Spinal cord injury (SCI) is a devastating ailment that results in drastic life style alterations for the patients and their family members (McDonald and Sadowsky, 2002). Damage post injury causes necrosis, edema, hemorrhage and vasospasm. Post injury, secondary damage is caused by ischemia, excitotoxicity, lipid peroxidation, free radicals production, and inflammation. Collectively, damage to multiple neuronal and glia sub-types leads to severed axonal connections, demyelination and scar tissue formation. Interventions therefore require regeneration of multiple axonal projections to recreate the lost neuronal diversity originally achieved through an elaborate, tightly regulated transcriptional code during development. Neuronal repair and regeneration post injury is impeded due to absence of such supportive environment in the adult spinal cord (Misra et al., 2014).
Impressive research advances using cell therapies have ushered in an era of new hope and today we are witnessing the translation of studies utilizing mesenchymal stem cells (MSCs) into mainline therapies for patients with SCIs. A testimonial to this trend is the surge in clinical studies using cellular transplantation for SCI (Tetzlaff et al., 2011). A search within (clinical trail.gov) using “MSCs transplantation” as search terms yielded ~400 studies, with only 6% of them related to SCI repair. Studies at EU clinical trial registry follow similar trends. Within the SCI domain, the majority of studies (69%) utilize BM-derived cells, followed by adipose tissue-derived MSCs (23%) and umbilical cord-derived cells (Figure 1a). While exciting at a first glance, a deeper analysis only highlights the confusion that prevails in the field of adult stem cells. The types of cells used and standards for study design and reporting are far from being well defined, despite organized efforts (Steeves et al., 2007). Given the variability in cell derivation protocols, it is extremely difficult to draw valid conclusions from these different (MSCs) cell therapy studies. Additionally, beyond the registered studies, there is alarming number of studies and treatments that are carried out without any controls or standards for safety and outcome measures, creating wrong perceptions in the public outlook. In this perspective, we have extrapolated the documented studies of cellular therapy for SCI to objectively draw inferences on the mechanisms of injury repair, and provide an outlook for bone marrow (BM)-derived cell therapy in the context of SCI.
Figure 1.
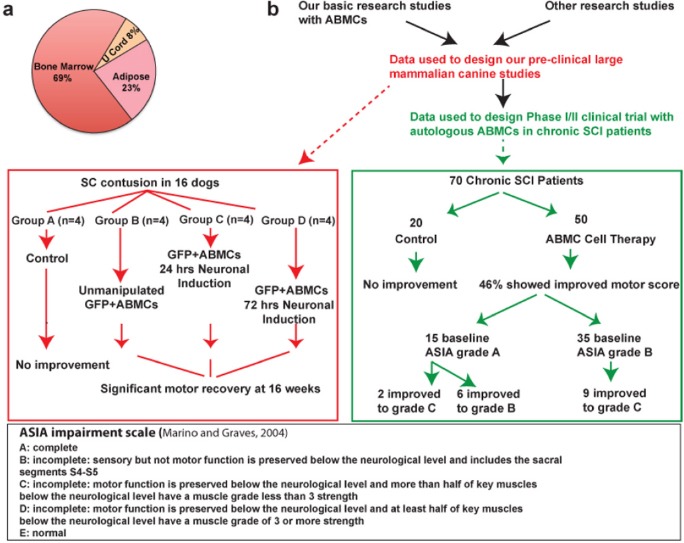
Bone marrow (BM)-derived cells for cell therapy of spinal cord injury (SCI).
(a) Sources of cells utilized for clinical cell therapy for SCI. (b) Study design and outcomes of preclinical and clinical studies demonstrating functional improvements when utilizing autologous bone marrow-derived cells (ABMCs) for cell therapy in the canine contusion model (Gabr et al., 2014) and phase I/II trial (NCT00816803) in chronic SCI patients (El-Kheir et al., 2014). GFP: Green fluorescent protein; ASIA impairment scale: The American Spinal Injury Association impairment scale.
Bone marrow derived cell populations in regenerative medicine: The human BM in vivo is made up of multiple cell subpopulations that could potentially contribute to injury repair. Besides hematopoietic stem cells (HSCs) and MSCs (also called multipotent mesenchymal stromal cells), BM contains other cell types such as adventitial reticular cells, vascular pericytes, BM fibroblasts, and bone lining cells. Some of these cells possess the ability to self-renew, maintain genetic and/or epigenetic profiles that make them more reprogrammable in vivo, and retain sufficient proliferative, secretory, and differentiation potentials to repair and/or reconstitute a specific tissue. While the beneficial effects of BM-derived cells for SCI repair are well recognized (Tetzlaff et al., 2011), it has been difficult to identify the mechanism(s) of repair or the specific cell type(s) that mediate the regenerative effects, since many BM cells display markers that are specific but not unique to any single cell subpopulation.
