Abstract
Background and Aims:
This study was conducted to determine the effectiveness of intravenous (IV) granisetron in the prevention of hypotension and bradycardia during spinal anesthesia in cesarean delivery.
Material and Methods:
A total of 200 parturients scheduled for elective cesarean section were included in this study. They were randomly divided into two groups. Group I was given 1 mg granisetron diluted in 10 ml normal saline slowly IV, 5 min before spinal anesthesia. Group II was given 10 ml of normal saline, 5 min before spinal anesthesia. Mean arterial blood pressure and heart rate (HR) were recorded every 3 min until the end of surgery (for 45 min). The total consumption of vasopressors and atropine were recorded. Apgar scores at 1 and 5 min were also assessed.
Results:
Serial mean arterial blood pressure and HR values for 45 min after onset of spinal anesthesia were decreased significantly in group II, P < 0.0001. The incidence of hypotension after spinal anesthesia was 64% in group II and 3% in group I (P < 0.0001). The total doses of ephedrine (4.07 ± 3.87 mg vs 10.7 ± 8.9 mg, P < 0.0001), phenylephrine (0.0 microg vs 23.2 ± 55.1 microg, P < 0.0001), and atropine (0.0 mg vs 0.35 ± 0.49 mg P < 0.0001) consumed in both the groups respectively, were significantly less in group I versus group II.
Conclusion:
Premedication with 1 mg IV granisetron before spinal anesthesia in an elective cesarean section significantly reduces hypotension, bradycardia and vasopressors usage.
Keywords: Cesarean, granisetron, hypotension, spinal anesthesia
Introduction
Although spinal anesthesia has been considered safe technique, it has many side effects, including hypotension, nausea, vomiting, bradycardia, and other dysrhythmias.[1] Large surveillance studies typically observed incidence of hypotension around 33% and bradycardia around 13% in nonobstetric population,[2,3] but in obstetrics the incidence of hypotension has been estimated to be as high as 50-60%.[3] Hypotension occurs from decrease in systemic vascular resistance and central venous pressure from sympathetic block.[3,4] Sudden bradycardia can occur from shift in cardiac autonomic balance toward the parasympathetic system from activation of left ventricular mechanoreceptors or chemoreceptors Bezold-Jarisch reflex (BJR) or from an increase in baroreflex activity.[4]
Serotonin released during low-volume states has been suggested as a possible trigger for the BJR.[5] Granisetron is a 5-HT3 receptor antagonist that offers potentially important therapeutic benefits due to its low-side effect profile.[5]
Many studies highlight the use of a 5-HT3 antagonist in prevention of hypotension and bradycardia caused by the BJR in animals, obstetric, and nonobstetric patients.[6,7,8] Further research is needed to determine the lowest effective dose of 5-HT3 antagonist in preventing hypotension and bradycardia post-spinal in the obstetric populations. Lower incidence of bradycardia and hypotension improve outcomes in obstetrics for the parturient and neonate.
This study was conducted to determine the effectiveness of the lower dose of intravenous (IV) granisetron (1 mg) in the prevention of hypotension and bradycardia during spinal anesthesia in caesarean delivery.
Material and Methods
A total of 200 patients American Society of Anesthiologists grades I and II patients, with ages between 18 and 30 years were included in this study. Approval of institutional medical Ethical Committee and written consent from patients were obtained. All patients were prepared for elective cesarean section. Exclusion criteria included refusal of patients, contra-indications for spinal anesthesia, known allergy to granisetron, patients receiving serotonin agonists or antagonists, ischemic heart disease, chronic hypertension or pregnancy induced hypertension.
All patients were prehydrated with IV 500 ml lactated ringer. Base line heart rate (HR) and mean arterial blood pressure (MABP) were measured before spinal anesthesia. Spinal anesthesia was performed in sitting position with 25-gauge spinal needle in 3-4 or 4-5 lumbar interspaces, 2 ml 0.5% hyperbaric bupivcaine was injected.
Sensory level was evaluated by testing for cold sensation, and motor block was assessed according to Bromage scale after 5 min and repeated again after 10 min.
Mean arterial blood pressure and HR were recorded every 3 min until the end of surgery (for 45 min). Blood pressure was measured every 1 min if vasopressors were needed until hypotension was controlled and then the predetermined protocol was followed.
