Abstract
A successful peripheral nerve block not only involves a proper technique, but also a thorough knowledge and understanding of the physiology of nerve conduction and pharmacology of local anesthetics (LAs). This article focuses on what happens after the block. Pharmacodynamics of LAs, underlying mechanisms of clinically observable phenomena such as differential blockade, tachyphylaxis, C fiber resistance, tonic and phasic blockade and effect of volume and concentration of LAs. Judicious use of additives along with LAs in peripheral nerve blocks can prolong analgesia. An entirely new group of drugs-neurotoxins has shown potential as local anesthetics. Various methods are available now to prolong the duration of peripheral nerve blocks.
Keywords: Local anesthesia, nerve blockade, regional anesthesia
Introduction
Peripheral Nerve blocks have gained much popularity in recent times due to widespread acceptance and adoption of ultrasound guided techniques. Improved success rates with reduced incidence of complications are contributory. However, the knowledge of what happens after the drug is deposited near a nerve requires a thorough understanding of physiology of nerve blockade and pharmacology of local anesthetic (LA) agents. Recent knowledge about structure and function of sodium channel, the primary site of action of LAs has enabled us to understand the mechanism of action of existing LA agents and develop newer agents that are longer acting, more potent and less toxic.
Anatomical Aspects
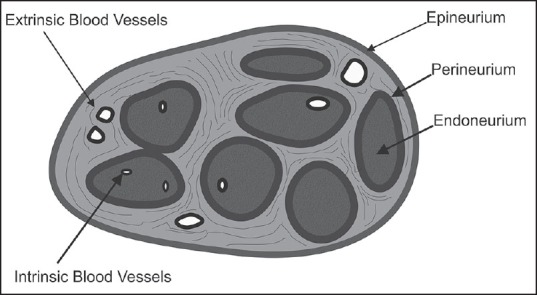
The structure of a peripheral nerve reveals individual axons covered by the endoneurium. Inside the axon, several nerve fibers are grouped together as fascicles which in turn are covered with the perineurium. The whole peripheral nerve is encased by the epineurium. Now, the fascicles are not continuous, but they divide every few millimeter and rejoin, thereby distributing the nerve fibers to the adjacent fascicles. The numbers of fascicles increase and their diameter decrease from proximal to distal.[1]
This complex plexiform topography of nerve fibers is the reason for poor recovery after disruption of the fascicles, as by accidental intraneural injections. Further damage is inflicted because of the hampering of blood supply and axonal transport of various functionally important substances, such as proteins. The perineurium is a tough membrane with several layers consisting of fibroblasts. It forms a formidable barrier to the diffusion of LAs.
Nonneural-neural Tissue Ratio
As the nerve leaves the spinal cord, the density of an epineurium (composed of stroma and connective tissue) decreases, but the total volume increases. Thus, the ratio of nonneural to neural tissue is approximately 1:1 in proximal plexuses, 2:1 in distal plexus and a peripheral nerve might contain up to 70% of connective tissue.[2] Clinically, even if a needle enters a peripheral nerve, it might still be inside the connective tissue, not necessarily within the fascicle.[3] This also explains the longer block onset times for peripheral nerve blocks as opposed to proximal plexus blocks [Figure 1].
Figure 1.
Intraneural arrangement
Extrinsic and Intrinsic System
Nerves have a dual blood supply. The extrinsic system is located in the epineurium, are nonnutritive and respond to adrenergic stimulation. On the other hand, the intrinsic system located in the perineurium is mainly nutritive and has minimal adrenergic receptors.[4] They have poorly developed smooth muscles and have limited ability to regulate intrafascicular blood flow.[5] Peripheral nerves are richly supplied by an extensive vascular network in which the endoneurial capillaries have endothelial “tight junctions,” a peripheral analogy to the “blood-brain barrier.” Autoregulation is notably absent in the blood vessels supplying peripheral nerves.[6]
Physiology of Nerve Impulse Conduction
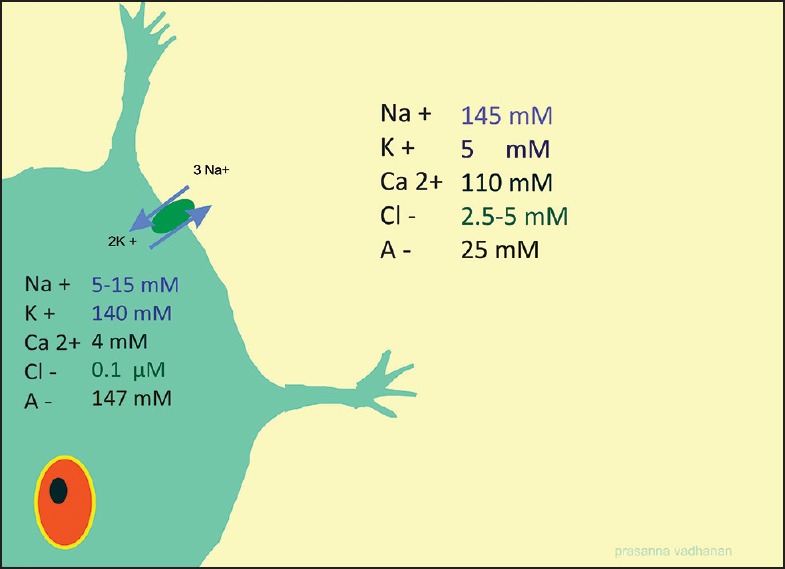
The resting membrane potential (RMP) of neuronal membrane is brought about by ionic disequilibria. The membrane is less permeable to Na+ as compared to K+. It is not due to the size factor of the ions. Na+ ions are 30% smaller in diameter than K+, yet the membrane of most cells is 20-30 times more permeable to K+. This is explained in part by the tendency of ions to be hydrated. As a result, the K+ ions which diffuse inside are retained by the negatively charged intracellular proteins; hence they maintain a steep gradient resulting in a negative RMP. The RMP varies across different type of nerve fibers [Figure 2].
Figure 2.
Ion distribution across neuronal membranes
Impulse Generation
During an impulse, the Na+ conductivity increases and the RMP becomes less negative. At threshold, more voltage gated Na channels (NaV) opens up and RMP shoots up to positive values. Now Nav channels close (or inactivated, as discussed subsequently) and K+ opens up, helping RMP to return to baseline values. Na+ K+ ATP-ase restores original ionic gradients by pumping 3 sodium ions out for two potassium ions.
