Abstract
Both genetic and environmental factors are important in the pathogenesis of Parkinson's disease. As α-synuclein is a major constituent of Lewy bodies, a pathologic hallmark of Parkinson's disease, genetic aspects of α-synuclein is widely studied. However, the influence of dietary factors such as quercetin on α-synuclein was rarely studied. Herein we aimed to study the neuroprotective role of quercetin against various toxins affecting apoptosis, autophagy and aggresome, and the role of quercetin on α-synuclein expression. PC12 cells were pre-treated with quercetin (100, 500, 1,000 μM) and then together with various drugs such as 1-methyl-4-phenylpyridinium (MPP+; a free radical generator), 6-hydroxydopamine (6-OHDA; a free radical generator), ammonium chloride (an autophagy inhibitor), and nocodazole (an aggresome inhibitor). Cell viability was determined using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltertazolium bromide (MTT) assay. Apoptosis was detected by annexin V-fluorescein isothiocyanate and propidium iodide through the use of fluorescence activated cell sorter. α-Synuclein expression was detected by western blot assay and immunohistochemistry. The role of α-synuclein was further studied by knocking out α-synuclein using RNA interference. Cell viability increased at lower concentrations (100 and 500 μM) of quercetin but decreased at higher concentration (1,000 μM). Quercetin exerted neuroprotective effect against MPP+, ammonium chloride and nocodazole at 100 μM. MPP+ induced apoptosis was decreased by 100 μM quercetin. Quercetin treatment increased α-synuclein expression. However, knocking out α-synuclein exerted no significant effect on cell survival. In conclusion, quercetin is neuroprotective against toxic agents via affecting various mechanisms such as apoptosis, autophagy and aggresome. Because α-synuclein expression is increased by quercetin, the role of quercetin as an environmental factor in Parkinson's disease pathogenesis needs further investigation.
Keywords: quercetin, Parkinson's disease, α-synuclein, Lewy body, PC12 cells, cell viability, cell death, neuroprotection
Introduction
Both genetic and environmental factors are important in the pathogenesis of Parkinson's disease (PD) (Mullin and Schapira, 2015). A mutation on α-synuclein (aSyn) gene was first reported in familial cases of PD (Polymeropoulos et al., 1997). Moreover, abnormal inclusion of aSyn was found in the brains of both sporadic and hereditary cases of PD as an essential constituent of Lewy body (LB), a pathological hallmark of PD (Polymeropoulos et al., 1997; Spillantini et al., 1997). Thus aSyn is considered as one of the key molecules in PD and its clinical implication has been extensively studied (Cookson and Bandmann, 2010). aSyn mRNA expression was reported to be related to age, but few studies have focused on the influence of dietary factors on the expression of aSyn (Kim et al., 2004).
Inverse correlation between coffee or caffeine consumption and the risk of PD was reported in Western countries (Costa, 2010). In Asians, intake of tea was also related to lower risk of PD (Tan et al., 2003; Tanaka, 2011). (-)-Epigallocatechin-3-gallate (EGCG) is a tea flavonoid and was widely studied in the context of PD pathogenesis, demonstrating the beneficial role in experimental studies (Mandel et al., 2004). EGCG could affect remodeling of amyloid fibrils and mitigate aSyn pathology in the substantia nigra (Mandel et al., 2004; Bieschke et al., 2010).
Quercetin (Qc) is one of the most abundant flavonoids in common diet and more prevalent than EGCG but less commonly studied in association with PD pathogenesis (Mullie et al., 2007). Qc is a strong antioxidant and was widely studied to prevent oxidative stress in experimental models (Terao, 2009). Only a few studies were done in relation to PD pathogenesis, showing mild neuroprotective effect and dualistic role in which Qc was protective in early period of treatment and became harmful to the cells after prolonged exposure (Kääriäinen et al., 2008; Ossola et al., 2008). As oxidative stress is one of the leading mechanisms of cell death in PD, affecting aSyn toxicity, it would be interesting to investigate the relationship between Qc and aSyn expression.
Qc may affect other intracellular machineries such as proteasome degradation, heat shock protein expression, and apoptosis in addition to oxidative stress (Chen et al., 2005; Zanini et al., 2007). Other mechanisms such as autophagy and aggresome pathway drew the attention of the previous studies because inclusion formation of aSyn may be an essential procedure of LB (Spillantini et al., 1998; Sulzer, 2010; Wakabayashi et al., 2013). The role of Qc on these cellular mechanisms was not reported. Interestingly, a recent study showed that Qc and oxidized Qc inhibited aSyn fibrillization by the 1:1 covalent bindings of Qc with aSyn (Zhu et al., 2013).
