Abstract
Background and Aims:
The role of clonidine as an adjuvant to regional blocks to hasten the onset of the local anesthetics or prolong their duration of action is proven. The efficacy of dexamethasone compared to clonidine as an adjuvant is not known. We aimed to compare the efficacy of dexamethasone versus clonidine as an adjuvant to 1.5% lignocaine with adrenaline in infraclavicular brachial plexus block for upper limb surgeries.
Material and Methods:
Fifty three American Society of Anaesthesiologists-I and II patients aged 18-60 years scheduled for upper limb surgery were randomized to three groups to receive 1.5% lignocaine with 1:200,000 adrenaline and the study drugs. Group S (n = 13) received normal saline, group D (n = 20) received dexamethasone and group C (n = 20) received clonidine. The time to onset and peak effect, duration of the block (sensory and motor) and postoperative analgesia requirement were recorded. Chi-square and ANOVA test were used for categorical and continuous variables respectively and Bonferroni or post-hoc test for multiple comparisons. P < 0.05 was considered significant.
Results:
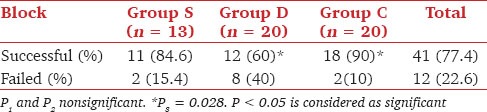
The three groups were comparable in terms of time to onset and peak action of motor and sensory block, postoperative analgesic requirements and pain scores. 90% of the blocks were successful in group C compared to only 60% in group D (P = 0.028). The duration of sensory and motor block in group S, D and C were 217.73 ± 61.41 min, 335.83 ± 97.18 min and 304.72 ± 139.79 min and 205.91 ± 70.1 min, 289.58 ± 78.37 min and 232.5 ± 74.2 min respectively. There was significant prolongation of sensory and motor block in group D as compared to group S (P < 0.5). Time to first analgesic requirement was significantly more in groups C and D as compared with group S (P < 0.5). Clinically significant complications were absent.
Conclusions:
We conclude that clonidine is more efficacious than dexamethasone as an adjuvant to 1.5% lignocaine in brachial plexus blocks.
Keywords: Clonidine, dexamethasone, regional anesthesia
Introduction
Several drugs like clonidine, opioids, tramadol, neostigmine, etc., have been used for prolongation of anesthesia and analgesia. The analgesic benefit of opioids added to brachial plexus block (BPB) has been inconclusive. Data regarding tramadol and neostigmine have not been sufficient to allow for any recommendations. Only clonidine appears to have a distinct benefit when administered as adjunct to BPB at doses of up to 150 μg without major side effects.[1] The addition of dexamethasone to lignocaine 1.5% solution in axillary BPB prolongs the duration of sensory and motor blockade.[2]
We aimed to compare the efficacy of clonidine versus dexamethasone as adjuvants on block characteristics (onset, peak and duration of sensory and motor blockade) and on duration of postoperative analgesia.
Material and Methods
The study protocol was approved by the institutional, departmental and ethics committee and written informed consent was obtained from all patients included in the study. The study population comprised of 53 American Society of Anaesthesiologists grade 1 and 2 patients of either sex, aged 18-60 years and scheduled for upper extremity surgery below the mid-humerus. Patients with history of head injury, psychiatric disorders, infection at site, severe pulmonary, cardiac, renal or endocrine disorders, coagulation disorders, on anticoagulation therapy, peptic ulcer disease, allergic reaction to any study drugs, peripheral neuropathies, chronic use of clonidine and refusal to consent were excluded from the study.
All patients included in the study were randomly allocated into one of the three groups based on computer generated list of random numbers. The placebo group received saline, the control group received clonidine and the test group received dexamethasone as an adjuvant in addition to the local anesthetic solution as follows: Placebo group: Group S (n = 13) received 1.5% lignocaine with adrenaline (0.6 ml/kg) + 2 ml of normal saline, the control group: Group C (n = 20) received 1.5% lignocaine with adrenaline (0.6 ml/kg) + 2 ml (150 micrograms) of clonidine and the test group: Group D (n = 20) received 1.5% lignocaine with adrenaline (0.6 ml/kg) + 2 ml (8 mg) of dexamethasone.
