The peripheral nervous system is a vital part of the body because it transfers information to coordinate all actions. Peripheral nerve injuries are detrimental to the proper function of this system and can cause loss of sense and movement. It is of utmost importance to research approaches to the treatment of peripheral nerve damage because such injuries can drastically change a person's life, and these traumatic injuries are a significant cause of physical disabilities that primarily affect the lives of young adults of working age. The aetiologies of traumatic peripheral nerve injury include penetrating injury, crush, traction, ischemia, and less common mechanisms, such as thermal-, electric shock-, radiation-, percussion-, and vibration-induced injuries (Robinson, 2004). Lacerations, for example, by glass, knives, fans, saw blades, auto metal, and long bone fractures, account for approximately 30% of serious nerve injuries. Another common injury mechanism is compression, which may involve mechanical deformation and ischemia. Manipulation of the nerve can generate a severe inflammatory reaction in the nerve, and these reactions have been likened to chemical burns with dense scarring accompanied by considerable pain (Bagheri et al., 2011).
Nerve injuries are classified into five degrees: (1) neurapraxia, (2) loss of continuity of the axons without breaching the endoneurial sheath of the nerve fibres, (3) loss of continuity of the nerve fibres, (4) involvement of the perineurium and the fasciculi, and (5) loss of continuity of the nerve trunk (Sunderland, 1978). Regeneration in the peripheral nervous system is possible, although appropriate surgical interventions are required for the recovery of a nerve that has been completely sectioned. Some procedures and material applications have been investigated to optimise the repair of damaged peripheral nerves, such as autologous nerve grafts, autologous stem cell transplants, tube techniques with various materials, and trophic agents (Winfree, 2005; Campbell, 2008).
Biomaterials are central to many regenerative medicine strategies; these materials serve a scaffolding function to create and maintain a space for tissue growth, provide mechanical stability, and support cell adhesion and migration. The rapid progress in the area of tissue engineering has encouraged researchers to propose therapeutic strategies for repairing peripheral nerves using biopolymers and neurotrophic factors. The use of microtubes as scaffolds can guide axonal growth during treatment to repair peripheral nerve injuries, and such scaffolding can be resorbable or non-resorbable. In this context, microtubes have been developed using poly-(2-hydroxyethylmethacrylate) and poly-L-lysine with different concentrations of neurotrophins. Carbon nanotubes are used to provide a structure that is capable of controlling the liberation of neurotrophins that aid in the process of peripheral nerve repair. In contrast, the use of synthetic biomaterials for tissue regeneration generally causes inflammatory reactions that may occasionally hinder or prevent healing and the consequent repair of the damaged structure; i.e., the injury incurred during the implantation process and the host inflammatory response to the implanted material can negatively influence the local environment (Boehler et al., 2011). This response can lead to the repair of the injury site through repopulation with granulation tissue that can result in fibrosis, and regeneration by local progenitor cells can produce fully functional tissue.
Thus, controlling the inflammatory reactions that are induced by both the trauma and the materials used for the regeneration appears to be crucial to the success of the proposed therapy. Anti-inflammatory therapies aim to modulate the magnitude and diversity of immune cell responses or to alter the phenotypes of the resident cells. The systemic delivery of anti-inflammatory cytokines has been employed to influence the immune cell response and cell phenotypes; however, this process has the potential to systemically deactivate the immune system and leave the body vulnerable to infection. Thus, approaches to nerve repair must control the redox balance because the relationship between injury damage and cellular adaptation is extremely fragile (Sen, 2003). Despite the simplicity of this concept, its application requires a broad knowledge of metabolic variables, the particularities of each tissue, and the interactions of the tissue/cells that surround the repair site. Our idea for a strategy for nerve repair is to combine the approaches mentioned above with antioxidants to promote protection and tissue repair on a scaffold that gradually releases its contents around the injured nerve.
Our strategy utilizes oxidative mediation with a locally applied agent. To this end, a biodegradable scaffold that contains an antioxidant compound and mineral salts and functions to mediating the process of in situ nerve repair was developed. The antioxidant compound was composed of β-carotene, α-tocopherol, B complex vitamins, selenium salts, zinc salts, magnesium salts, phosphor salts, glutamic acid, soy lecithin, hydrolysed collagen, glycosaminoglycan sulphate and chondroitin sulphate. The amounts of selenium, zinc and magnesium varied from 10% to 15% of the total weight of the active components. Selenium varied from 20% to 30% of the relative weights of zinc and magnesium, which were 1.8% and 1.2%, respectively. β-Carotene and α-tocopherol varied between 15% and 20%, and the α-tocopherol content was 64% that of the β-carotene content. The B complex varied from 20% to 30% of the total weight of the active components; vitamins B1 and B6 comprised 30% of the complex, and glutamic acid comprised 50% of the vitamin B6. Vitamin D (calciferol) was added in the final process at a maximum of 40 parts per million of the final weight. Hydrolysed collagen, glycosaminoglycan sulphate and chondroitin sulphate are agglutinating agents, and they were added in proportions of 60%, 20% and 20%, respectively, until the material attained an adequate consistency for easy manipulation.
