Abstract
Ischemic edema can alter the structure and permeability of the blood-brain barrier. Recent studies have reported that progesterone reduces cerebral edema after cerebral ischemia. However, the underlying mechanism of this effect has not yet been elucidated. In the present study, progesterone effectively reduced Evans blue extravasation in the ischemic penumbra, but not in the ischemic core, 48 hours after cerebral ischemia in rats. Progesterone also inhibited the down-regulation of gene and protein levels of occludin and zonula occludens-1 in the penumbra. These results indicate that progesterone may effectively inhibit the down-regulation of tight junctions, thereby maintaining the integrity of the blood-brain barrier and reducing cerebral edema.
Keywords: nerve regeneration, brain injury, gonadal hormone, cerebral ischemia, permeability, occludin, zonula occludens-1, Evans blue dye, penumbra, ischemic core, rats, neural regeneration
Introduction
A number of animal studies have suggested that progesterone (PROG) reduces edema formation after experimental traumatic brain injury (TBI) (Roof and Hall, 2000; Stein, 2001; Wright et al., 2001; Robertson et al., 2015) and stroke (Betz and Coester, 1990; Jiang et al., 2009; Perez-Alvarez et al., 2014; Wong et al., 2014). PROG has been shown to reduce cerebral edema and modulate aquaporins (particularly aquaporins 4 and 9), which are molecules that control water drainage into the ventricles of the brain (Amiry-Moghaddam et al., 2003). PROG regulates swelling and ion exchange (Badaut et al., 2002; Guo et al., 2006). This hormone also reduces the cytotoxic phase of edema caused by the accumulation of fluid inside neurons and reactive astrocytes (Roof et al., 1994, 1996; Shear et al., 2002; Guo et al., 2006). This form of edema can disrupt cells and cause the release of additional toxic agents into the brain parenchyma, producing an ongoing cycle of secondary cell death (Guo et al., 2006).
The blood-brain barrier (BBB) is composed of a continuous layer of cerebrovascular endothelial cells connected by tight junctions. The main structures of the BBB are tight junctions, which are composed of transmembrane proteins. Occludin, claudins, and junction-associated proteins are transmembrane proteins that seal the inter-endothelial space between adjacent endothelial cells. Zonula occludens-1 (ZO-1) is a cytoplasmic protein associated with tight junctions and located at the cytoplasmic surface of endothelial cells. It connects tight junctions to actin and other cytoskeletal proteins (Bauer et al., 2014).
Ischemic injury alters the permeability and structure of the BBB. Moreover, post-ischemic inflammatory cascades and counteracting repair processes can result in further modification of the BBB (Fujimura et al., 1999). The integrity and potency of tight junction proteins for sealing the space between endothelial cells modulates the response to these pathologic conditions (Vaccarino and Ment, 2004). Therefore, in response to injury, tight junctions in the BBB may help to maintain central nervous system homeostasis.
Most cases of human stroke are caused by permanent occlusion of cerebral arteries (Sarkaki et al., 2015). Therefore, in the present study, we assessed the neuroprotective effect of PROG on BBB leakage and the expression of occludin and ZO-1 in permanent middle cerebral artery occlusion (pMCAO) rats.
Materials and Methods
Animals and treatment
A total of 168 clean 70-day-old male Sprague-Dawley rats (License No. HNXK-2014002) weighing 200–250 g were used. They were randomly and equally divided into four groups: control, ischemia, vehicle, and PROG. Animals in each group were then assigned to subgroups for detection of Evans blue dye extravasation (n = 72), reverse transcription-polymerase chain reaction (RT-PCR) (n = 48) and western immunoblot analysis (n = 48). The rats were quarantined for at least 7 days before the experiment and were housed under standard conditions, a 12-hour light/dark cycle, and allowed access to food and water ad libitum. PROG (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 22.5% 2-hydroxypropyl-β-cyclodextrin and given at a dose of 8 mg/kg by intraperitoneal (i.p.) injection 1 and 6 hours post-occlusion to ensure more rapid absorption following injury. Additional injections of 15 mg/kg PROG were administered subcutaneously 24 and 48 hours post-occlusion (Kumon et al., 2000; Djebaili et al., 2005; Sayeed et al., 2006). In the vehicle group, PROG was replaced with 22.5% 2-hydroxypropyl-β-cyclodextrin at the same volume. In the ischemia and control groups, rats were not treated with PROG or 2-hydroxypropyl-β-cyclodextrin.