No premedication was given. Infraclavicular brachial plexus block (IBPB) was achieved through Wilson's approach[3] (entry point was 1.5-2 cm medial and inferior to coracoid process) with dual injection technique. An insulated 10 mm short bevelled needle compatible with the nerve stimulator Stimuplex-DIG (B. Braun, Melsungen AG, Germany) was inserted perpendicular to the skin, directed laterally toward the emergence of the axillary artery. Contractions were elicited starting at 2.5-≤0.5 mA and at frequency of 1-2 Hz. Nerve response of either a proximal (musculocutaneous, axillary) or distal (radial, ulnar, median) type was accepted. The needle was withdrawn and directed posterior and medial to locate the second nerve. For each nerve thus stimulated, half of the volume of local anesthetic solution was injected. The nerves thus located and the current required for it were noted.
Intraoperative standard monitoring of pulse-oximetry, electrocardiogram, noninvasive blood pressure monitoring and respiratory rate was done every 5 min interval till 30 min and then every 10 min till end of surgery. Onset and peak of sensory and motor blockade was monitored every 5 min till 30 min. Sensory block was tested with ice: 0 = no block (patient can feel cold), 1 = analgesia (patient can feel touch, not cold), 2 = anesthesia (patient cannot feel touch). Motor block was measured on modified Bromage's scale: 0 = no block (normal function with full flexion and extension of elbow, wrist, and fingers), 1 = paresis (decreased motor strength with ability to move fingers only), 2 = paralysis (complete motor block with inability to move fingers) (musculocutaneous - flexion at elbow, radial - thumb abduction, ulnar nerve - thumb adduction and median - flexion at wrist). Score of 1 denoted the onset, 2- the peak of block.
Intraoperative sedation was given with incremental doses of 1 mg of midazolam (maximum of 3 mg) and fentanyl boluses of 25 mg (maximum of 2 μ/kg) and total amount was noted. Patients were observed for complete effect of the blocks (defined as peak effect of sensory and motor effect in radial, ulnar, median and musculocutaneous nerve areas) partial blocks (defined as onset of sensory or motor block but could not reach the peak effect in the nonsurgical area with peak effect of sensory and motor block in the surgical area) and failed blocks (defined as no effect of block or only onset of sensory and motor block in the surgical area and hence surgery could not be performed). The block was defined as successful if there was either complete or partial effect of the sensory and motor block in the radial, ulnar, median and musculocutaneous nerves by 30 min and the surgery could be carried out without general anesthesia and with sedative supplementation. After 30 min failed cases were converted to general anesthesia and included in the study but excluded from the statistical analysis. Side effects and complications of technique and drugs were monitored and treated.
Postoperatively patients were monitored every 30 min till 6 h and then at 6 h intervals till 24 h. Pain was assessed with numeric rating scale (NRS) (0-10) and analgesia provided with patient-controlled analgesia (PCA) morphine intravenously (PCA settings: 1 mg/ml of morphine solution, bolus of 1 ml, lock out interval of 5 min, 4 h limit of 10 mg, without background infusion and total morphine consumption and time to first analgesia (time interval between onset of sensory and first PCA bolus) was noted. Duration of sensory block (time interval between the onset of sensory block and the first postoperative pain) and duration of motor block (time interval between the onset of motor block and complete recovery of motor functions) were noted. Patient, anesthesiologist's and surgeon's satisfaction score [Appendix 1] were recorded at the end of study.
Appendix 1.
-
Patient satisfaction score:
- 1 = Not satisfied, will not come to same hospital for same procedure
- 2 = Satisfied but would have preferred another technique
- 3 = Satisfied but would have preferred more analgesia
- 4 = Very satisfied.
-
Anesthesiologist's satisfaction score:
- 1 = Converted to general anesthesia
- 2 = Patient complained, needed treatment with supplemental analgesic
- 3 = Minor complaint needing no analgesic, moderately successful
- 4 = No complaint, good.
-
Surgeon's satisfaction score
- 1 = Unsuccessful
- 2 = Poor
- 3 = Acceptable
- 4 = Perfect.