The acronym MSCs has been utilized in multiple contexts within the field of regenerative medicine. While commonly used to describe culture expanded MSCs, it is also used for mesenchymal stromal cells and multipotent stem cells. Earlier studies have focused on bulk culturing of BM stromal cells (BMSCs), but majority of studies diversified into expanding the subpopulation of cells that might represent stem cells, that in this context, were called MSCs. Therefore, MSCs are the primary cell types that overgrow (and adapt) to in vitro cultures. Unfortunately, growth expansion and culture adaptation likely come at the expense of altered biological and secretory features that completely distinguish MSCs from their in vivo counterparts (Prockop, 2009).
While significant research efforts have focused on identifying phenotypic markers that are expressed by cultured MSCs, in vivo characterization of BMSCs has been limited. Moreover, MSCs express markers that are likely to be very different from markers of BMSCs that are expressed in vivo. Understandably, BMSCs require their BM microenvironment to maintain these phenotypic markers, and retain the beneficial effects of being a potential in vivo source of tissue stem cells. Prevalent protocols for MSCs expansion require exposure to culture materials (frequently of animal origin) for several weeks, criteria that would likely cause loss of (most of) the (beneficial) properties as well as changes in phenotypic markers. MSCs classically express surface markers such as CD90, CD105, CD73, but lack expression of CD14, CD34, CD45 and have some (non-standardized) capacity to differentiate into adipocytes, chondrocytes and osteocytes, however, differentiation into other tissue types remains frequently not examined.
Minimally manipulated adherent bone marrow cells (ABMCs): Keeping in mind the probability of loss of in vivo phenotypes along with beneficial potentials post culturing of MSCs in the absence of in vivo niche, we have developed protocols for rapid isolation of BMSCs by “minimal manipulation” utilizing their expression of adhesion molecules to isolate cells that we called adherent bone marrow cells (ABMCs). We utilize the “ABMC” term to distinguish these cells from culture expanded (and adapted) MSCs. ABMCs from human, canine, and murine BM maintain their in vivo phenotypic characteristics and are phenotypically and functionally distinct from culture-expanded MSCs. Our ABMC isolation protocol involves very little manipulations, and as such might qualify ABMCs as “minimally manipulated cells” under FDA regulation. Unlike the fibroblast-like shape of traditional human MSCs, ABMCs have a flat oblong morphology. ABMCs are positive for CD29, CD44, CD73, CD90, CD105, CD117 (C-Kit), CD166, CD271 but have very low (< 0.01%) to non-detectable expression of CD45, CD34, and CD13 (El-Kheir et al., 2014). These cells retain better abilities to be induced into adipocytes, osteocytes, chondrocytes and their culture in neural induction media results in formation of atypical neurospheres. Based on successful preclinical work, we designed large animal studies followed by clinical testing in chronic SCI patients. In the former, ABMCs were tested in a canine contusion model of SCI (Gabr et al., 2014). Both unmanipulated and neurally pre-differentiated ABMCs were utilized. Unlike controls, significant recoveries of motor functions were observed in ABMCs-treated animals (Figure 1b). This large mammal study demonstrated the safety and efficacy of cell therapy with ABMCs and supported progression towards testing in human trials.
Our phase I/II trial with a fixed dose autologous ABMCs in chronic SCI patients also yielded promising results (Figure 1b). In treated patients, 17 out of 50 showed improvements measured by the American Spinal Injury Association (ASIA) Impairment Scale (AIS), whereas none of 20 controls showed any significant improvement (Figure 1b). Within the cell therapy subgroup of 15 patients with a baseline AIS A (complete lack of motor and sensory function below injury level), 2 patients converted to AIS C (some muscle movement is spared below injury site, but 50% of the muscles caudally to injury level cannot move against gravity) and 6 patients improved to AIS B (Some sensation below injury level). Similarly, within the cell therapy subgroup of 35 patients with a baseline AIS B, 9 patients converted to AIS C (Figure 1b). In addition, 46% of treated patients, but none of controls, had their ASIA motor score increased by ≥10 points (Tables 2 and Table 3, El-Kheir et al., 2014). These preclinical and clinical studies validate our model that minimally manipulated ABMCs are capable of inducing significant repair post-transplantation in SCI patients.
Mechanisms underlying bone marrow induced repair and regeneration: BM-derived MSCs and other stem cell types have been extensively studied in SCI models. Although modest beneficial effects were consistently observed (Tetzlaff et al., 2011), the mechanisms of repair and regeneration are still poorly understood. We have demonstrated that crosstalk between MSCs and endogenous SCI stem cells enhances axonal regeneration (Patel et al., 2012). Direct conversion of transplanted cells into functional neurons was reported earlier by many studies. This concept however remains debatable, despite being supported by the documented potential of adult fibroblasts to be reprogrammed into neuronal or pluripotent (iPS) cells (Takahashi and Yamanaka, 2006). Therefore, it is possible that endogenous reprogramming of ABMCs by factors within the injured tissues occurs, this however remains to be directly elucidated. More and more data support the roles for BMSCs/BM-cell-derived paracrine factors (Figure 2) in mediating the relatively rapid regeneration rather than the slow and inefficient transdifferentiation and/or reprogramming that remain difficult to elucidate. BMSCs are known to possess neuroprotective activities by secreting trophic factors, cytokines, exosomes and microvesicles (Liang et al., 2014) in response to appropriate stimuli. For instance, hypoxia, inflammation, and 3D culture induce the transcription of multiple growth factors and anti-inflammatory modulators (Figure 2). Moreover, there is growing evidence that the regenerative capabilities are associated with BMSCs-derived exosomes (40–100 nm nanoparticles) and microvesicles (50–1,000 μm cell membrane buddings) that are secreted upon bi-directional communications between injury resident and therapeutic cells. These trends collectively indicate that the therapeutic benefits associated with BMSCs are mediated, at least in part, via secreted factors, or by mitochondrial transfer, and not necessarily only by direct differentiation into specialized cells.