All animal experiments were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
Establishing pMCAO in rats
Rats were anesthetized with 10% chloral hydrate (0.1 mL/100 g, i.p. injection). Body temperature was monitored throughout the surgery using a rectal probe and maintained at 37.0 ± 0.5°C by a heating pad. Permanent cerebral ischemia was induced by occlusion of the left middle cerebral artery using the intraluminal filament technique (Takaba et al., 2004). The left common carotid artery, external carotid artery, and internal carotid artery were isolated through a midline cervical skin incision under a microscope. A nylon monofilament (diameter of 220 μm) coated with thermomelting glue (4 mm in length and 280 μm in diameter) was introduced through an arteriotomy performed on the external carotid artery, and it was placed into the internal carotid artery. Occlusion of the middle cerebral artery was controlled by monitoring the cerebral blood flow in the middle cerebral artery territory using a laser Doppler flowmetry for 5 minutes after the insertion of the filament. Rats with less than a 50% drop in blood flow were excluded from the study. After surgery, the wound was sutured and rats were returned to their cage, which was maintained at 25°C, with free access to food and water. Sham-operated rats were subjected to the same surgical procedure, except that the filament was not advanced to occlude the middle cerebral artery.
Characterization of the ischemic core and penumbra in rats
The ischemic penumbra was defined as the ischemic region protected by PROG 2 days after stroke, and the ischemic core was designated as the infarct region (Figure 1).
Figure 1.
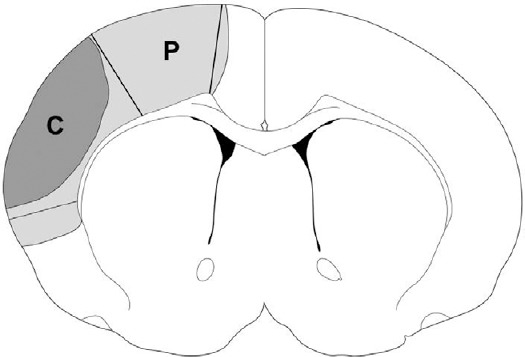
Characterization of the ischemic core and the penumbra.
The light gray region (P) plus the dark gray region (C) represents ischemic injury in a rat with ischemia alone. The P region spared by progesterone was defined as the penumbra, and the C region was defined as the ischemic core.
Evaluation of BBB integrity
BBB integrity was studied using Evans blue dye in a spectrophotometric assay (Yang et al., 2007). The time course of BBB leakage in rats receiving ischemia was studied 6, 24, and 48 hours post-stroke onset. The effect of PROG on BBB was examined 24 and 48 hours after stroke. Evan's blue dye (4%, 2.5 mL/kg) was injected intravenously into rats, which were then perfused with heparinized saline solution 2 hours later. The rat brains were then harvested, and the ischemic core and penumbra in the ipsilateral hemisphere and the contralateral cortex were dissected, weighed, homogenized, and incubated in 500 μL formamide at 54°C for 2 hours. The solution was centrifuged (12,000 × g for 15 minutes) and the supernatant was removed and Evan's blue extravasation was measured using a spectrophotometer (Spectro Max 340, Molecular Devices, Sunnyvale, CA, USA) at 620 nm.