Sodium Channels (NaV)
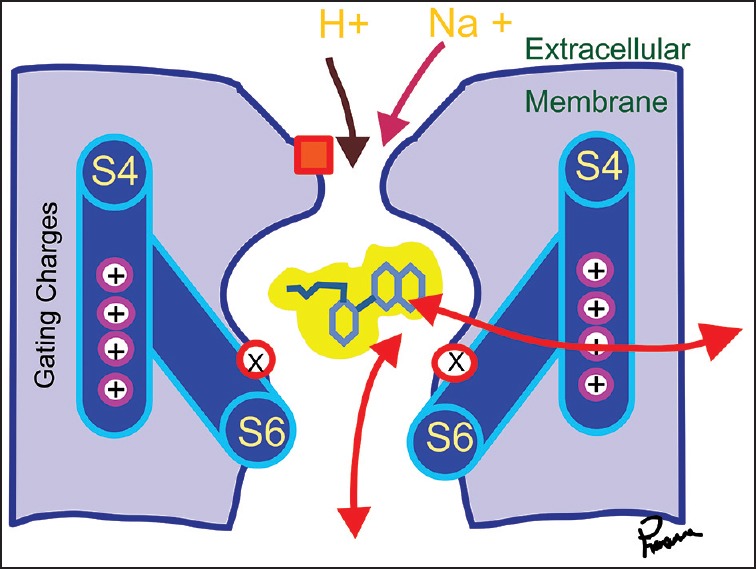
It is obvious that Na channels play a vital role in impulse propagation and is the site of action of drugs exhibiting LA actions. Electron microscopic studies reveal the NaV channel as a bell-shaped transmembrane glycoprotein with 4 domains, D1-D4. Each domain spans the membrane 6 times, s1-s6. The NaV channel belongs to the superfamily of other transmembrane voltage-gated channels like calcium and potassium channels [Figure 3].
Figure 3.
NaV channel cross-section
The channels can exist in three states, closed (or resting-below 70 mv) open (above 40 mV) and inactivated. Only the open state can conduct Na ions through them. Specific sites of the NaV channel for sensing voltage and binding LAs have been elucidated (domain IV s6).[7,8] Point mutations at these sites alter nerve impulse conduction as expected. More recent studies show other residues in D1 S6 and D3-S6 are also critical for LA binding. Overall multiple S6 segments of various domains together form the LA binding site, most prominent being those of D3 and D4.[9]
Mechanism of Local Anesthetic Action
Local anesthetic binds to specific sites at the inner pore creating an electrostatic field that repels positively charged ions like Na. They don’t physically occlude the pores as previously thought.[10] All LAs are weak bases and exist in ionized and unionized forms, the percentage of which varies depending on their pKa. Three factors determine the clinical differences among the LAs.
Pka: Lower the Pka means more unionized drug at physiological pH, hence the onset is faster as the unionized drug diffuses freely across lipid membranes.
Protein binding: More protein bound drugs has a longer duration of action, as the protein bound fraction acts like a reservoir.
Lipid solubility (expressed as octanol buffer partition coefficient): Determines the potency of the drug.
The Aftermath
What happens after the LA is injected in close proximity to the nerve? Two factors determine the amount of drug that actually reaches the nerve.[11]
Relative mass
This is the mass of the nerve as opposed to the mass of the tissues surrounding the volume of the LA agent injected. The LA has to reach equilibrium with all these tissues. The tissues include muscles, bone, connective tissue etc., the mass of other tissues is 5-10 times more than the mass of the nerve, hence only a small portion of the drug is taken up by the nerve.
Perineurium
As already mentioned the perineurium forms a formidable barrier for the diffusion of drugs. The rate of diffusion across the perineurium is much slower than the rate at which the drug is absorbed into the systemic circulation. Thicker the perineurium and more vascular the area, lesser the amount of drug that reaches the actual site of action, that is the inner pore of the Nav channels clustered around the Nodes of Ranvier.
The effective concentration of lignocaine to block 50% maximum obtainable impulses (IC50) is 10 times more if injected percutaneously when compared to the concentration required for equilibrating the nerves when perfused with lignocaine.[12]
Perception of Conducted Impulses and Effective Blocks
As we have seen NaV channels are clustered around the nodes of Ranvier. The internodal distance varies according to the nerve fiber size. Furthermore, impulses are known to “jump” across blocked nodes. Hence the concept of minimum length of nerve fiber to be exposed to LA was created. The more the length of the nerve exposed to the LA, more the percentage of impulses blocked.[13] However, ultrasound guided (USG) techniques have demonstrated even volumes <1 ml per nerve can cause clinical results.[14]
It is possible for the block to be successful even if nerve conduction is not blocked completely. This brings the question — Are all conducted impulses perceived? Pinprick sensation, carried by Aδ-nociceptors may be totally absent when conduction remains in C-nociceptors, which are only activated by more intense noxious stimuli. Furthermore, repeated activation of C-nociceptors “sensitizes the receiving neurons in the dorsal horn, setting up a situation where a partial block, reduces impulse activity below the perceived minimal” level at first, later becomes ineffectual as the dorsal horn neurons become sensitized. There is evidence from clinical blocks that afferent activity still arrives at the central nervous system during local anesthesia in patients who are completely pain-free that is, acutely, all sensation can be lost without blocking all impulses.[15]
C Fiber Resistance
Traditionally myelinated small fibers are most sensitive to LAs and dull visceral pain mediated by unmyelinated C fibers are resistant to LAs. The clinical picture is wherein patient is unresponsive to skin incision but complains of visceral pain as the surgery proceeds. Several mechanisms are proposed for the apparent C fiber resistance. NaV isoforms and tetrodotoxin (TTX) resistant channels are the most plausible explanation. TTX is a toxin which binds to the NaV channel from its outside unlike LA, and their use is limited to experimental in vitro studies because of their high systemic toxicity. There are a group of NaV channels which are resistant to TTX[16] particularly NaV 1.8.[17] They require more than the usual concentrations of LA to, block them (3-4 times more).[18] These channels are predominant in C fibers.
Differential Blockade
The ability of the LA to block sensory impulses while sparing motor and proprio reception is called a differential blockade. This is highly desirable where motor function needs to be preserved as in labor analgesia. Several theories were put forth for selective sensory blockade. They include length of the nerve fiber (in epidural space), frequency of firing (motor fibers fire slower, they need higher concentration of drug) and ‘Use dependent blockade’ as described later. Currently, a combination of factors are deemed responsible for the differential blockade, that include NaV isoforms, use dependent blockade and particular LA used.