In this study, we aimed to investigate the role of Qc on cell survival against various toxicants including the agents affecting the protein degradation pathway, which are thought to be involved in the pathogenesis of PD (Sulzer et al., 2010). We also planned to study aSyn expression by Qc and ubiquitination of aSyn because aggregated and ubiquitinated aSyn is a major component of LB (Kuzuhara et al., 1988).
Materials and Methods
Cell survival assay
Undifferentiated PC12 cells were purchased from ATCC (Manassas, VA, USA) and maintained in Dulbecco's modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA). PC12 cells were plated (3 × 104) in a 96-well plate. Cell viability was determined using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltertazolium bromide (MTT; Sigma, St. Louis, MO, USA) assay. After 24 hour treatment of Qc (Sigma) at different concentrations (100, 500, 1,000 μM), MTT solution (5 mg/mL in PBS) was added to the wells. MTT solution was removed after 3 hours of incubation and formazan precipitate was dissolved in 200 μL of dimethyl sulfoxide (DMSO; Sigma). Absorbance was measured at 540 nm (Beckman spectrophotometer; Beckman, Brea, CA, USA) using DMSO as a blank. Cell survival was expressed as the ratio of the optical density (percent survival).
To investigate mechanisms of Qc on cell survival, cells were treated for 24 hours with various toxicants such as 1-methyl-4-phenylpyridinium (MPP+; Sigma), 6-hydroxydopamine (6-OHDA; Sigma), ammonium chloride (NH4Cl, AC; Sigma), and nocodazole (Nc; Sigma) in DMEM after Qc pre-treatment for 24 hours. MPP+ (a derivative of 1-methyl-4-phenyl-1,2,3,6-tetrahydropiridine, MPTP) and 6-OHDA damage neurons via various mechanisms such as free radical generation or mitochondrial dysfunction, which are frequently used in PD studies (Singer et al., 1990; Glinka et al., 1997). AC impairs autophagy via intervening lysosomal degradation whereas Nc interferes aggresome-lysosome fusion (Yamada et al., 2012). Fixed dose of various drugs such as MPP+(0.75 mM), 6-OHDA (50 μM), AC (15 mM), Nc (100 mM) were used. z-VAD-fmk (zVf, 200 μM; Sigma) was used to rescue apoptosis after Qc treatment.
Flow cytometry
PC12 cells treated with Qc for 24 hours were harvested into tubes for flow cytometry. They were washed with phosphate buffer solution (PBS) and centrifuged for 2 minutes at 1,500 r/min. Annexin V-fluorescein isothiocyanate (AV; Sigma) was added to the cells on the ice for 30 minutes and then propidium iodide (Sigma; PI) was used. The cells stained with AV and PI were analyzed through the use of fluorescence activated cell sorter (FACS; BD Biosciencs, San Hose, CA, USA). Those stained with only AV, only PI and both AV and PI were counted, respectively. The results were expressed as fractions.
Western blot analysis
Western blot for aSyn was done at 24 hours after Qc treatment. After PC12 cells treated with Qc were harvested and washed with ice-cold PBS and protein was extracted in a lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, protease inhibitor cocktail (Sigma)]. Protein concentration was determined using bicinchoninate (BCA; Pierce, Rockford, IN, USA). Twenty micrograms of protein was loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. Incubation with primary antibodies [rabbit anti-aSyn antibody (1:1,000; Cell Signaling Technology, Beverly, MA, USA) and rabbit anti-β-actin (1:500; Sigma)] was performed overnight at 4°C. Incubation with an anti-rabbit-conjugated horseradish peroxidase antibody was performed at room temperature for 1 hour. Blots were visualized by chemiluminescence (Pierce). Band density was measured with a densitometer (GS700 model, BioRad, Hercules, CA, USA).
Immunofluorescent staining
For immunofluorescent staining, the cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature, treated with 4% normal goat serum in PBS with 0.2% Triton X-100 for 1 hour at room temperature, and then incubated with primary antibodies against aSyn (1:500) and ubiquitin (produced in mouse, 1:500; Chemicon, Temecula, CA, USA) at 4°C overnight. After washing the plates, cells were incubated with secondary antiobodies, biotinylated anti-rabbit IgG and anti-mouse IgG (1:100; Vector Laboratories, Burlingame, CA, USA) for 1 hour at room temperature. Visualization was done with Cy3-tagged streptavidin or FITC-tagged streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The images were photographed using confocal microscopy (Leica Microsystems, Wetzlar, Germany).
