Nerve regeneration in the central nervous system (CNS) has become a holy grail of biomedical research. To understand nerve growth that would be required for efficient regeneration, many scientists have turned to developing systems where nerve growth is abundant and normal neural connections are established. One aspect of this neural development, which would also be important in nerve regeneration, is axon guidance – the process by which a growing axon, through its growth cone, is guided to the correct target for synaptic connections.
There has been considerable work done on axon guidance in a variety of systems, but one system that has presented many opportunities is the visual system. In the visual system, there is one cell type that provides the output nerve from the eye, the optic nerve. These cells are the retinal ganglion cells (RGCs). They grow from one distinct location, the eye, along a stereotypical and well-defined pathway to specific targets in the brain. Axon guidance is crucial as these axons navigate this pathway, both at intermediate choice points (e.g., optic chiasm) as well as forming the specific innervations at the target, the lateral geniculate nucleus (LGN) and superior colliculus in mammals or the tectum in lower vertebrates.
There has been significant work done investigating molecules that act as axon guidance cues at various points in the visual system. This work has yielded a number of proteins now established as axon guidance cues, including Ephs and ephrins, netrins, semaphorins, wnts, etc. (for review, see Erskine and Herrera, 2007). However, there is a novel set of molecules, the signaling lysophospholipids, that are being investigated as potential axon guidance cues. The two best studied signaling lysophospholipids are lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P). Observations a number of years ago demonstrated that LPA would cause growth cone collapse and neurite retraction in PC12 cells (Jalink et al., 1993). Further work has reproduced these results in PC12 cells as well as in some other neural types, including growth cone collapse of primary embryonic retinal axons in vitro from mouse, chick, and Xenopus (Strochlic et al., 2008; Birgbauer and Chun, 2010; Fincher et al., 2014). The ability to induce growth cone collapse is a hallmark of several well-established inhibitory axon guidance cues, such as semaphorins and ephrins. Although not actual evidence of axon guidance, growth cone collapse by LPA suggests the hypothesis that LPA may be an axon guidance cue for RGCs. S1P may also serve a role as an axon guidance cue for RGC axons as it also causes growth cone collapse of retinal axons in vitro in both chickens and Xenopus (Strochlic et al., 2008; Fincher et al., 2014); interestingly, S1P does not cause growth cone collapse of mouse retinal axons in vitro (Birgbauer and Chun, 2010). Furthermore, in what may be the strongest evidence that lysophospholipids are involved in axon guidance, Strochlic et al. (2008) have shown a role of S1P for entry of RGC axons into the tectum in Xenopus; this guidance into the tectum was found to be perturbed by exogenous S1P or S1P receptor agonists and antagonists (Strochlic et al., 2008).
LPA and S1P act by binding to and activating specific receptors and have been shown to be involved in many biological processes, including cell proliferation, vascular development, neurogenesis, and morphological changes, including in the nervous system (for a recent review, see Yung et al., 2015). The LPA and S1P receptors are part of the family of G-protein coupled receptors (GPCRs) and activate the classic G-protein cell signaling pathways Gi, Gs, Gq, and G12/13, depending on the receptor and cell type assayed. There are currently six characterized LPA receptors and five known S1P receptors (Chun et al., 2010). Investigating the role of these GPCRs in retinal growth cone collapse, Fincher et al. (2014) recently characterized the intracellular G-protein pathways that lead to retinal growth cone collapse by LPA and S1P (see Figure 1). Not surprisingly, based on a well-established role for