RNA isolation and RT-PCR analysis
Forty-eight rats (n = 6 per group) were euthanized 48 hours after pMCAO and total RNA was extracted from the brain ischemic penumbra using the Trizol Kit (Invitrogen, Carlsbad, CA, USA). The RNA was measured and verified with an ultra-violet spectrophotometer (LASPEC, Shanghai, China). The primer sequences are shown in Table 1. RT-PCR was performed using the Takara RNA LA PCR kit (Takara, Kyoto, Japan), according to the manufacturer's instructions. Each amplification used 200 μg of total RNA, and each experiment was repeated three times. The reaction mixture was denatured at 96°C for 1 minute followed by 30 cycles for 1 minute at 94°C, 61°C, and 72°C. The final extension step occurred at 72°C for 10 minutes. The internal control, beta (β)-actin, was amplified under similar conditions. The PCR products were separated on a 0.8% agarose gel (Borunlaite, Beijing, China). Analysis was carried out using a gel imaging system (Glyko, Novato, CA, USA). The optical densities (OD) were measured as a function of the expression levels of occludin and ZO-1, which were then calculated and expressed as percentages relative to β-actin (OD of target proteins/OD of β-actin).
Table 1.
Sequences of primers and the predicted sizes of PCR products

Western immunoblot assay
Six rats from each group were euthanized 48 hours post-occlusion. The brains were carefully removed and placed in chilled saline. Total protein was isolated directly from the ischemic penumbra after cell lysis and then added to Laemmli buffer (75 mM Tris-HCl containing 2% sodium dodecyl sulfate (SDS), 10% glycerol, 2% 2-mercaptoethanol, 0.002% bromphenol blue). The samples were heated to 95°C for 10 minutes and then separated on 10% Tris/glycine/SDS acrylamide gels (Bio-Rad, Hercules, CA, USA). The proteins were subsequently transferred to polyvinylidene difluoride (Minipore, Billerica, MA, USA) membranes and blocked for 2 hours at room temperature in 5% non-fat milk. The immunoblots were incubated for 2 hours at 37°C with mouse anti-occludin or mouse anti-ZO-1, both with β-actin monoclonal antibody (serving as the control) (all at 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing (3 × with Tris-buffered saline/0.05% Tween-20), the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (1:1,000; Santa Cruz Biotechnology) for 1 hour at 37°C. The membranes were then stained with tetrazotized o-dianisidine and β-naphthyl acid phosphate and analyzed with an electrophoresis gel imaging system (Bio-Rad) to obtain OD values. The levels of occludin and ZO-1 were then expressed as percentages relative to β-actin.
Statistical analysis
All data are presented as the mean ± SD and were analyzed by one-way analysis of variance followed by the least significant difference post hoc test. Results were analyzed by SPSS Statistics 19.0 software (IBM, Armonk, NY, USA). A level of P < 0.05 was considered statistically significant.
Results
PROG reduced BBB leakage
BBB permeability was measured using Evans blue dye in a spectrophotometric assay. In the ischemic penumbra, Evans blue extravasation significantly (P < 0.05) increased as early as 6 hours after stroke and increased further (P < 0.05) at the 48-hour time point (Figure 2). However, in the ischemic core, BBB leakage reached a peak at 6 hours (i.e., no further increase in BBB leakage was detected at 24 or 48 hours). A significantly (P < 0.01) greater quantity of Evans blue dye was detected in the ischemic core compared with the ischemic penumbra (Figure 2A). PROG markedly (P < 0.01) reduced Evans blue leakage 48 hours, but not 24 hours, after stroke in the penumbra. PROG did not affect BBB leakage in the ischemic core (Figure 2B). Because the level of Evans blue dye reached its peak at 48 hours in the ischemic penumbra, this time point was chosen to measure the expression of occludin and ZO-1.
Figure 2.