In a recent rat study (Wistar rat) reported by our team (Salles et al., 2015), an injury in the ischiatic nerve was induced in all animals by suturing three distinct regions of the right nerve, with approximately 3 mm intervals for each suture knot with nylon, was treated with the deposition (around the trauma area) of the antioxidant compound (test group) and not-treated sites (control group). The antioxidant compound favoured the process of nerve repair 30 days after its application to the area around nerve injuries and produced an obvious contrast to the controls when observed macroscopically. A disorganization of the structures was observed in the control group. The experimental group that was treated with the antioxidant compound presented integrity of the nerve strand and a neoformation under the suture wire, as a “bridge” formed between the distal and proximal portions of the nerve. Fifteen days after the surgery, the histological samples of the animals that were treated with the antioxidant compound revealed no signs of nervous degeneration, and this result was possibly due to the control of the local inflammatory process due to the surgical intervention and related manipulation of the ischiatic nerve. In the experimental group, neoformation of the nerve was observed despite the signs of morphological alterations that indicated damage to the nerve strand (Figure 1).
Figure 1.
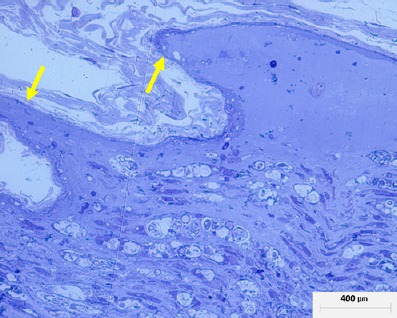
Histological image of a neurovascular bundle 15 days after surgery.
The region shown was located near the trauma. A marked local inflammatory process has occurred; however, the newly fromed cones of new nerve formation are clearly visible which indicates a positive response to treatment (arrows).
After 30 days, the experimental groups exhibited nerve neoformation in the presence of well-formed epinerves that separate the neoformed or secondary strand from the main strand. Moreover, the secondary strand possessed blood vessels and a myelin sheath that was narrower than normal (as indicated by the ratio of the strand to the sheath), denoting an immature nerve strand. The experimental animals exhibited an organization of the secondary nerve strand that was parallel to the main strand and the formation of a well-organized epinerve separating the main strand from the secondary strand (Figure 2).
Figure 2.
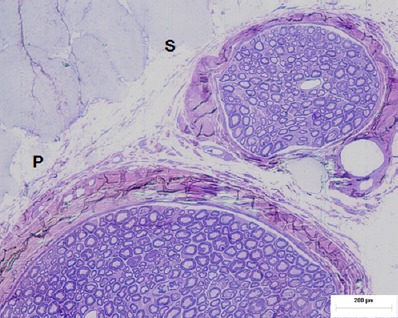
Histological image of a neurovascular bundle 30 days after surgery.
Note that in this image, a newly for med cone present at 15 days has assumed a secondary beam (S) structure that detached from the main beam (P), which indicates the effectiveness and feasibility of the studied material.
Our hypothesis was based on three findings: I) after trauma, many cells return to their embryonic state (Sen, 2003); II) many cytokines that are expressed in the early stages of the process of repair are present during organogenesis (Kumar et al., 2005); and III) the healing of damaged tissue is defined in the early stages of the repair process (Tosh and Slack, 2002). In the beginning of the repair process, there are substantial cellular and tissue potentials that are proportionate to the favourability of the conditions to the repair of the damaged tissue, which are linked directly to the time and extent of the damage.