Statistical analysis
As no previous research on the topic was available, thus “effect size” variable to calculate sample size did not exist; therefore, a sample size prior to study could not be validated for the present investigation. Our outcomes will provide these values for any future projects planned on the topic. As this was a pilot study (one of the reason for no power given), an interim analysis showed that we were able to delineate a statistically significant difference with already included 13 patients in the control group. Thus no further recruitment into the trial was made. The data was analyzed statistically using SPSS 15 software (IBM Inc. Chicago, IL, USA). Chi-square test was used for categorical variables and one-way ANOVA test for continuous variables. Multiple comparisons between groups were done using Bonferroni test or post-hoc test whenever necessary. All data are presented as mean ± standard deviation The level of significance was set at P < 0.05. The level of significance (P value) was denoted as P1 while comparing between group S and D, P2 while comparing between group S and C and P3 while comparing between groups D and C.
Results
The three groups were comparable with respect to demographic variables [Table 1]. There was statistically significant difference in the success rate of clonidine and dexamethasone groups (P = 0.028) [Table 2]. The three groups were comparable in terms of volume of drug injected, the nerve responses obtained as first or second nerves, the current required for the nerve stimulations, occurrence of partial blocks, onset and peak action of motor and sensory block in the radial, median, musculocutaneous and ulnar nerve territories and intraoperative sedation requirements [Table 1]. Musculocutaneous nerve was the most frequent nerve stimulated in first attempt (15/41) and radial nerve with the second nerve stimulation (20/41). The requirement for intraoperative sedation for midazolam and fentanyl was comparable between the three groups.
Table 1.
Demographic, surgical and block data

Table 2.
Success rate of the block
Group C had significantly less systolic blood pressure between 30 and 100 min as compared with group D but was clinically insignificant and could be managed with intravenous fluid administration [Figure 1]. Other intraoperative vital parameters were comparable between the groups. The duration of sensory and motor block was significantly more in group D as compared with group S (P1 sensory = 0.047, P1 motor = 0.031) [Figure 2]. The time to first analgesic requirement was significantly more in groups C and D as compared with group S (P1= 0.006, P2= 0.016) [Figure 3]. There was no difference in 24 h morphine requirement, postoperative NRS scores, satisfaction scores between the 3 groups. Group C recorded hypotension postoperatively which was clinically insignificant [Figure 4]. No clinically significant side effects were observed.
Figure 1.
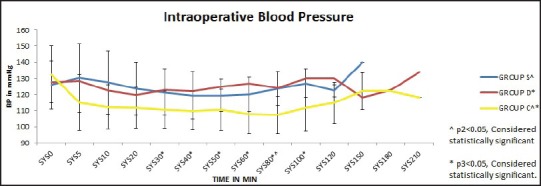
Intraoperative blood pressure
Figure 2.

Duration of block
Figure 3.

Time to first analgesia
Figure 4.

Postoperative blood pressure
Discussion
This study showed that clonidine is more efficacious than dexamethasone in terms of success of the block. Dexamethasone increases the duration of sensory and motor block which is comparable with clonidine and more than the duration provided with lignocaine. The three groups have been comparable in terms of demographic and surgical profile and block characteristics and postoperative analgesia requirement.
This study had an overall success rate of 77.4% with highest being the clonidine group (90%) and minimum in dexamethasone group (60%) while in lignocaine group it was 84.6%. The success with IBPB is reported to vary from 40% to 100% in different studies[3,4,5,6,7,8,9] which is explained by different definitions of a successful block, landmarks and variable direction of needle insertion. IBPB with double stimulation technique has resulted in satisfactory success rate and is well tolerated.[10] Single injection techniques have produced inconsistent anesthesia[9,11,12,13] and triple injection technique has offered no advantage over dual injection.[12,14] We found no correlation between the chronology of the nerve stimulated and the success of block.
The presence of septae as shown by Partridge et al.[15] could have prevented the spread of the drug thus explaining the failure in few of the cases. Morimoto et al.,[16] using ultrasound guidance demonstrated the presence of such septation in 4 of 6 patients having unilateral local anesthetic spread in IBPB. Inspite of using double injection technique, Koscielniak-Nielsen et al.[8] found only 57% of patients had complete analgesia distal to the elbow. However the presence of septae alone cannot explain the difference in the success of blocks between the three groups in this study.