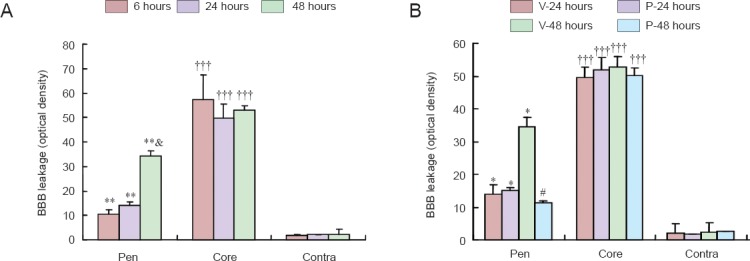
Effects of progesterone (PROG) on blood-brain barrier (BBB) leakage after permanent middle cerebral artery occlusion.
(A) Evans blue dye penetrates the ischemic brain tissue as early as 6 hours and persists up to 48 hours. More leakage occurs in the ischemic core than in the ischemic Pen. **P < 0.01, vs. corresponding Contra; †††P < 0.001, vs. corresponding Pen and Contra; &P < 0.05, vs. 6 and 24 hours in the Pen. (B) PROG reduces BBB leakage 48 hours, but not 24 hours, after stroke in the Pen. PROG does not affect BBB leakage in the ischemic core (n = 6 per time point). *P < 0.05, vs. corresponding Contra; #P < 0.05, vs. V-48 hours Pen. V: Vehicle; P: progesterone-treated; Pen: penumbra; Contra: contralateral hemisphere.
PROG inhibited the down-regulation of occludin mRNA and protein in the penumbra after pMCAO
Quantitative RT-PCR revealed that the expression of occludin mRNA was higher in the control group 48 hours after ischemia. The expression of occludin mRNA in the penumbra was significantly (P < 0.05) higher in the PROG group compared with the ischemia and vehicle groups, and was markedly (P < 0.05) lower than in the control group (Figure 3A and B).
Figure 3.
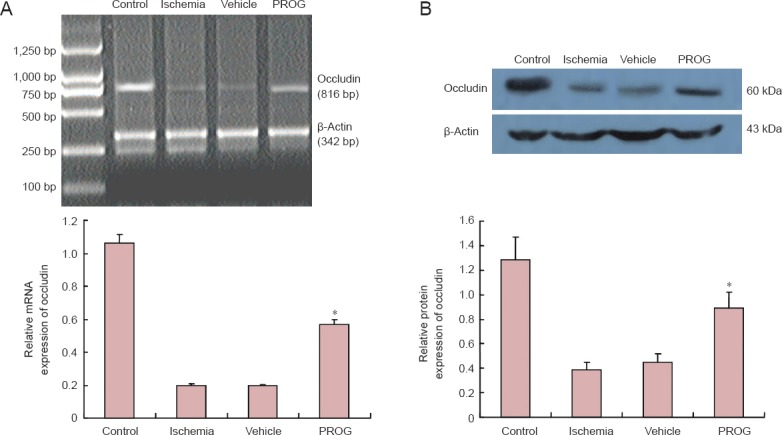
Progesterone (PROG) inhibited the down-regulation of occludin after permanent middle cerebral artery occlusion (pMCAO).
(A) Occludin mRNA extracted from the ischemic penumbra 48 hours after pMCAO (β-actin served as the internal standard). Occludin mRNA was upregulated in the PROG group compared with the ischemia and vehicle groups (n = 6 per group, *P < 0.05). (B) PROG inhibited the down-regulation of occludin protein in the penumbra after pMCAO (β-actin served as the loading control). The expression of occludined protein was represented as a ratio of the optical density of occludin/β-actin. Occludin protein was markedly upregulated in the PROG group compared with the ischemia and vehicle groups (n = 6 per group, *P < 0.05).
Western immunoblotting analysis revealed that the expression of occludin protein was very high in the penumbra of the control rats (Figure 3A and B). Furthermore, the expression of occludin was higher in PROG-treated rats compared with the ischemia- and vehicle-treated rats (P < 0.05), and was lower in the PROG group compared with the control group (P < 0.01).