rho in cytoskeletal rearrangements, including growth cone collapse, blocking the G12/13-rho-ROCK pathway with a ROCK inhibitor prevented both LPA and S1P induced growth cone collapse of chick retinal axons (Fincher et al., 2014). However, inhibition downstream of Gq, either by a phospholipase C inhibitor or calcium chelation, did not prevent retinal growth cone collapse by LPA and S1P. The most interesting finding was inhibition of Gi by pertussis toxin, which partially blocked LPA and S1P induced retinal growth cone collapse; there was still significant LPA induced growth cone collapse in the presence of pertussis toxin, but significantly less than with LPA or S1P alone (in the absence of pertussis toxin). Since the growth cone collapse assay scores growth cones in a binary fashion as either collapsed or spread, these results suggest that the responsiveness of embryonic chick retinal growth cones to LPA (and S1P) through the Gi pathway was variable – some growth cones responded when Gi was blocked by pertussis toxin while others did not. What these different growth cone states were was not determined, but a significant body of work has shown that cyclic nucleotide levels in a growth cone influence the responsiveness of those growth cones to axon guidance cues, even reversing responses to specific cues. In addition, it is possible that other factors, either extracellular or intracellular, modulate growth cone responses to guidance cues. For example, chick retinal growth cone collapse by Slit-2 can be modulated by the chemokine SDF-1 (Chalasani et al., 2003). This modulation does not abolish growth cone collapse, but rather reduces the sensitivity to Slit-2, seen as lower growth cone collapse at a normally effective concentration of Slit-2. Furthermore, this modulation effect by SDF-1 was shown to be pertussis toxin sensitive, demonstrating a role for Gi. Interestingly, SDF-1 signaling through Gi was shown to increase cyclic adenosine monophosphate (cAMP), which suggested a noncanonical pathway compared to the usual role of Gi in lowering cAMP levels (Chalasani et al., 2003). As the study by Fincher et al. (2014) as well as others (e.g., Manns et al., 2012) have shown, there are clearly multiple pathways that lead to growth cone collapse. Activation of these different pathways depends on a variety of factors, many of which are just being elucidated. For instance, the signaling pathways leading to dorsal root ganglion (DRG) growth cone collapse by semaphorin 3A are dependent not only on the concentration of semaphorin 3A, but also the levels of growth factor (NGF) in the media, which was seen clearly by varying levels of partial growth cone collapse (Manns et al., 2012). Thus, growth cone responses to axon guidance cues is complex and can be affected by a variety of intrinsic and extrinsic factors, and this study by Fincher et al. (2014) indicates that there is a heterogeneity of retinal growth cone responses in vitro to signaling molecules.
Figure 1.
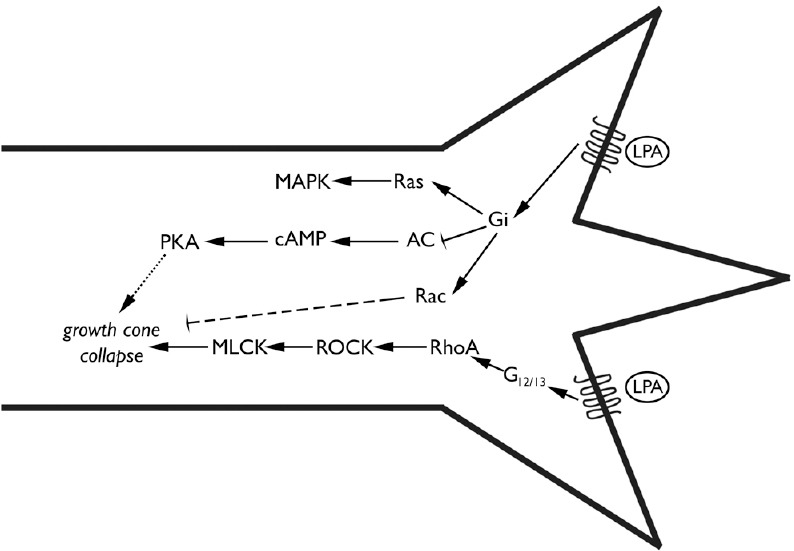
Signaling pathways leading to retinal growth cone collapse by lysophosphatidic acid (LPA).
Schematic representation of the cell signaling pathways demonstrated by Fincher et al. (2014) to lead to growth cone collapse by LPA and S1P on embryonic chicken retinal axons. Binding of LPA (or S1P, not shown) to G-protein coupled receptors activates the G12/13 pathway (bottom) which leads to RhoA and Rho kinase (ROCK) activation proceeding to myelin light chain kinase (MLCK) phosphorylation which leads to growth cone collapse, a well-established pathway. Furthermore, Fincher et al. (2014) showed that growth cone collapse was partially inhibited by pertussis toxin, which blocks Gi. Gi is known to activate a variety of cell pathways, including Ras and Rac, as well as inhibiting adenlyyl cyclase (AC), lowering cyclic adenosine monophosphate (cAMP) levels; cAMP can activate protein kinase A (PKA). Although the pathway of growth cone collapse via Gi is not established, the dashed lines from Rac or cAMP and PKA indicate probable mechanisms that Gi could effect growth cone collapse.