Delayed effect or evolving block even after 30 min can result in partial effect. Raj et al.[4] had observed in his study that 30 min were required for the complete effect of the block. This was explained by the spread of the local anesthetic occurring from outer fibers to distal fibers in core and anesthesia spreading from proximal to distal area. Rettig et al.[17] also showed that IBPB continued to evolve even after 1 h of the block placement. However most sensory blocks become fixed at 30 min whereas motor distal blocks continue to progress. Niemi et al.[18] evaluated the IBPB at 60 min after the injection of the block for the efficacy and found adequate surgical anesthesia ranging from 90% to 97% in the various nerve territories with absence of maximum block until 45 min. Delayed effect or evolving block even after 30 min could have resulted in partial effect in the patients receiving dexamethasone, particularly in the failed cases and cannot be ruled out since we waited only 30 min for the effect of the block. Movafegh et al.[2] had 6 failures in the dexamethasone group as compared to 10 in lignocaine group with axillary BPB demonstrating 80% success with dexamethasone group.
Clonidine is an α2-agonist used in combination with local anesthetics. The effect of prolongation of anesthesia and analgesia in brachial plexus with clonidine is peripherally mediated and dose dependent, as is its side effect profile. Pratap et al.[19] studied the mechanism of action of clonidine by infiltrating the skin with lignocaine and clonidine and concluded that clonidine prolongs anesthetic duration by a peripheral action. Kopacz and Bernards[20] studied the effect of clonidine on lignocaine clearance in vivo with help of micro dialysis probe and found that addition of clonidine significantly prolonged the duration of anesthesia to pin prick, touch and cold sensation by a pharmacokinetic mechanism. The exact role of dexamethasone in peripheral blocks has not been studied and hence factors such as mechanism of action and site of action is still unknown. Reinhart et al.[21] measured pH along with chemical analysis by capillary electrophoresis and found no significant change in solution's physico-chemical properties when clonidine was mixed with lignocaine and stored at 4°C for 1-week. The addition of dexamethasone to lignocaine could have resulted in change in drug properties and delayed the block effect. However presently there is no literature available predicting the interaction of dexamethasone with lignocaine in vivo or in vitro. Hence the possibility of dexamethasone - lignocaine mixture causing significant change in solutions cannot be ruled out. These could probably explain the difference in the success rates between the groups inspite of employing similar techniques in all the groups.
An intermediate acting local anesthetic lignocaine was used to bring out any analgesic effect from clonidine and dexamethasone when added as adjuvant. Also clonidine and epinephrine are known to be most effective when added to intermediate local anesthetics such as lignocaine.[22]
Adding clonidine did not offer any advantage over lignocaine with epinephrine mixture in our study as shown by Gaumann et al.[23] They suggested that clonidine could prove to be a useful adjunct to lignocaine in cases where epinephrine is contraindicated.
The addition of clonidine resulted in prolonged sensory blockade as compared to motor blockade. Iskandar et al.[24] found that 50 μg of clonidine added to mepivacaine when applied to mid-humeral block enhanced the sensory blockade without prolonging the motor block. Similar results have been found by other authors.[25,26] Such a differential block has clinical applications particularly in outpatient setting where patients can leave the hospital setting while being pain free and with complete recovery of motor function. It also allows earlier assessment of nerve injuries by the surgeons and can facilitate pain free physiotherapy.
Dexamethasone was found to increase the duration of sensory and motor block equivalent to that of clonidine and more than that of lignocaine. Movafegh et al.[2] found significantly increased duration of sensory and motor block when dexamethasone was added to lignocaine as compared with control.
Clinically significant complications such as pneumothorax, respiratory dysfunction, Horner's syndrome, recurrent laryngeal nerve block and vascular punctures etc., were absent in our study. The Wilson's approach of IBPB has not been associated with any significant adverse effects.[3,7,10] This restricted distribution of local anesthetic solution avoids recurrent nerve block, phrenic nerve block or Horner's syndrome. Clonidine group had hypotension in the perioperative period but it was clinically insignificant. One patient in lignocaine group and three patients in clonidine group had developed transient tachycardia. However there was no vascular puncture and the drugs were injected carefully after repeated aspiration. The tachycardia did not result during the injection of the block but after sometime and was transient.
Niemi et al.,[18] had observed mild tachycardia with ST segment depression in two patients who had received the axillary BPB, 10-15 min after the administration of local anesthetic. He suggested that the relative late appearance of symptoms were caused by absorption of epinephrine and attributed it to the large dose of epinephrine used (168-240 μg). The doses of epinephrine used in our study (150-285 μg) correlate with these findings.