Use Dependent Blockade-Tonic and Phasic Blockade
Tonic block
All LAs decrease Na current (I Na) as determined by infrequent stimuli — due to binding of LA to resting or closed Na channels. This is called tonic block.
Phasic block
When stimuli become more frequent, further inhibition of I Na binding to open channels occur. This phasic block is lesser in ‘neutral’ drugs like benzocaine. Phasic block is also seen with volatile agents.
Hille-modulated Receptor Theory and Hydrophilic Pathway
Modulated receptor theory was proposed by Hille[19] to explain tonic and phasic blockade. It was proposed that the LA receptor site is modulated by state transitions during membrane depolarization. Also the open and inactivated states of voltage-gated Na+ channels have higher affinities toward LA drugs than that of the resting state. In fact as the Na+ channel cycles from resting to open and inactivated states during a pulse, the LA receptor site also changes its configuration and thereby displaying higher affinity to LAs.[20] More recently, Sheets and Hanck[21] show that stabilizing the S4 segments into the outward configuration locks the sodium channels into the high affinity state, essentially providing proof for the concept behind the modulated receptor hypothesis. Hille's hypothesis has a wide-reaching application in ion channel physiology. Numerous ion channel receptors are modulated by ion channel gating.
LA binds to NaV channel in inactivated and open states preferentially than closed states. In other words fibers which fire faster are blocked earlier. Motor fibers whose frequency of impulse generation is slower is the last sensory modality to be blocked.
Clinically can it be used to hasten the onset of block by increasing the activity? Isometric hand contraction after interscalene block for shoulder surgery has failed to appreciably hasten the onset of block for shoulder arthroscopy.[22]
Guarded Receptor Hypothesis
According to the Guarded receptor hypothesis proposed by Starmer et al.,[23] the LA binds to a constant affinity receptor, but the access to it is regulated by channel gates. During an action potential, the gates change conformation in response to the transmembrane potential. Conducting channels have their gates open and expose the binding site to the drugs, whereas the nonconducting channel restrict drug access or trap the drug if it is already bound. The guarded receptor hypothesis is mathematically identical to the Modulated receptor hypothesis, they differ only in the reasons why drug binding is state dependent.
Volume and Concentration for Nerve Blocks
It is well-known that if the volume of LA agents is increased to improve the diffusion of the drug, the density of the block decreases. If the concentration of LA is decreased due to excessive dilution, the steep concentration gradient needed to penetrate the perineurium might not be achieved. Also, sparing of certain sensory modalities and motor function ensues. This has given way to the concept of mean effective volume(MEV) and minimum effective anaesthetic concentration (MEAC).
MEV and MEAC
The minimum effective volume to abolish all sensory modalities is denoted by the term MEV. This varies according to the site of injection. In short, tighter the space, lesser the volume required. For example sciatic nerve block needs lesser volume than popliteal nerve block.
Effect of ultrasound-guidance on MEV
Studies have shown the minimum volume for brachial plexus block is around 32 ml irrespective of the technique used.[24] However, volumes as low as 1.8 ml and 2 ml has been administered per nerve (radial, ulnar, median and axillary) by USG guided techniques with success.[25] The total volume in these cases were only around 10 ml. But the surgeries done did not include major bone manipulation, and patients needed opioid supplementations intravenously.
What if the volume is kept constant and concentration decreased? Thicker the nerve, more the concentration required. As already mentioned, a steep concentration gradient is needed to penetrate thicker perineurium. Thus sciatic nerve blocks need more concentration and lesser volume[26] than popliteal nerve blocks. Hence, blocks where the nerves are ‘tightly packed’ like sciatic nerve block depends mainly on concentration, whereas blocks like popliteal nerve block and plexus blocks also depend on sufficient volume.
Mixture of Local Anesthetics
It is a common practice to mix Lignocaine with bupivacaine to hasten the onset and prolong the duration of the block. But studies using this mixture reveal lesser duration, when compared to bupivacaine alone. Also, the plasma levels of the long-acting agent were lower than when given alone.[27] A mixture of LAs do not provide any significant benefit and cannot be recommended routinely.
Tachyphylaxis
Tachyphylaxis is the clinically observed decrease in drug's effectiveness when given repeatedly. Bromage et al. described it as “a form of acute tolerance or tachyphylaxis, develops in response to repeated applications of LAs.”[28] Tachyphylaxis is established when the block is waning, and the noxious stimuli persists.[29]
The key factor in tachyphylaxis appears to be the timing between subsequent doses. If the second dose is delayed till the effect of the first dose has been completely worn off, tachyphylaxis is definitely possible. But if the time interval between loss of analgesia and reinjection is less than few minutes, tachyphylaxis can be avoided.[30] However the exact molecular mechanism is still debatable. On the contrary, pseudotachyphylaxis is a term used to denote time-dependent variations in pain or circadian changes in the duration of LA.[31]
Probable pharmocokinetic mechanisms include: Formation of local edema, an increase in epidural protein concentrations, an alteration in the distribution of the drug or a decrease in perineural pH. All of these mechanisms could result in decreased diffusion of the LAs to their binding sites.[31,32] In terms of pharmacodynamics, antagonistic effects of nucleotides, increased sodium concentrations, increased afferent input from nociceptors, or receptor down-regulation of the sodium channels have been suggested as possible mechanisms.
More recently, Nitric oxide has been implicated in the development of tachyphylaxis.[33] N-methyl-D-aspartic acid (NMDA)-antagonists as well as NO-synthase-inhibitors appear to prevent the development of tachyphylaxis. Nitric oxide could be a potential second messenger for NMDA pathways in the spinal cord and involved in spinal mechanisms of hyperalgesia.[34]
Similar to opioids, changing over to lignocaine from bupivacaine has been shown to be successful in managing tachyphylaxis to the latter drug.[35]
Extra Axonal Effects of Local Anesthetic
A variety of extra axonal actions of LAs has been identified. They exert potent anti-inflammatory effects, particularly on neutrophil priming reactions.[36] LAs inhibit local inflammatory response to injury that can sensitize nociceptive receptors and contribute to pain and hyperalgesia. LAs reduce the release of inflammatory mediators from neutrophils, reduce neutrophil adhesion to the endothelium, reduce the formation of free oxygen radicals, and decrease edema formation.[37] Antithrombotic and neuroprotective actions of intravenous LAs are independent of Na+ channel blockade and may account for additional pain relief after surgery.[37,38]
Lignocaine also has some modulatory effect on NMDA receptors.[39] LAs have long been known to inhibit the growth of different species in vitro[39] Bupivacaine was shown to have the most efficient activity against microorganisms.[40] Intravenous lignocaine has shown to be effective in enhancing bowel function recovery after surgery.[41] Surgery induced immune alterations are also reduced by perioperative lignocaine.[39]
Adjuvants
Adrenaline, sodium bicarbonate, clonidine, opioids and several other drugs have been used as additives to improve the duration of the block and to enhance postoperative analgesia.