PROG inhibited the down-regulation of ZO-1 mRNA and protein after pMCAO
The expression of ZO-1 mRNA decreased in the penumbra of ischemia- and vehicle-treated rats compared with the control group. In contrast, PROG markedly (P < 0.05) inhibited the down-regulation of ZO-1 mRNA 48 hours after ischemia. The low level of ZO-1 mRNA was not affected by vehicle (Figure 4A and B).
Figure 4.
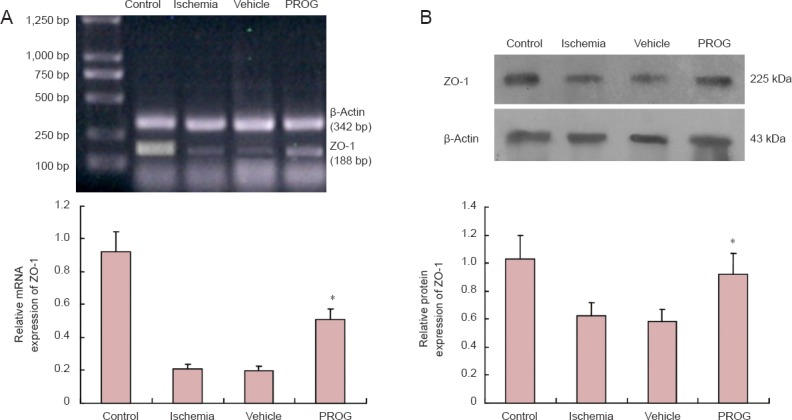
Progesterone (PROG) inhibited the down-regulation of zonula occludens-1 (ZO-1) mRNA and protein after permanent middle cerebral artery occlusion (pMCAO).
(A) High expression of ZO-1 mRNA was observed in the control group, and ischemia induced littled transcription of ZO-1 mRNA. ZO-1 mRNA was more highly expressed in the PROG group than in the ischemia and vehicle groups, and was less than that that of the control group. There was a marked up-regulation of ZO-1 mRNA compared with the ischemia and vehicle groups (n = 6 per group; *P < 0.05). (B) PROG inhibited the down-regulation of the expression of ZO-1 protein after pMCAO. There was a marked up-regulation of ZO-1 protein in the PROG group compared with the ischemia and vehicle groups (n = 6 per group; *P < 0.05).
Western immunoblotting analysis revealed that the expression of ZO-1 protein was higher (P < 0.01) in control rats (Figure 4A and B). Furthermore, ZO-1 was higher (P < 0.05) in the PROG group compared with the ischemia and vehicle groups 48 hours after ischemia, and was lower than the control group.
Discussion
Results of the present study showed that damage to the BBB in the ischemic brain occurred as early as 6 hours after injury, and this damage persisted up to 48 hours. BBB leakage was higher in the ischemic core than in the ischemic penumbra. Our findings also showed that PROG reduced BBB leakage only 48 hours after stroke in the ischemic penumbra, and did not affect BBB leakage in the ischemic core. Furthermore, PROG inhibited the down-regulation of protein and mRNA levels of occludin and ZO-1. Taken together, our findings suggest that PROG administration differentially modifies the response of the tight junction complex to ischemia in the ischemic core and ischemic penumbra. Moreover, the results indicate that PROG regulates the composition of tight junction proteins expressed in the BBB of rats.