Silencing aSyn gene
Silencing aSyn gene (Genebank Accession No. NM 019169) was done by small interfering RNAs (siRNAs; Allele Biotechnology, San Diego, CA, USA). Target sequence was CAG TGA GGC TTA TGA AAT and siRNAs was purchased from JCBiotech (Seoul, Korea). siRNA was cloned using SilenCircle™ RNAi system (Allele Biotechnology, San Diego, CA, USA). Briefly, top and bottom oligo (1 μg/μL, respectively) was prepared and annealed in annealing buffer. For ligation, annealed insert was incubated with pre-cut vector and T4 DNA ligase in cold block (4°C) for 12 hours. E. coli competent cells were mixed with the ligation product, which was treated with heat (42°C, 45 seconds). After heat treatment, the mixture was plated in ampicillin-containing media and incubated. Colonies containing tranformed E. coli was selected and plasmids were harvested using mimi-prep kit (Agilent Technology, Santa Clara, CA, USA). Plasmids were treated with restriction enzyme and self-ligated ones were eliminated after electrophoresis in 1.5% agarose gel. Transfection was done using AvantGene™ 2 transfection reagent (Allele Biotechnology) according to the manufacturer's manual. Transfected cells were incubated for 72 hours and western blot assay was done to confirm the down-regulation of aSyn.
Statistical analysis
The results were analyzed by the SPSS 19.0 package for Windows (IBM Corp. Armonk, NY, USA). Data were presented as the mean ± SD. Analysis of variance (ANOVA) followed by post-hoc pairwise comparison (Turkey method) was done. Student's t-test was done to compare two independent samples. The chi-square test was done to test distribution of samples. The cutoff value of all the statistical analyses was set at P = 0.05.
Results
Cell survival
Qc increased cell survival at the lower concentrations (Figure 1). The neurotoxicity of Qc became evident at 1,000 μM Qc, which was reverted by a pan-caspase inhibitor zVf (Figure 1). The pattern of cell survival after co-treatment of Qc and neurotoxic drugs including AC (an autophagy inhibitor) and Nc (an aggresome inhibitor) was similar, leaving that of survival after 6-OHDA treatment exceptional (Figure 2). In the pairwise comparison, the increase in cell survival by Qc was significant in the cells treated with MPP+ and AC treatment at 100 μM (Figure 2).
Figure 1.
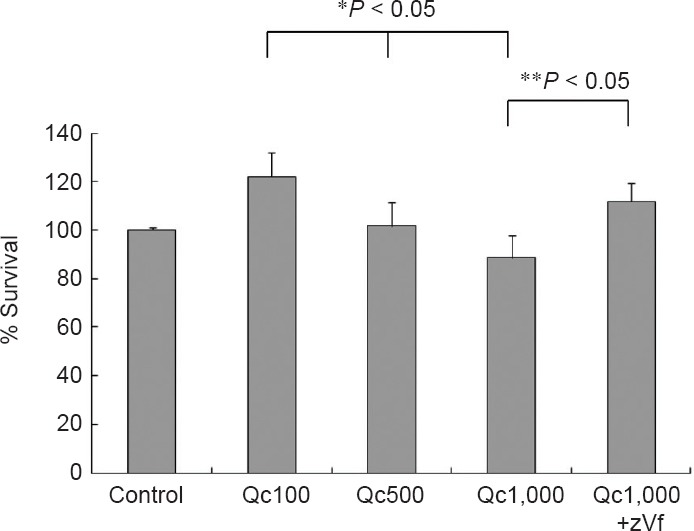
PC12 cell survival after quercetin (Qc) treatment (MTT assay).
Qc increases cell survival at lower concentrations (100 and 500 μM) and is neurotoxic at higher concentration (1,000 μM; * indicates analysis of variance). The neurotoxic effects of 1,000 μM of Qc is reversed by co-treatment of z-VDF-fmk (zVf) (**indicates Student's t-test). Data represent the mean ± SD of five independent experiments.
Figure 2.