Adrenaline
Adrenaline is useful as a marker for accidental intravascular injection, decrease systemic absorption, and increase the duration of action. Adrenaline itself has been shown to exhibit analgesic propertiesin central neuraxial blockade. The duration and intensity of peripheral nerve blocks are consistently enhanced by adrenaline, especially with lignocaine. A pharmacodynamic action that transiently enhances LA and a pharmacokinetic action that prolongs the duration have been proposed.[42] But such a consistent action has not been observed when adrenaline is added to other LAs like ropivacaine.[43]
Adrenaline and nerve damage
Concerns arise due to possible neural ischemia due to vasoconstriction mediated by adrenaline. But adrenaline in usual doses does not decrease spinal cord blood flow as long as mean arterial blood pressure is maintained within autoregulatory limits.(50 mmHg). On the other hand, peripheral nerve blood flow (PNBF) has been shown to be consistently decreased by adrenaline, especially when used in doses more than 2.5 μg/ml. But this is usually inconsequential in healthy adults. The extrinsic system of blood vessels respond to sympathetic stimuli, hence drugs with alpha 1 agonistic action decreases PNBF. In rats Lignocaine decreases PNBF to 81% of control and 1 in 200000 adrenaline decreased it to 55%. Their combination decreased PNBF to 20% of control.[44] On the other hand, increasing doses of bupivacaine has been shown to cause lesser decrease in PNBF.[45]
Lower plasma levels of LA if administered with adrenaline is supposed to be due to perineural vasoconstriction decreasing the drug clearance, but an opposite effect was observed in muscle arterioles where low concentration of Lignocaine caused vasodilation and higher doses caused vasoconstriction.[46]
It is clear that Lignocaine and adrenaline combination decreases PNBF upto 20% of baseline but is well tolerated by healthy individuals. This reduction is comparable to pneumatic tourniquets.[47] However, it is prudent to use lesser concentration of adrenaline in diabetics, peripheral vascular disease patients, geriatric patients etc. Similarly, injured peripheral nerves have been shown to be more susceptible to adrenaline-induced damage. PNBF was transiently increased with low concentrations of adrenaline (2.5 μg/ml) in rat sciatic nerve.[45] In conclusion, a 1 in 400,000 adrenaline can be preferred in patients with diabetes, arteriosclerosis, and postchemotherapy state etc. Here PNBF is transiently increased, and the duration of block prolongation is only slightly less as compared to 1 in 200,000 adrenaline.[47]
Some authors even recommend adrenaline as an additive only for blocks done without ultrasound guidance, as a safety measure against accidental intravascular injection.[48]
Sodium Bicarbonate
Sodium bicarbonate is frequently added in nerve blocks to hasten the onset. It raises the pH of the solution making more of the drug in its base form available that makes it easier to penetrate neural membranes. In peripheral nerve blocks, the results are not uniform. Some studies show a rapid onset of motor block, without affecting other parameters.[49] The degree and duration of block was decreased when sodium bicarbonate was added with lignocaine plain, but not with adrenaline, even though the onset was hastened.[50] Currently, there are no evidences to warrant routine use of sodium bicarbonate as an adjunct in peripheral nerve blocks.
Clonidine
The alpha 2 agonist clonidine has been claimed to prolong the analgesia when mixed with LAs. However, alpha 2 receptors are not clearly demonstrated in a normal peripheral nerve. Hyperpolarization activated cation current has been suggested as a possible mechanism, independent of alpha 2 receptors.[51] clinical studies have consistently demonstrated enhancement of analgesia by adding clonidine to nerve and plexus blocks. In a 2009 meta-analysis, clonidine as an adjuvant in peripheral nerve or plexus block with long and intermediate onset agents do prolong analgesia and motor block by 2 h.[52] The side-effects like orthostatic hypotension, bradycardia and sedation might affect its usefulness in day care surgeries. Similar analgesic prolongation has been observed with dexmedetomidine also.[53]
Calcium and Magnesium
Calcium and magnesium has been known to affect nerve impulse conduction. In the presence of LAs, the effects are different. These may be relevant in certain pathological states affecting their levels.
Decreasing calcium concentrations enhanced both tonic and phasic blocks with or without the lignocaine, whereas increasing Ca2+ concentrations decreased both tonic and phasic blocks. The proposed mechanism is that Ca2+ ions can occupy NaV and stabilize them in closed states, thereby making them unavailable to LA, as LA affinity of open channels is more than closed channels (modulated receptor hypothesis). The absence of calcium causes an increase in the number of open and inactivated channels, thereby enhancing LA binding.[54] The clinical projection would be enhanced action of LAs in hypocalcemia and resistance in hypercalcemia.
On the other hand, magnesium has been claimed to potentiate the action of LAs by a different mechanism. Mg potentiates only use-dependent block, which can be explained by the surface charge hypothesis.[55] This theory suggests the external surface of the nerve membrane has a net negative charge.
Mg2+ gets attracted by these charges and interferes with the gating of the NaV channels. They neutralize the negative charge and alter the local field near the voltage sensing parts of the NaV channel. The membrane is hyperpolarized making it difficult to reach the threshold hence conduction block ensues. Also Mg2+ has been suggested, to delay the closing of NaV channels and enhance the number of inactivated channels, hence phasic blockade is enhanced.[54]
More recently magnesium sulfate has been shown to decrease the effects of amide LAs on rat sciatic nerves in vivo.[56] The previous studies demonstrating potentiation of LA by Mg are mainly in vitro studies. This apparent discrepancy may be due to study design and species selection. However, magnesium induced vasodilation may be responsible for shortening the block duration observed in vivo. Also, magnesium has several other actions including action on NMDA, K+ channels etc., the relative contribution of which can vary. Some studies have shown magnesium potentiates and prolongs LA action. This might be due to its action on NMDA receptors. Hence, currently magnesium cannot be recommended as an adjuvant to enhance nerve blocks.