PROG has been shown to be neuroprotective in cerebral ischemia by decreasing lesion volume and improving functional recovery (Goss et al., 2003; Sayeed et al., 2007). Moreover, PROG reduces edema (Galani et al., 2002; Guo et al., 2006) following traumatic brain injury and stroke. Further studies are necessary to elucidate the mechanism(s) by which PROG is neuroprotective against cerebral edema in rats that have undergone direct surgical middle cerebral artery occlusion
The BBB acts as a selective barrier to maintain homeostasis of the brain parenchymal microenvironment. The BBB comprises brain microvascular endothelial cells that line the cerebral capillaries and are ensheathed by astrocytic end-feet. Brain endothelial cells are distinguished from endothelial cells of other organs by inter-endothelial tight junctions and pinocytic vesicles. For many central nervous system pathologies, such as stroke, disruption of the BBB results, in part, from the structural and functional variation of tight junctions (Ballabh et al., 2004).
The physiological and pathological molecular structures of the tight junctions have been investigated. However, there is very little information about the pharmacological regulation of these complexes and/or ischemia-related alterations in their molecular composition. In vivo studies, in particular, are highly required.
Tight junctions are complexes consisting of transmembrane proteins and cytoplasmic accessory proteins linked to an actin-based cytoskeleton. Occludin is one such transmembrane protein associated with tight junctions (Kago et al., 2006) and decreased cellular permeability. Occludin has an M-shaped topology with four transmembrane domains, cytoplasmic N- and C-termini, and two extracellular loops that facilitate its performance for both signaling and structural roles at the tight junction (Blasig et al., 2011; Cummins, 2012). Occludin extends into the interendothelial space to interact with homologous segments of occludin molecules on adjacent microvascular endothelial cells. This interaction helps to fuse apposing cell membranes, thereby forming a tight seal that restricts paracellular diffusion. Occludin is capable of self-association. Occludin oligomerization is facilitated by the presence of a primary sequence motif of approximately 200 amino acids, which has a similarity to myelin, lymphocyte, and related proteins for vesicle trafficking and membrane linking (Raleigh et al., 2010; Yaffe et al., 2012). Through its C-terminus, occludin interacts with tight junction accessory proteins, such as the zonula occludens proteins (ZO-1, ZO-2, and ZO-3), which anchor multi-protein tight junction complexes to the underlying actin cytoskeleton. At different sites within the N- and C-termini and the intracellular loop that are exposed to the cytoplasm, occludin interacts with a variety of signaling, regulatory, and vesicle trafficking proteins, including numerous kinases and phosphatases, growth factor receptors, caveolin, rab13, the ubiquitin-protein ligase itch, and proteins containing a ubiquitin interacting motif (Lochhead et al., 2010; Dorfel and Huber, 2012).
ZO-1 is the most widely studied member of the cytoplasmic accessory protein family. It is crucial in stabilizing the complex structure of tight junctions. ZO-1 is a member of the membrane-associated guanylate kinase-like protein family. It interacts with both occludin and claudin, as well as actin anchoring the transmembrane proteins to the cytoskeletal scaffold of the endothelial cell. ZO-1 serves as a recognition protein for tight junction placement and as a support structure for signal transduction (Huber et al., 2001).
Previous studies of the BBB have shown that hypoxia and ischemia alter its permeability, as well as the expression, phosphorylation, and localization of various tight junction proteins (Plateel et al., 1997; Witt et al., 2003; Brown and Davis, 2005). Mechanisms underlying these changes have been suggested to include the increase in oxygen free radicals, hypoxia-inducible factor, vascular endothelial growth factor, and nitric oxide, as well as the activation of phosphokinases, nuclear factor kappa B, adenylate cyclase, and intracellular calcium pathways (Fischer et al., 1999). Other potential mechanisms include oxygen and metabolite deprivation, the cessation of shear blood flow followed by leukocyte invasion into the brain, and the induction of local inflammatory cascades (Dux et al., 1984; Fischer et al., 1999, 2004; Brown et al., 2004; Witt et al., 2005).
Overall, our findings show that PROG significantly mitigates BBB damage by blocking the down-regulation of occludin and ZO-1 after experimental stroke. This finding may therefore support the development of new therapeutic approaches directed at the BBB for stroke and other neurodegenerative disorders.