The patients were randomly classified into two groups (100 patients in each group): Group I was given 1 mg granisetron diluted in 10 ml normal saline slowly IV (over 1 min) 5 min before spinal anesthesia. Group II was given 10 ml of normal saline (placebo) slowly IV (within 1 min) 5 min before spinal anesthesia.
Randomization was performed using a computer-based random number generator in permutated blocks of varying sizes and the assignment entered in sealed envelopes that were not opened till informed consent was obtained.
The study was double-blind where the anesthetists, data collectors and patients were blinded to the assignment groups. The syringes were prepared by anesthetist who was blinded to study protocol.
Parturients were placed on the operating table in the supine position with 15° of left lateral tilt with supplemental oxygen through a face mask. Maintenance fluid of lactated Ringer's solution was infused at a rate of 10 ml/kg/h in both groups during the surgical procedure. An additional rapid bolus infusion (100 ml) of lactated Ringer's was given at each episode of hypotension.
Vasopressors were administered if MABP <70 mmHg. IV vasopressors consisted of ephedrine given in a bolus dose of 5 mg if HR was <90 beat/min, or phenylephrine 0.1 mg IV bolus if HR was >90 beat/min. Vasopressors treatment was repeated every 1 min if hypotension persisted or recurred. If bradycardia occurred (HR <60 beat/min), 0.5 mg atropine was given IV if bradycardia was not associated with hypotension. The total dose of vasopressors and atropine administered was recorded. Apgar scores at 1 and 5 min were assessed by a pediatric resident.
Statistical analysis
Sample size calculation was based on assumption that the incidence of hypotension of 55%[9] with a relative 40% change considered to be clinically significant and as such a sample size of 88 per group was required using a two-tailed test. However, to enable detection of potential variations and avoid potential errors, 100 patients were included in each group. Demographic and continuous data (MABP and HR) were analyzed using Student's t-test. Categorical data (incidence of hypotension) were analyzed using Fisher's exact test. Nonparametric data were analyzed using Mann-Whitney U-test. P < 0.05 was considered as significant.
Results
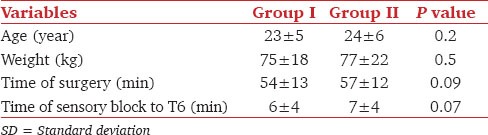
A total of 200 patients were enrolled in the study. There was no significant difference in demographic data between the groups [Table 1]. Sensory blockade extended to T6 and above within 10 min in all patients. There were no differences in the extent of sensory blockade or time-intervals. Five (3 in group I and 2 in group II) parturients were excluded from statistical analysis because they were in need for general anesthesia due to patchy spinal anesthesia.
Table 1.
Demographic data in both groups (data expressed as mean±SD)
Both groups had similar baseline MABP and HR. Serial MABP and HR values for 45 min after onset of spinal anesthesia decreased significantly in group II, P < 0.0001 [Figures 1 and 2]. The incidence of hypotension after spinal anesthesia was 3% in group I and 64% in group II (P < 0.0001). The median (range) time elapsed to incidence of hypotension was 16 (12-42) min in the group I and 7 (3-45) min in group II (P < 0.0001).
Figure 1.
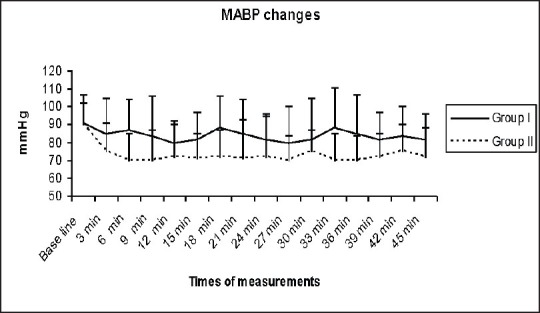
Mean arterial blood pressure (mmHg) changes in both groups (data was expressed as mean ± standard deviation
Figure 2.