Corticosteroids
Corticosteroids have been shown to specifically inhibit C-fibre transmission.[57] Dexamethasone has been shown to prolong peripheral nerve and plexus blocks.[58] The addition of 8 mg of dexamethasone extends the duration of analgesia after interscalene block using ropivacaine or bupivacaine by up to 22 h.[59] In supraclavicular block with mepivacaine, dexamethasone has been shown to provide analgesia for 5 h.[60] However concerns about neurotoxicity of perineural dexamethasone has been put forth.[61]
Opioids
Opioid receptors have been demonstrated in peripheral afferent nerves,[62,63] especially upregulated after inflammation. A review article examining the efficacy of adding opioids to LAs for brachial plexus blocks considering ten trials involving 413 patients found that six were supportive and four negative. The authors concluded that there was little evidence for any analgesic benefit of using opioid analgesics in brachial plexus block over systemic administration.[64] But more recently buprenorphine has been shown to prolong analgesia in peripheral nerve and plexus blocks.[65,66,67]
To summarize, the only adjuvants that can be considered in peripheral nerve blocks are adrenaline, alpha 2 agonists and possibly buprenorphine.
Future of Local Anesthetics
Newer local anesthetics
The ideal LA would have the following attributes. Long acting, better sensory motor separation and lesser systemic side-effects. To this, longer shelf life, stability, less tissue irritation can also be added.
Future local anesthetics-neurotoxins?
By the current knowledge of NaV isoforms, it would be ideal for LAs to specifically block neuronal Na channels. The main drawback of currently available LAs is they are nonspecific blockers of NaV channels. Hence prone for cardiac and neurological side-effects. The affinity of LAs for NaV channels is quite low. Low affinity clinically translates to more systemic toxicity.
Neurotoxins have gained much attention and there are few agents which show promise. They differ from conventional LAs in these key aspects:
Neurotoxins have very high affinity for neuronal NaV channels as compared to LAs. The dissociation constants are in the range of 10−9 to 10−6 mol/L (neurotoxins) as of 10−4 to 10−3 mol/L (LAs).[68]
They are very specific to neuronal NaV channels, hence cardiac and neurological side effects are low. For example, The affinity of cardiac purkinje fibers to neurotoxin is 200 fold lesser than that of axons.[69] Hence, cardiac side-effects should be lesser.
They are extremely potent, hence need very small doses. At pH 7.2, 2 nM neo-saxitoxin (neo-STX) produces 50% inhibition of compound action potentials in peripheral nerves.[70] In other words, neo-STX is roughly 1 million fold more potent than lignocaine.
The site of action is different. While LAs inhibit sodium channel activity by binding to the inner pore entering from the intracellular side, the toxins bind to the outer pore of the channel.[71]
Neo-saxitoxin
Saxitoxin is a neurotoxin produced by certain species of dinoflagellates and cyanobacteria. Ingestion of STX (usually through shellfish contaminated by toxic algal blooms) is responsible for the human illness known as paralytic shellfish poisoning. Neo-STX differs from STX by the addition of an oxygen atom wherein the hydrogen (-H) at nitrogen 1 of STX is replaced by a hydroxyl (-OH) group. Neo STX has been shown to be more potent than STX and TTX both in vitro and in vivo.[71,72]
In a first ever study to assess the clinical efficacy of neo STX on human volunteers 50 μg of neo-STX was injected subcutaneously on the skin of the calf and sensory parameters were assessed. All sensory modalities (warmth, cold, heat pain, cold pain, touch) were reliably abolished for an average of 3 h. Return to baseline values took as long as 9-12 h.[73]
Wound infiltration with neo-STX after laparoscopic cholecystectomy provided lower pain scores after 12 hours as compared to bupivacaine, and adverse events were no more frequent in the neo-STX group.[74]
Tetrodotoxin
Tetrodotoxin is a naturally occurring neurotoxin of puffer fish. They are specific blockers of Na channel, but differently from LAs. TTX is known to plug the Na+ channel from the external side of the permeation pathway adjacent to the narrow selectivity filter region.[75] When combined with adrenaline, it caused prolonged block of rat sciatic nerve. Considerable systemic toxicity precludes its clinical use.[76]
Other such toxins which are of interest include ralfinamide, 5 Arryl 2 furfurramides, ziconotide and pro TX II. Among these, ziconotide is a conotoxin (neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.) blocks neuronal calcium channels and operates at the spinal cord level. Prolonged intrathecal administration of ziconotide has been shown to be useful in chronic pain and does not cause addiction or tolerance.[77]
Prolonging the Block
Analgesia for days to weeks is highly desirable for certain patient sub sects. The following modalities are available.
Liposomes
Liposomes act as reservoirs for drugs. Lysosomal vesicles are sealed sacs containing a lipid bilayer, usually phospholipids. Following a single injection at the time of surgery, they remain in subcutaneous tissues (around the surgical incision) around a neural plexus or in the epidural space for a much longer period of time compared to the free drug.[78,79] Liposomal bupivacaine has been approved by the US Food and Drug Administration for local infiltration for pain relief after bunionectomy and hemorrhoidectomy.
