes to perceptual stability across eye movements. Proc Natl Acad Sci USA 107: 1229–1234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, Rubin DB. Comparing correlated but nonoverlapping correlations. Psychol Methods 1: 178–183, 1996. [Google Scholar]
- Richard A, Churan J, Whitford V, O'Driscoll GA, Titone D, Pack CC. Perisaccadic perception of visual space in people with schizophrenia. J Neurosci 34: 4760–4765, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Cohen BM, Ongur D. Functional connectivity of left Heschl's gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res 143: 260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science 296: 1480–1482, 2002. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol 91: 1403–1423, 2004. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31: 317–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spering M, Dias EC, Sanchez JL, Schutz AC, Javitt DC. Efference copy failure during smooth pursuit eye movements in schizophrenia. J Neurosci 33: 11779–11787, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim C, Medoff D. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Res 59: 221–237, 1996. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Heckers S, Park S. Disrupted saccadic corollary discharge in schizophrenia. J Neurosci 35: 9935–9945, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Jablenski A, Regier D, Sartorius N. SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 47: 589–593, 1990. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64: 663–667, 2003. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169: 1092–1099, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T. Visual space is compressed in prefrontal cortex before eye movements. Nature 507: 504–507, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]