Acknowledgments
We express our thanks to Xiu-hua Ren (School of Basic Medical Sciences, Zhengzhou University, China) for her help in establishing the rat models of cerebral ischemia.
Footnotes
Funding: This study was financially supported by the National Natural Science Foundation of China, No. 81301006; a grant from Henan Provincial Scientific and Technological Research Projects of China, No. 132102310092.
Conflicts of interest: None declared.
Copyedited by Ponce-Lopez T, Wilkinson J, Mark F, Raye W, Wang J, Li CH, Song LP
References
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain:distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Krizbai IA, Bauer H, Traweger A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front Neurosci. 2014;8:392. doi: 10.3389/fnins.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21:1199–1204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15:1195–1219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol. 2004;286:C1045–1052. doi: 10.1152/ajpcell.00360.2003. [DOI] [PubMed] [Google Scholar]
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Dorfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/807356. 807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux E, Temesvári P, Joó F, Adám G, Clementi F, Dux L, Hideg J, Hossmann KA. The blood-brain barrier in hypoxia: ultrastructural aspects and adenylate cyclase activity of brain capillaries. Neuroscience. 1984;12:951–958. doi: 10.1016/0306-4522(84)90182-9. [DOI] [PubMed] [Google Scholar]
- Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol. 2004;198:359–369. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- Fleegal MA, Hom S, Borg LK, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2012–2019. doi: 10.1152/ajpheart.00495.2005. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebrale edema induced by cortical contusion in rats. Restor Neurol Neurosci. 2001;18:161–166. [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury, a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sayeed, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm Res. 2009;58:619–624. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Commun. 2006;339:1197–1203. doi: 10.1016/j.bbrc.2005.11.133. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez MJ, Mateos L, Alonso A, Wandosell F. Estradiol and progesterone administration after pMCAO stimulates the neurological recovery and reduces the detrimental effect of ischemia mainly in hippocampus. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8963-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem. 1997;68:874–877. doi: 10.1046/j.1471-4159.1997.68020874.x. [DOI] [PubMed] [Google Scholar]
- Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Fidan E, Stanley RM, Noje C, Bayir H. Progesterone for neuroprotection in pediatric traumatic brain injury. Pediatr Crit Care Med. 2015;16:236–244. doi: 10.1097/PCC.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke:neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema:treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- Sarkaki A, Farbood Y, Hashemi S, Rafiei Rad M. Pomegranate seed hydroalcoholic extract improves memory deficits in ovariectomized rats with permanent cerebral hypoperfusion/ischemia. Avicenna J Phytomed. 2015;5:43–55. [PMC free article] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Takaba H, Fukuda K, Yao H. Substrain differences, gender, and age of spontaneously hypertensive rats critically determine infarct size produced by distal middle cerebral artery occlusion. Cell Mol Neurobiol. 24:589–598. doi: 10.1023/B:CEMN.0000036400.55503.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92:203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- Wong R, Gibson CL, Kendall DA, Bath PM. Evaluating the translational potential of progesterone treatment following transient cerebral ischaemia in male mice. BMC Neurosci. 2014;15:131. doi: 10.1186/s12868-014-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe Y, Shepshelovitch J, Nevo-Yassaf I, Yeheskel A, Shmerling H, Kwiatek JM, Gaus K, Pasmanik-Chor M, Hirschberg K. The MARVEL transmembrane motif of occludin mediates oligomerization and targeting to the basolateral surface in epithelia. J Cell Sci. 2012;125:3545–2556. doi: 10.1242/jcs.100289. [DOI] [PubMed] [Google Scholar]
- Yang DY, Pan HC, Chen CJ, Cheng FC, Wang YC. Effects of tissue plasminogen activator on cerebral microvessels of rats during focal cerebral ischemia and reperfusion. Neurol Res. 2007;29:274–282. doi: 10.1179/016164107X159171. [DOI] [PubMed] [Google Scholar]