Microspheres and nanospheres
Micro and nano spheres are prepared from biodegradable synthetic hydrophobic materials such as homo-or copolymers of polylactic and polyglycolic acids. Clinically, in human intercostal blockade studies, dexamethasone added to microcapsules containing bupivacaine showed a longer duration of anesthesia affect compared with microcapsules without dexamethasone.[80]
Hyaluronic acid-based hydrogels
Hyaluronic acid is a nonimmunogenic naturally occurring mucopolysaccharide, used as a viscous carrier solution to prolong LA action. Cross linked hyaluronic acid has been shown to double the duration of action of bupivacaine.[81]
Controlled-release local anesthetic matrix
An absorbable, controlled-release, LA delivery system containing 16% (w/w) lignocaine (Xybrex) is capable of providing up to several days of reversible rat sciatic nerve block in a dose-(mass-) dependent fashion.[82]
Injectable liquid polymers
There are three types of polymers for encapsulation, namely, nondegradable synthetic polymers, natural biodegradables (that degrade to nontoxic products that are completely eliminated from the body), and drug-conjugated polymers (where a drug is attached to water-soluble polymer by a cleavable bond). The use of a 15% bupivacaine lactic acid-co-castor oil copolymer prolonged the in vivo effect to 96-h sensory block.[83]
Conclusion
A fine knowledge of LA action and nerve conduction physiology and sodium channels will help is in administering better blocks. The future of LAs hold much promise with possible development of drugs with prolonged action, low systemic toxicity and better sensory-motor separation. Also, prolonged analgesia is possible by long-acting preparations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bonnel F. Microscopic anatomy of the adult human brachial plexus: an anatomical and histological basis for microsurgery. Microsurgery. 1984;5:107–18. doi: 10.1002/micr.1920050302. [DOI] [PubMed] [Google Scholar]
- 2.Moayeri N, Bigeleisen PE, Groen GJ. Quantitative architecture of the brachial plexus and surrounding compartments, and their possible significance for plexus blocks. Anesthesiology. 2008;108:299–304. doi: 10.1097/01.anes.0000299433.25179.70. [DOI] [PubMed] [Google Scholar]
- 3.Neal JM, Gerancher JC, Hebl JR, Ilfeld BM, McCartney CJ, Franco CD, et al. Upper extremity regional anesthesia: Essentials of our current understanding, 2008. Reg Anesth Pain Med. 2009;34:134–70. doi: 10.1097/AAP.0b013e31819624eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers RR, Heckman HM. Effects of local anesthesia on nerve blood flow: Studies using lidocaine with and without epinephrine. Anesthesiology. 1989;71:757–62. doi: 10.1097/00000542-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Bell MA, Weddell AG. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain. 1984;107(Pt 3):871–98. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- 6.Witt NJ, Zochodne DW, Bolton CF, Grand’Maison F, Wells G, Young GB, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–84. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- 7.Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–22. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 8.Sunami A, Tracey A, Glaaser IW, Lipkind GM, Hanck DA, Fozzard HA. Accessibility of mid-segment domain IV S6 residues of the voltage-gated Na+ channel to methanethiosulfonate reagents. J Physiol. 2004;561(Pt 2):403–13. doi: 10.1113/jphysiol.2004.067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nau C, Wang GK. Interactions of local anesthetics with voltage-gated Na+ channels. J Membr Biol. 2004;201:1–8. doi: 10.1007/s00232-004-0702-y. [DOI] [PubMed] [Google Scholar]
- 10.McNulty MM, Edgerton GB, Shah RD, Hanck DA, Fozzard HA, Lipkind GM. Charge at the lidocaine binding site residue Phe-1759 affects permeation in human cardiac voltage-gated sodium channels. J Physiol. 2007;581(Pt 2):741–55. doi: 10.1113/jphysiol.2007.130161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strichartz G. Pathways and obstacles to local anesthesia, a personal account: The 2000 Gaston Labat lecture. Reg Anesth Pain Med. 2000;25:447–51. doi: 10.1053/rapm.2000.8575. [DOI] [PubMed] [Google Scholar]
- 12.Huang JH, Thalhammer JG, Raymond SA, Strichartz GR. Susceptibility to lidocaine of impulses in different somatosensory afferent fibers of rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:802–11. [PubMed] [Google Scholar]
- 13.Raymond SA, Steffensen SC, Gugino LD, Strichartz GR. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth Analg. 1989;68:563–70. [PubMed] [Google Scholar]
- 14.O’Donnell B, Riordan J, Ahmad I, Iohom G. Brief reports: A clinical evaluation of block characteristics using one milliliter 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesth Analg. 2010;111:808–10. doi: 10.1213/ANE.0b013e3181e79965. [DOI] [PubMed] [Google Scholar]
- 15.Benzon HT, Toleikis JR, Dixit P, Goodman I, Hill JA. Onset, intensity of blockade and somatosensory evoked potential changes of the lumbosacral dermatomes after epidural anesthesia with alkalinized lidocaine. Anesth Analg. 1993;76:328–32. [PubMed] [Google Scholar]
- 16.Strassman AM, Raymond SA. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. J Neurophysiol. 1999;81:413–24. doi: 10.1152/jn.1999.81.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Kistner K, Zimmermann K, Ehnert C, Reeh PW, Leffler A. The tetrodotoxin-resistant Na channel Na (v)1.8 reduces the potency of local anesthetics in blocking C-fiber nociceptors. Pflugers Arch. 2010;459:751–63. doi: 10.1007/s00424-010-0785-5. [DOI] [PubMed] [Google Scholar]
- 18.Scholz A, Vogel W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain. 2000;89:47–52. doi: 10.1016/S0304-3959(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 19.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GK, Strichartz GR. State-dependent inhibition of sodium channels by local anesthetics: A 40-year evolution. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2012;6:120–127. doi: 10.1134/S1990747812010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol. 2007;582(Pt 1):317–34. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langen KE, Candido KD, King M, Marra G, Winnie AP. The effect of motor activity on the onset and progression of brachial plexus block with bupivacaine: A randomized prospective study in patients undergoing arthroscopic shoulder surgery. Anesth Analg. 2008;106:659–63. doi: 10.1213/ane.0b013e31815edad6. [DOI] [PubMed] [Google Scholar]