Dexamethasone and clonidine significantly prolonged the time for first analgesic requirement as compared with lignocaine. However the total analgesic consumption in the three groups was similar at 24 h. Dexamethasone did not result in any advantage over clonidine in improving postoperative analgesic requirement or time to first analgesia.
The pain scores (NRS) were not significantly different between the three groups in the 24 h period. Clonidine group reported the maximum score of 4.11 ± 1.71 at 5 h postoperatively which coincided with the mean duration of sensory block effect (304 ± 139 min) and the time for first analgesia requirement. The lack of sympathetic activity due to absence of pain and central inhibition of sympathetic pathway by clonidine could have resulted in low blood pressure perioperatively. With the cessation of the effect of block the patients had an increase in the pain which was manifested as increased NRS and probably increase in blood pressure. Gaumann et al.,[23] found maximal pain scores to be higher for 6 h after the bock in the clonidine group as compared with epinephrine but the total amount of mephenamic acid consumption was similar on the day after surgery. Stan et al. found no difference in the number of pain pills consumed by the steroid group and control group till second postoperative day. However the patients receiving the steroid had significantly less visual analogue scale on the day of the surgery. The satisfaction scores reported by the patient, anesthesiologist and surgeon did not differ statistically between the three groups in our study.
Conclusions
Double stimulation IBPB using Wilson's approach is a safe and effective block for surgeries of the upper limb below the mid-humerus. Addition of dexamethasone does not offer any advantage over clonidine in terms of increasing the success rate, block characteristics and postoperative analgesic requirement. Clonidine prolongs the sensory block more than motor block which can help in early neurological assessment and physiotherapy while the patient remains pain free. Larger prospective randomized trials are required to evaluate the role of dexamethasone as an adjuvant to IBPB.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006;102:263–7. doi: 10.1213/01.ane.0000189055.06729.0a. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JL, Brown DL, Wong GY, Ehman RL, Cahill DR. Infraclavicular brachial plexus block: Parasagittal anatomy important to the coracoid technique. Anesth Analg. 1998;87:870–3. [PubMed] [Google Scholar]
- 4.Raj PP, Montgomery SJ, Nettles D, Jenkins MT. Infraclavicular brachial plexus block — A new approach. Anesth Analg. 1973;52:897–904. [PubMed] [Google Scholar]
- 5.Whiffler K. Coracoid block — A safe and easy technique. Br J Anaesth. 1981;53:845–8. doi: 10.1093/bja/53.8.845. [DOI] [PubMed] [Google Scholar]
- 6.Borgeat A, Ekatodramis G, Dumont C. An evaluation of the infraclavicular block via a modified approach of the Raj technique. Anesth Analg. 2001;93:436–41. doi: 10.1097/00000539-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 7.Desroches J. The infraclavicular brachial plexus block by the coracoid approach is clinically effective: An observational study of 150 patients. Can J Anaesth. 2003;50:253–7. doi: 10.1007/BF03017794. [DOI] [PubMed] [Google Scholar]
- 8.Koscielniak-Nielsen ZJ, Rotbøll Nielsen P, Risby Mortensen C. A comparison of coracoid and axillary approaches to the brachial plexus. Acta Anaesthesiol Scand. 2000;44:274–9. doi: 10.1034/j.1399-6576.2000.440309.x. [DOI] [PubMed] [Google Scholar]
- 9.Gaertner E, Estebe JP, Zamfir A, Cuby C, Macaire P. Infraclavicular plexus block: Multiple injection versus single injection. Reg Anesth Pain Med. 2002;27:590–4. doi: 10.1053/rapm.2002.36456. [DOI] [PubMed] [Google Scholar]
- 10.Minville V, N’Guyen L, Chassery C, Zetlaoui P, Pourrut JC, Gris C, et al. A modified coracoid approach to infraclavicular brachial plexus blocks using a double-stimulation technique in 300 patients. Anesth Analg. 2005;100:263–5. doi: 10.1213/01.ANE.0000142119.20284.9E. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez J, Bárcena M, Rodríguez V, Aneiros F, Alvarez J. Infraclavicular brachial plexus block effects on respiratory function and extent of the block. Reg Anesth Pain Med. 1998;23:564–8. [PubMed] [Google Scholar]