Fincher et al. (2014) also examined the mitogen-activated protein kinases (MAPK) signaling requirements for LPA and S1P induced growth cone collapse of chick retinal axons compared to previous published work on Xenopus retinal axons (Campbell and Holt, 2003). LPA-induced growth cone collapse was inhibited by a p38 inhibitor, but not a p42/44 inhibitor, in the chick system similar to the Xenopus system. Interestingly, S1P-induced retinal growth cone collapse was shown to be different in the intracellular signaling pathway compared to LPA-induced growth cone collapse; S1P-induced retinal growth cone collapse was neither sensitive to a p38 inhibitor nor a p42/44 inhibitor. Although it shouldn’t be surprising, this indicates that there are differences in the intracellular signaling downstream of LPA and S1P receptors in a specific response, growth cone collapse, that they both elicit.
Although this study focused on axon growth and guidance during development, these lysophospholipids could have significant roles in nerve regeneration. LPA is released during an injury response, including being produced at high levels by platelets. Other evidence has demonstrated that LPA receptors play a significant role in neuropathic pain (for review, see Ueda et al., 2013), and they could be involved in inhibition of nerve regeneration.
Furthermore, the cell signaling pathways activated by LPA and S1P receptors have clear demonstrated roles in inhibition of nerve regeneration. Neural regeneration inhibitors in CNS myelin act by activating the RhoA/Rho-associated protein kinase (ROCK) pathway. Treatment with antagonists of RhoA or ROCK have been shown to increase sprouting and nerve regeneration in both optic nerve and spinal cord injury models (for review, see Fujita and Yamashita, 2014). Potential clinical application has been demonstrated in a human phase I/IIa clinical trial for the cell permeable Rho antagonist BA-210 (Cethrin®) which suggested efficacy in patients with severe cervical spinal cord injury (Fehlings et al., 2011). Furthermore, combination therapies may be more effective, such as the report of a combination of a ROCK inhibitor with Stat3 inhibition in an optic nerve injury model which showed significant regeneration in the combined therapy compared to either single therapy (Pernet et al., 2013). In addition, as Fincher et al. (2014) found a role of Gi, and thus possibly cAMP, in LPA and S1P mediated retinal growth cone collapse, there appears to be effects of cAMP levels in nerve regeneration as well (Qiu et al., 2002).
In conclusion, Fincher et al. (2014) adds to our understanding that there may be a new molecular paradigm for axon guidance as well as potentially for nerve regeneration, and that is the role of signaling lysophospholipids such as LPA and S1P. Although the work so far has examined axonal responses during development, these lysophospholipids could have significant roles in nerve regeneration. Thus, the roles for lysophospholipids in the nervous system, especially nerve regeneration, are promising but still need to be elucidated.
EB is supported by the National Eye Institute of the National Institutes of Health under Award Number R15EY024453. (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.)
References
- Birgbauer E, Chun J. Lysophospholipid receptors LPA1-3 are not required for the inhibitory effects of LPA on mouse retinal growth cones. Eye Brain. 2010;2010:1–13. doi: 10.2147/EB.S7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011;28:787–796. doi: 10.1089/neu.2011.1765. [DOI] [PubMed] [Google Scholar]
- Fincher J, Whiteneck C, Birgbauer E. G-protein-coupled receptor cell signaling pathways mediating embryonic chick retinal growth cone collapse induced by lysophosphatidic acid and sphingosine-1-phosphate. Dev Neurosci. 2014;36:443–453. doi: 10.1159/000364858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- Manns RPC, Cook GMW, Holt CE, Keynes RJ. Differing semaphorin 3A concentrations trigger distinct signaling mechanisms in growth cone collapse. J Neurosci. 2012;32:8554–8559. doi: 10.1523/JNEUROSCI.5964-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013;4:e734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Strochlic L, Dwivedy A, van Horck FP, Falk J, Holt CE. A role for S1P signalling in axon guidance in the Xenopus visual system. Development. 2008;135:333–342. doi: 10.1242/dev.009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Matsunaga H, Olaposi OI, Nagai J. Lysophosphatidic acid: chemical signature of neuropathic pain. Biochim Biophys Acta. 2013;1831:61–73. doi: 10.1016/j.bbalip.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J. Lysophosphatidic acid signaling in the nervous system. Neuron. 2015;85:669–682. doi: 10.1016/j.neuron.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