- 23.Starmer CF, Grant AO, Strauss HC. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984;46:15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggan E, El Beheiry H, Perlas A, Lupu M, Nuica A, Chan VW, et al. Minimum effective volume of local anesthetic for ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med. 2009;34:215–8. doi: 10.1097/AAP.0b013e31819a9542. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell BD, Iohom G. An estimation of the minimum effective anesthetic volume of 2% lidocaine in ultrasound-guided axillary brachial plexus block. Anesthesiology. 2009;111:25–9. doi: 10.1097/ALN.0b013e3181a915c7. [DOI] [PubMed] [Google Scholar]
- 26.Taboada Muñiz M, Rodríguez J, Bermúdez M, Valiño C, Blanco N, Amor M, et al. Low volume and high concentration of local anesthetic is more efficacious than high volume and low concentration in Labat's sciatic nerve block: A prospective, randomized comparison. Anesth Analg. 2008;107:2085–8. doi: 10.1213/ane.0b013e318186641d. [DOI] [PubMed] [Google Scholar]
- 27.Cuvillon P, Nouvellon E, Ripart J, Boyer JC, Dehour L, Mahamat A, et al. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: A double-blind randomized study. Anesth Analg. 2009;108:641–9. doi: 10.1213/ane.0b013e31819237f8. [DOI] [PubMed] [Google Scholar]
- 28.Bromage PR, Pettigrew RT, Crowell DE. Tachyphylaxis in epidural analgesia: I. Augmentation and decay of local anesthesia. J Clin Pharmacol J New Drugs. 1969;9:30–8. [PubMed] [Google Scholar]
- 29.Baker CE, Berry RL, Elston RC. Effect of pH of bupivacaine on duration of repeated sciatic nerve blocks in the albino rat. Local Anesthetics for Neuralgia Study Group. Anesth Analg. 1991;72:773–8. doi: 10.1213/00000539-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Strichartz GR, Pastijn E, Sugimoto K. Neural physiology and local anesthetic action. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors. Cousins and Bridenbaugh's Neural Blockade in Clinical Anaesthesia and Pain Medicine. 4th ed. Lippincott Williams & Wilkins; 2012. pp. 41–3. [Google Scholar]
- 31.Kottenberg-Assenmacher E, Peters J. Mechanisms of tachyphylaxis in regional anesthesia of long duration. Anasthesiol Intensivmed Notfallmed Schmerzther. 1999;34:733–42. doi: 10.1055/s-1999-228. [DOI] [PubMed] [Google Scholar]
- 32.Choi RH, Birknes JK, Popitz-Bergez FA, Kissin I, Strichartz GR. Pharmacokinetic nature of tachyphylaxis to lidocaine: Peripheral nerve blocks and infiltration anesthesia in rats. Life Sci. 1997;61:PL177–84. doi: 10.1016/s0024-3205(97)00664-4. [DOI] [PubMed] [Google Scholar]
- 33.Wilder RT, Sholas MG, Berde CB. NG-nitro-L-arginine methyl ester (L-NAME) prevents tachyphylaxis to local anesthetics in a dose-dependent manner. Anesth Analg. 1996;83:1251–5. doi: 10.1097/00000539-199612000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Sholas MG, Berde CB, DiCanzio J, Zurakowski D, Wilder RT. Evidence that spinal segmental nitric oxide mediates tachyphylaxis to peripheral local anesthetic nerve block. Acta Anaesthesiol Scand. 2001;45:945–53. doi: 10.1034/j.1399-6576.2001.450805.x. [DOI] [PubMed] [Google Scholar]
- 35.Mercadante S, Villari P, Ferrera P, Arcuri E. Local anesthetic switching for intrathecal tachyphylaxis in cancer patients with pain. Anesth Analg. 2003;97:187–9. doi: 10.1213/01.ane.0000067403.15926.bd. [DOI] [PubMed] [Google Scholar]
- 36.Shipton EA. New formulations of local anaesthetics-part I. Anesthesiol Res Pract 2012. 2012:546409. doi: 10.1155/2012/546409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–8. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Wang CF, Pancaro C, Gerner P, Strichartz G. Prolonged suppression of postincisional pain by a slow-release formulation of lidocaine. Anesthesiology. 2011;114:135–49. doi: 10.1097/ALN.0b013e3182001996. [DOI] [PubMed] [Google Scholar]
- 39.Borgeat A, Aguirre J. Update on local anesthetics. Curr Opin Anaesthesiol. 2010;23:466–71. doi: 10.1097/ACO.0b013e328339eef2. [DOI] [PubMed] [Google Scholar]
- 40.Coghlan MW, Davies MJ, Hoyt C, Joyce L, Kilner R, Waters MJ. Antibacterial activity of epidural infusions. Anaesth Intensive Care. 2009;37:66–9. [PubMed] [Google Scholar]
- 41.Harvey KP, Adair JD, Isho M, Robinson R. Can intravenous lidocaine decrease postsurgical ileus and shorten hospital stay in elective bowel surgery?. A pilot study and literature review. Am J Surg. 2009;198:231–6. doi: 10.1016/j.amjsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Sinnott CJ, Cogswell III LP, Johnson A, Strichartz GR. On the mechanism by which epinephrine potentiates lidocaine's peripheral nerve block. Anesthesiology. 2003;98:181–8. doi: 10.1097/00000542-200301000-00028. [DOI] [PubMed] [Google Scholar]
- 43.Weber A, Fournier R, Van Gessel E, Riand N, Gamulin Z. Epinephrine does not prolong the analgesia of 20 mL ropivacaine 0.5% or 0.2% in a femoral three-in-one block. Anesth Analg. 2001;93:1327–31. doi: 10.1097/00000539-200111000-00060. [DOI] [PubMed] [Google Scholar]
- 44.Myers RR, Heckman HM. Effects of local anesthesia on nerve blood flow: studies using lidocaine with and without epinephrine. Anesthesiology. 1989;71:757–62. doi: 10.1097/00000542-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Partridge BL. The effects of local anesthetics and epinephrine on rat sciatic nerve blood flow. Anesthesiology. 1991;75:243–50. doi: 10.1097/00000542-199108000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Johns RA, DiFazio CA, Longnecker DE. Lidocaine constricts or dilates rat arterioles in a dose-dependent manner. Anesthesiology. 1985;62:141–4. doi: 10.1097/00000542-198502000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Neal JM. Effects of epinephrine in local anesthetics on the central and peripheral nervous systems: Neurotoxicity and neural blood flow. Reg Anesth Pain Med. 2003;28:124–34. doi: 10.1053/rapm.2003.50024. [DOI] [PubMed] [Google Scholar]
- 48.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–16. doi: 10.1097/AIA.0b013e31820e4a49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ririe DG, Walker FO, James RL. Effect of alkalinisation of lidocaine on median nerve block. Br J Anaesth. 2000;84:163–8. doi: 10.1093/oxfordjournals.bja.a013397. [DOI] [PubMed] [Google Scholar]
- 50.Sinnott CJ, Garfield JM, Thalhammer JG, Strichartz GR. Addition of sodium bicarbonate to lidocaine decreases the duration of peripheral nerve block in the rat. Anesthesiology. 2000;93:1045–52. doi: 10.1097/00000542-200010000-00028. [DOI] [PubMed] [Google Scholar]
- 51.Kroin JS, Buvanendran A, Beck DR, Topic JE, Watts DE, Tuman KJ. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology. 2004;101:488–94. doi: 10.1097/00000542-200408000-00031. [DOI] [PubMed] [Google Scholar]