- 12.Rodríguez J, Bárcena M, Taboada-Muñiz M, Lagunilla J, Alvarez J. A comparison of single versus multiple injections on the extent of anesthesia with coracoid infraclavicular brachial plexus block. Anesth Analg. 2004;99:1225–30. doi: 10.1213/01.ANE.0000131724.73956.8E. [DOI] [PubMed] [Google Scholar]
- 13.Fanelli G, Casati A, Garancini P, Torri G. Nerve stimulator and multiple injection technique for upper and lower limb blockade: Failure rate, patient acceptance, and neurologic complications. Study Group on Regional Anesthesia. Anesth Analg. 1999;88:847–52. doi: 10.1097/00000539-199904000-00031. [DOI] [PubMed] [Google Scholar]
- 14.De Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: A review of approaches and techniques. Can J Anaesth. 2007;54:662–74. doi: 10.1007/BF03022962. [DOI] [PubMed] [Google Scholar]
- 15.Partridge BL, Katz J, Benirschke K. Functional anatomy of the brachial plexus sheath: Implications for anesthesia. Anesthesiology. 1987;66:743–7. doi: 10.1097/00000542-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto M, Popovic J, Kim JT, Kiamzon H, Rosenberg AD. Case series: Septa can influence local anesthetic spread during infraclavicular brachial plexus blocks. Can J Anaesth. 2007;54:1006–10. doi: 10.1007/BF03016635. [DOI] [PubMed] [Google Scholar]
- 17.Rettig HC, Gielen MJ, Boersma E, Klein J. A comparison of the vertical infraclavicular and axillary approaches for brachial plexus anaesthesia. Acta Anaesthesiol Scand. 2005;49:1501–8. doi: 10.1111/j.1399-6576.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- 18.Niemi TT, Salmela L, Aromaa U, Pöyhiä R, Rosenberg PH. Single-injection brachial plexus anesthesia for arteriovenous fistula surgery of the forearm: A comparison of infraclavicular coracoid and axillary approach. Reg Anesth Pain Med. 2007;32:55–9. doi: 10.1016/j.rapm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Pratap JN, Shankar RK, Goroszeniuk T. Co-injection of clonidine prolongs the anesthetic effect of lidocaine skin infiltration by a peripheral action. Anesth Analg. 2007;104:982–3. doi: 10.1213/01.ane.0000257949.46444.a8. [DOI] [PubMed] [Google Scholar]
- 20.Kopacz DJ, Bernards CM. Effect of clonidine on lidocaine clearance in vivo: A microdialysis study in humans. Anesthesiology. 2001;95:1371–6. doi: 10.1097/00000542-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Reinhart DJ, Wang W, Stagg KS, Walker KG, Bailey PL, Walker EB, et al. Postoperative analgesia after peripheral nerve block for podiatric surgery: Clinical efficacy and chemical stability of lidocaine alone versus lidocaine plus clonidine. Anesth Analg. 1996;83:760–5. doi: 10.1097/00000539-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Joseph M. Neal, James R. Hebl, Gerancher J. C., Quinn H. Understanding. Regional Anesthesia and Pain Medicine; 27 (4), 2002. :402–428. doi: 10.1053/rapm.2002.34377. [DOI] [PubMed] [Google Scholar]
- 23.Gaumann D, Forster A, Griessen M, Habre W, Poinsot O, Della Santa D. Comparison between clonidine and epinephrine admixture to lidocaine in brachial plexus block. Anesth Analg. 1992;75:69–74. doi: 10.1213/00000539-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Iskandar H, Guillaume E, Dixmérias F, Binje B, Rakotondriamihary S, Thiebaut R, et al. The enhancement of sensory blockade by clonidine selectively added to mepivacaine after midhumeral block. Anesth Analg. 2001;93:771–5. doi: 10.1097/00000539-200109000-00043. [DOI] [PubMed] [Google Scholar]
- 25.Bernard JM, Macaire P. Dose-range effects of clonidine added to lidocaine for brachial plexus block. Anesthesiology. 1997;87:277–84. doi: 10.1097/00000542-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Singelyn FJ, Gouverneur JM, Robert A. A minimum dose of clonidine added to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Anesth Analg. 1996;83:1046–50. doi: 10.1097/00000539-199611000-00025. [DOI] [PubMed] [Google Scholar]