Thus, our strategy was to develop a biomaterial that could locally provide functional microelements with the abilities to mediate the repair process via the gradual liberation of antioxidants and a metabolic substrate capable of positively interfering with the cellular activity and protein synthesis to aid the process of nervous tissue repair. The antioxidant organic compounds were composed of substances that favour the process of nerve repair via two possible mechanisms of action, i.e., oxidative protection and the supply of a metabolic substrate. The vitamin components of β-carotene, α-tocopherol, B complex and calciferol (vitamin D) have direct or indirect protective functions against reactive oxygen species. Carotenoids and tocopherols are major natural protective agents against free radical-mediated liver damage. The B complex is involved in the production of metabolic substrates related to the production of α-amino acids and β-acetic acids, which are substrates for the formation of proteins and ATP, which, in turn, indirectly protect cells from the action of reactive oxygen species and limit cellular damage. Vitamin D has been associated with increases in the synthesis of anti-inflammatory/repairing cytokines, such as transforming growth factor-β and interleukin-8, and with a reduction in TNF-α that limits the local inflammatory process (Gurlek et al., 2002).
The inorganic compounds of zinc and selenium are members of the prosthetic groups of the superoxide dismutase (SOD) enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. These compounds are an important antioxidant defence mechanism in nearly all cells that are exposed to oxygen and glutathione and act as enzymatic co-factors in cellular protection (Sidhu et al., 2005). There is an active effort to identify a path to cellular protection that is mediated by oxidative agents, such as tocopherols, but that does not interfere with the production of free radicals that signal the neo-vascularisation of the damaged tissue (Jiang et al., 2011). Via another path, a greater locally available concentration of amines from the B group, which are related to the synthesis of α-amino acids and α-acetic acids (proteins and ATP), could favour the repair of damaged tissue. This process results in reductions of cellular damage via antioxidants and another path that supplies metabolic substrates that are capable of sustaining the metabolism of local cells.
Finally, the additions of the hydrolysed collagen, glycosaminoglycan and chondroitin sulphate were linked to the formation of the application matrix and intended to facilitate its use in surgical procedures by making the material plastic and allowing for its easy manipulation.
Multiple strategies, such as the induction of growth factors and the inhibition of deleterious factors, have been applied to protect peripheral nerves with the objective of enhancing the repair of these structures and is well referred in the revision about of this subject in the recent article by Ramburrun et al. (2014); however, all of these strategies have important limitations that are primarily related to the time after the initial trauma. The temporal limitation also applies to the approach proposed here; i.e., the organic material must be used quickly to reduce the damage to the peripheral nerve bundle and reduce the extension of the nerve damage and its consequences.
Although these initial results are promising, the current data do not support the efficacy of the product in all cases, and the effects and mechanisms of this organic compound are strongly justified by the critical roles of nerve injury in the pathophysiology and the efficacy of this organic compound in improving nerve regeneration in terms of the structure and function. These findings might represent the beginning of a study that that will result in an alternative treatment for peripheral nerve injury.
References
- Bagheri SC, Meyer RA. Management of mandibular nerve injuries from dental implants. Atlas Oral Maxillofacial Surg Clin N Am. 2011;19:47–61. doi: 10.1016/j.cxom.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51:239–240, 242, 244. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha, 25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. 2002;23:763–786. doi: 10.1210/er.2001-0044. [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. In: Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier Saunders; 2005. Cellular Adaptations, Cell Injury, and Cell Death; pp. 3–46. [Google Scholar]
- Ramburrun P, Kumar P, Choonara YE, Bijukumar D, du Toit LC, Pillay V. A review of bioactive release from nerve conduits as a neurotherapeutic strategy for neuronal growth in peripheral nerve injury. Biomed Res Int 2014. 2014 doi: 10.1155/2014/132350. 132350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LR. Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol. 2004;57:173–186. doi: 10.1016/s1567-424x(09)70355-1. [DOI] [PubMed] [Google Scholar]
- Salles MB, Gehrke SA, Koo S, Allegrini S, Jr, Rogero SO, Ikeda TI, Cruz AS, Shinohara EH, Yoshimoto M. An alternative to nerve repair using an antioxidant compound: a histological study in rats. J Mater Sci Mater Med. 2015;26:5340. doi: 10.1007/s10856-014-5340-z. [DOI] [PubMed] [Google Scholar]
- Sen CK. The general case for redox control of wound repair. Wound Repair Reg. 2003;11:431–438. doi: 10.1046/j.1524-475x.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- Sidhu P, Garg ML, Dhawan DK. Protective effects of zinc on oxidative stress enzymes in liver of protein-deficient rats. Drug Chem Toxicol. 2005;28:211–230. doi: 10.1081/dct-52551. [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JMW. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- Winfree CJ. Peripheral nerve injury evaluation and management. Curr Surg. 2005;62:469–476. doi: 10.1016/j.cursur.2005.03.008. [DOI] [PubMed] [Google Scholar]