- 52.Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology. 2009;111:406–15. doi: 10.1097/ALN.0b013e3181aae897. [DOI] [PubMed] [Google Scholar]
- 53.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 54.Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Effects of calcium and magnesium on peripheral nerve conduction. Pol J Pharmacol. 2003;55:25–30. [PubMed] [Google Scholar]
- 55.Kiss T, Osipenko ON. Toxic effects of heavy metals on ionic channels. Pharmacol Rev. 1994;46:245–67. [PubMed] [Google Scholar]
- 56.Hung YC, Chen CY, Lirk P, Wang CF, Cheng JK, Chen CC, et al. Magnesium sulfate diminishes the effects of amide local anesthetics in rat sciatic-nerve block. Reg Anesth Pain Med. 2007;32:288–95. doi: 10.1016/j.rapm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams BA, Murinson BB, Grable BR, Orebaugh SL. Future considerations for pharmacologic adjuvants in single-injection peripheral nerve blocks for patients with diabetes mellitus. Reg Anesth Pain Med. 2009;34:445–57. doi: 10.1097/AAP.0b013e3181ac9e42. [DOI] [PubMed] [Google Scholar]
- 58.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–8. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 59.Cummings KC, Jr, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–53. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 60.Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35:422–6. doi: 10.1097/AAP.0b013e3181e85eb9. [DOI] [PubMed] [Google Scholar]
- 61.Fredrickson Fanzca MJ, Danesh-Clough TK, White R. Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: Results from 2 randomized placebo-controlled trials. Reg Anesth Pain Med. 2013;38:300–7. doi: 10.1097/AAP.0b013e318292c121. [DOI] [PubMed] [Google Scholar]
- 62.Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980;284:351–3. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- 63.Al-Khrasani M, Lackó E, Riba P, Király K, Sobor M, Timár J, et al. The central versus peripheral antinociceptive effects of μ-opioid receptor agonists in the new model of rat visceral pain. Brain Res Bull. 2012;87:238–43. doi: 10.1016/j.brainresbull.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 65.Candido KD, Hennes J, Gonzalez S, Mikat-Stevens M, Pinzur M, Vasic V, et al. Buprenorphine enhances and prolongs the postoperative analgesic effect of bupivacaine in patients receiving infragluteal sciatic nerve block. Anesthesiology. 2010;113:1419–26. doi: 10.1097/ALN.0b013e3181f90ce8. [DOI] [PubMed] [Google Scholar]
- 66.Candido KD, Franco CD, Khan MA, Winnie AP, Raja DS. Buprenorphine added to the local anesthetic for brachial plexus block to provide postoperative analgesia in outpatients. Reg Anesth Pain Med. 2001;26:352–6. doi: 10.1053/rapm.2001.23931. [DOI] [PubMed] [Google Scholar]
- 67.Modi M, Rastogi S, Kumar A. Buprenorphine with bupivacaine for intraoral nerve blocks to provide postoperative analgesia in outpatients after minor oral surgery. J Oral Maxillofac Surg. 2009;67:2571–6. doi: 10.1016/j.joms.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Butterworth JF., 4th Will conventional local anesthetics soon be replaced by neurotoxins? Reg Anesth Pain Med. 2011;36:101–2. doi: 10.1097/AAP.0b013e31820db23e. [DOI] [PubMed] [Google Scholar]
- 69.Baer M, Best PM, Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976;263:344–5. doi: 10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- 70.Strichartz G. Structural determinants of the affinity of saxitoxin for neuronal sodium channels. Electrophysiological studies on frog peripheral nerve. J Gen Physiol. 1984;84:281–305. doi: 10.1085/jgp.84.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohane DS, Lu NT, Gökgöl-Kline AC, Shubina M, Kuang Y, Hall S, et al. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg Anesth Pain Med. 2000;25:52–9. doi: 10.1016/s1098-7339(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 72.Strichartz G, Rando T, Hall S, Gitschier J, Hall L, Magnani B, et al. On the mechanism by which saxitoxin binds to and blocks sodium channels. Ann N Y Acad Sci. 1986;479:96–112. doi: 10.1111/j.1749-6632.1986.tb15564.x. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Navarro AJ, Lagos N, Lagos M, Braghetto I, Csendes A, Hamilton J, et al. Neosaxitoxin as a local anesthetic: Preliminary observations from a first human trial. Anesthesiology. 2007;106:339–45. doi: 10.1097/00000542-200702000-00023. [DOI] [PubMed] [Google Scholar]
- 74.Rodríguez-Navarro AJ, Berde CB, Wiedmaier G, Mercado A, Garcia C, Iglesias V, et al. Comparison of neosaxitoxin versus bupivacaine via port infiltration for postoperative analgesia following laparoscopic cholecystectomy: A randomized, double-blind trial. Reg Anesth Pain Med. 2011;36:103–9. doi: 10.1097/aap.0b013e3182030662. [DOI] [PubMed] [Google Scholar]
- 75.Wang GK, Strichartz GR. State-dependent inhibition of sodium channels by local anesthetics: A 40-year evolution. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2012;6:120–127. doi: 10.1134/S1990747812010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–31. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 77.McGivern JG. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr Dis Treat. 2007;3:69–85. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boogaerts JG, Lafont ND, Declercq AG, Luo HC, Gravet ET, Bianchi JA, et al. Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: A first study. J Clin Anesth. 1994;6:315–20. doi: 10.1016/0952-8180(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 79.Chahar P, Cummings KC., 3rd Liposomal bupivacaine: A review of a new bupivacaine formulation. J Pain Res. 2012;5:257–64. doi: 10.2147/JPR.S27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopacz DJ, Lacouture PG, Wu D, Nandy P, Swanton R, Landau C. The dose response and effects of dexamethasone on bupivacaine microcapsules for intercostal blockade (T9 to T11) in healthy volunteers. Anesth Analg. 2003;96:576–82. doi: 10.1097/00000539-200302000-00050. [DOI] [PubMed] [Google Scholar]
- 81.Weiniger CF, Golovanevski M, Sokolsky-Papkov M, Domb AJ. Review of prolonged local anesthetic action. Expert Opin Drug Deliv. 2010;7:737–52. doi: 10.1517/17425241003767383. [DOI] [PubMed] [Google Scholar]
- 82.Wang CF, Djalali AG, Gandhi A, Knaack D, De Girolami U, Strichartz G, et al. An absorbable local anesthetic matrix provides several days of functional sciatic nerve blockade. Anesth Analg. 2009;108:1027–33. doi: 10.1213/ane.0b013e318193596a. [DOI] [PubMed] [Google Scholar]
- 83.Sokolsky-Papkov M, Golovanevski L, Domb AJ, Weiniger CF. Prolonged local anesthetic action through slow release from poly (lactic acid co castor oil) Pharm Res. 2009;26:32–9. doi: 10.1007/s11095-008-9699-8. [DOI] [PubMed] [Google Scholar]