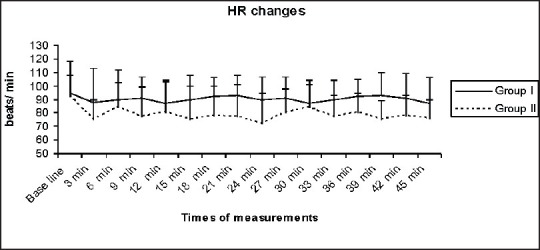
Heart rate (beat/min) changes in both groups (data was expressed as mean ± standard deviation
The total doses of ephedrine (4.07 ± 3.87 mg, 10.7 ± 8.9 mg, P < 0.0001) in both groups respectively), phenylephrine (0.0 μg, 23.2 ± 55.1 μg in both groups respectively, P < 0.0001), and atropine (0.0 mg, 0.35 ± 0.49 mg in both groups respectively, P < 0.0001) were significantly less in group I versus group II.
Neonatal outcomes were comparable in both groups at 1 and 5 min.
Discussion
This study revealed that the granisetron significantly reduced the decrease in MABP and use of vasopressor, when used as a premedicant before spinal anesthesia in cesarean section.
Hypotension occurs frequently during spinal anesthesia. Physiologic research indicated that hypotension results from peripheral pooling of blood that decrease venous return to the heart and decrease cardiac output from a decrease in systemic vascular resistance or from a combination of both.[10]
The decrease in preload caused by spinal anesthesia may initiate vagally mediated cardiac-depressant reflexes. Later, bradycardia and hypotension from stimulation of cardiac chemoreceptor and mechanoreceptor were established.[11,12]
Spinal anesthesia related triggering of BJR is known to result from stimulation of 5-HT3 receptors in vagal nerve endings,[13] resulting in release of serotonin from activated thrombocyte.[13,14]
A study in rabbits evaluated the effects of exogenous serotonin administration on systemic hemodynamics. It revealed that hypotension and bradycardia similar to that associated with BJR occurred.[15]
Bolus IV injection of 5-HT causes transient bradycardia in many species.[16,17]
Other study reported this reflex in anesthetized rats which are mediated through activation of 5-HT3 receptors located on endings of vagal afferent nerves.[18] Furthermore, 5-HT3 receptors have been implicated in anxiety, vomiting and stress-induced gastrointestinal disorders.[19]
Another study revealed that the grainsteron was significantly effective, at preventing paradoxical bradycardia and preventing a fall in the systolic blood pressure (SBP) due to bleeding.[6]
Tsikouris et al.[7] revealed that IV granisetron decreased the change in HR and prevented recurrence of Tilt-table syncope in 47% of 17 patients but did not alter the time to syncope or presyncope.
Similar data have already been reported using ondansetron 4 mg,[8] given intravenously 5 min before subarachnoid block and in other study where patients received 8 mg IV ondansetron.[20]
However, in the present study 1 mg of granisetron was used which was much lower than that of the previous studies to decrease the incidence of neonatal toxicity of granisetron because of the presence of benzyl alcohol. Although usual therapeutic doses of this drug deliver benzyl alcohol in amount that is substantially lower than that reported for its association with central nervous system toxicity but the minimum amount of benzyl alcohol at which toxicity may occurs is not known.
One of the limitations of the present study is that, the protocol allowed the use of ephedrine because of availability issues for phenylephrine in our hospitals and therefore it was used only for severe or resistant cases. The protocol should have focused on phenylephrine only as the risk of fetal acidosis is higher with ephedrine.