Figure 2.
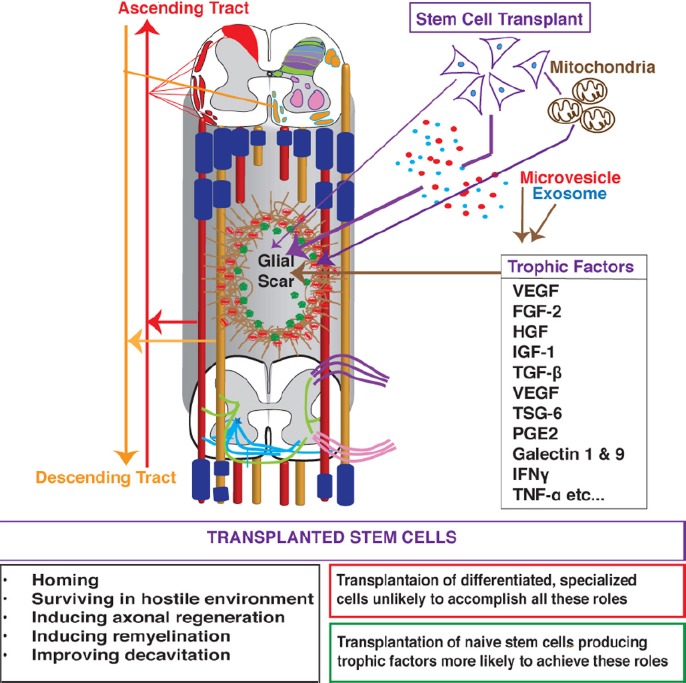
Mechanisms of spinal cord injury (SCI) repair and axonal regeneration that are induced by cell therapy with autologous bone marrow-derived cells (ABMCs).
ABMCs home to and survive at injury site, and induce axonal regeneration and remyelination. Trophic factors, cytokines, exosomes, and microvesicles that are secreted by ABMCs, or mitochondrial transfer induce SCI repair. VEGF: Vascular endothelial growth factor; FGF-2: fibroblast growth factor-2; HGF: hepatocyte growth factor; IGF-1: insulin-like growth factor 1; TGF-β: transforming growth factor beta; TSG-6: tumor necrosis factor-inducible gene 6 protein; PGE2: prostaglandin E2; IFNγ: interferon gamma; TNF-α: tumor necrosis factor alpha.
Despite the benefits, concerns about associated risk profile when utilizing stem cells prevail. Uncontrolled growth risk associated with the use of embryonic stem cells is mitigated with adult stem cells like ABMCs. Risk for mutations and other genetic modifications associated with in vitro expansion and differentiation are also mitigated with ABMCs due to minimal expansion. Immune response induction is another risk factor prevented by autologous ABMC administration. Collectively, our pre-clinical as well as clinical data demonstrates that ABMCs possess therapeutic repair capabilities via a number of tissue regeneration mechanisms along with a superior safety profile relative to cells derived from iPS or embryonic stem cells. Harnessing the full regenerative powers of ABMCs would be further achieved upon standardization of cell preparation, handling, and cell transplantation procedures.
References
- El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- Gabr H, El-Kheir WA, Farghali HA, Ismail ZM, Zickri MB, Maadawi ZM, Kishk NA, Sabaawy HE. Intrathecal transplantation of autologous adherent bone marrow cells induces functional neurological recovery in a canine model of spinal cord injury. Cell Transplant. 2014 doi: 10.3727/096368914X683025. doi:10.3727/096368914X683025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Graves DE. Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Arch Phys Med Rehabil. 2004;85:1804–1810. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- Misra K, Luo H, Li S, Matise M, Xiang M. Asymmetric activation of Dll4-Notch signaling by Foxn4 and proneural factors activates BMP/TGFbeta signaling to specify V2b interneurons in the spinal cord. Development. 2014;141:187–198. doi: 10.1242/dev.092536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Klassert TE, Greco SJ, Patel SA, Munoz JL, Reddy BY, Bryan M, Campbell N, Kokorina N, Sabaawy HE, Rameshwar P. Developmental regulation of TAC1 in peptidergic-induced human mesenchymal stem cells: implication for spinal cord injury in zebrafish. Stem Cells Dev. 2012;21:308–320. doi: 10.1089/scd.2011.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]