Conclusion
Premedication with 1 mg IV granisteron before spinal anesthesia in an elective cesarean section significantly reduces the hypotension, bradycardia and the need for vasopressors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–16. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Arndt JO, Bömer W, Krauth J, Marquardt B. Incidence and time course of cardiovascular side effects during spinal anesthesia after prophylactic administration of intravenous fluids or vasoconstrictors. Anesth Analg. 1998;87:347–54. doi: 10.1097/00000539-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Norris MC. Spinal anesthesia for cesarean delivery. In: Norris MC, editor. Handbook of Obstetric Anesthesia. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 309–12. [Google Scholar]
- 4.Butterworth J. Physiology of spinal anesthesia: What are the implications for management? Reg Anesth Pain Med. 1998;23:370–3. doi: 10.1016/s1098-7339(98)90008-6. [DOI] [PubMed] [Google Scholar]
- 5.Adams VR, Valley AW. Granisetron: The second serotonin-receptor antagonist. Ann Pharmacother. 1995;29:1240–51. doi: 10.1177/106002809502901211. [DOI] [PubMed] [Google Scholar]
- 6.White CM, Chow MS, Fan C, Kluger J, Bazunga M. Efficacy of intravenous granisetron in suppressing the bradycardia and hypotension associated with a rabbit model of the Bezold-Jarisch reflex. J Clin Pharmacol. 1998;38:172–7. doi: 10.1002/j.1552-4604.1998.tb04407.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsikouris JP, Kluger J, Chow MS, White CM. Usefulness of intravenous granisetron for prevention of neurally mediated hypotension upon head upright tilt testing. Am J Cardiol. 2000;85:1262–4. doi: 10.1016/s0002-9149(00)00743-8. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo T, SenDasgupta C, Goswami A, Hazra A. Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: A double-blind randomised, placebo-controlled study. Int J Obstet Anesth. 2012;21:24–8. doi: 10.1016/j.ijoa.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Ilies C, Kiskalt H, Siedenhans D, Meybohm P, Steinfath M, Bein B, et al. Detection of hypotension during Caesarean section with continuous non-invasive arterial pressure device or intermittent oscillometric arterial pressure measurement. Br J Anaesth. 2012;109:413–9. doi: 10.1093/bja/aes224. [DOI] [PubMed] [Google Scholar]
- 10.Critchley LA, Conway F. Hypotension during subarachnoid anaesthesia: Haemodynamic effects of colloid and metaraminol. Br J Anaesth. 1996;76:734–6. doi: 10.1093/bja/76.5.734. [DOI] [PubMed] [Google Scholar]
- 11.Mark AL. The Bezold-Jarisch reflex revisited: Clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1:90–102. doi: 10.1016/s0735-1097(83)80014-x. [DOI] [PubMed] [Google Scholar]
- 12.Stienstra R. Mechanisms behind and treatment of sudden, unexpected circulatory collapse during central neuraxis blockade. Acta Anaesthesiol Scand. 2000;44:965–71. doi: 10.1034/j.1399-6576.2000.440812.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinek RM. Witnessed asystole during spinal anesthesia treated with atropine and ondansetron: A case report. Can J Anaesth. 2004;51:226–30. doi: 10.1007/BF03019100. [DOI] [PubMed] [Google Scholar]
- 14.Gyermek L. Pharmacology of serotonin as related to anesthesia. J Clin Anesth. 1996;8:402–25. doi: 10.1016/0952-8180(96)00093-1. [DOI] [PubMed] [Google Scholar]
- 15.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305–18. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 16.Zucker IH, Cornish KG. Reflex cardiovascular and respiratory effects of serotonin in conscious and anesthetized dogs. Circ Res. 1980;47:509–15. doi: 10.1161/01.res.47.4.509. [DOI] [PubMed] [Google Scholar]
- 17.Saxena PR, Mylecharane EJ, Heiligers J. Analysis of the heart rate effects of 5-hydroxytryptamine in the cat; mediation of tachycardia by 5-HT1-like receptors. Naunyn Schmiedebergs Arch Pharmacol. 1985;330:121–9. doi: 10.1007/BF00499904. [DOI] [PubMed] [Google Scholar]
- 18.Fozard JR. Failure of 5-methoxytryptamine to evoke the Bezold-Jarisch effect supports homology of excitatory 5-HT receptors on vagal afferents and postganglionic sympathetic neurones. Eur J Pharmacol. 1983;95:331–2. doi: 10.1016/0014-2999(83)90659-3. [DOI] [PubMed] [Google Scholar]
- 19.Jones BJ, Costall B, Domeney AM, Kelly ME, Naylor RJ, Oakley NR, et al. The potential anxiolytic activity of GR38032F, a 5-HT3-receptor antagonist. Br J Pharmacol. 1988;93:985–93. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owczuk R, Wenski W, Polak-Krzeminska A, Twardowski P, Arszulowicz R, Dylczyk-Sommer A, et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: A double-blind, placebo-controlled study. Reg Anesth Pain Med. 2008;33:332–9. doi: 10.1016/j.rapm.2008.01.010. [DOI] [PubMed] [Google Scholar]