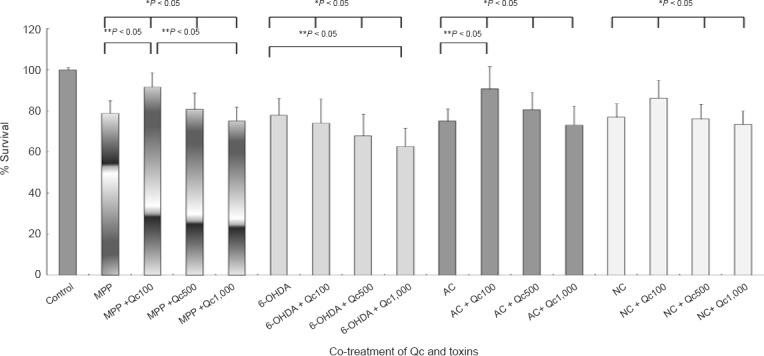
PC12 cell survival after co-treatment of quercetin (Qc) and various drugs (MTT assay).
Qc treatment enhances cell survival against 1-methyl-4-phenylpyridinium (MPP+, 0.75 mM), ammonium chloride (AC, 15 mM) and nocodazole (Nc, 100 mM). 6-Hydroxydopamine (6-OHDA, 50 μM) induced cell death is not prevented by Qc. * Indicates analysis of variance, and ** indicates post hoc analysis by Turkey method. Data represent the mean ± SD of five independent experiments.
Cell apoptosis
FACS showed decreased fraction of apoptotic cells (stained with AV) at lower concentration of Qc (100 μM) and increased at highest concentration of Qc (1,000 μM; Figure 3A). The apoptotic fraction in the cells treated with MPP+ was larger than that in those treated with 6-OHDA in the absence of Qc. Apoptotic fraction was markedly decreased when Qc was added to the cells treated with MPP+(Qc = 100 μM; Figure 3B). In the cells treated with 6-OHDA, co-treatment with Qc had no effect on apoptotic fractions (Figure 3C).
Figure 3.
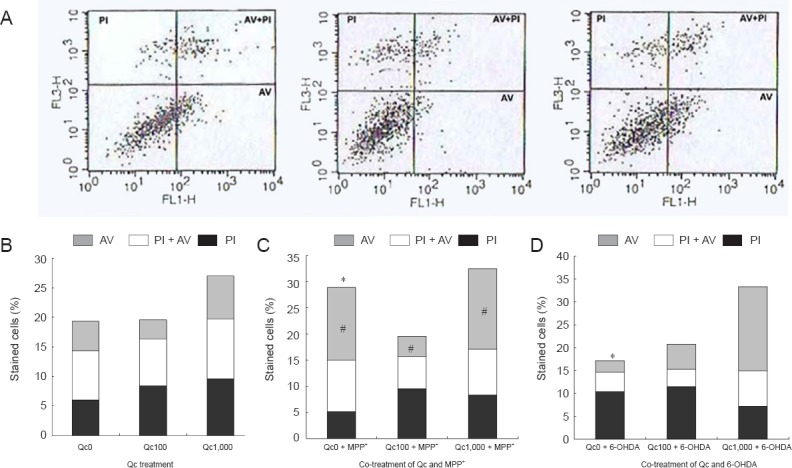
PC12 cell apoptosis after co-treatment of quercetin (Qc) and MPP+ or 6-OHDA (fluorescence activated cell sorter (FACS) analysis).
(A) A representative result of FACS. AV: Annexin V immunoreactive cells; PI: propidium iodide immunoreactive cells; AV + PI: annexin V and propidium iodide immunoreactive cells. (B) Early apoptotic fraction increases at higher concentration of quercetin (Qc) (C, D). In the baseline (Qc = 0 μM), apoptosis is more dominant with 1-methyl-4-phenylpyridinium 1-methyl-4-phenylpyridinium (MPP+) treatment than 6-hydroxydopamine (6-OHDA) (chi-square test, *P < 0.05). Co-treatment of Qc and MPP+ decreases apoptotic fraction at 100 μM of Qc (analysis of variance, #P < 0.05). In the cells treated with 6-OHDA, co-treatment of Qc has no effect. Each experiment was performed in triplicate.
aSyn expression
The expression of aSyn increased by Qc but decreased even below the expression of control at 1,000 μM of Qc (Figure 4A1, A2). aSyn was rarely co-localized with ubiquitin (Figure 4B). Western blot assay confirmed a 90 % down-regulation of aSyn using RNA silencing (Figure 5A). The survival pattern of aSyn-ko was similar to that of the wild-type, with no significant difference in the cell survival at each concentration of Qc (Figure 5B, data not shown).
Figure 4.
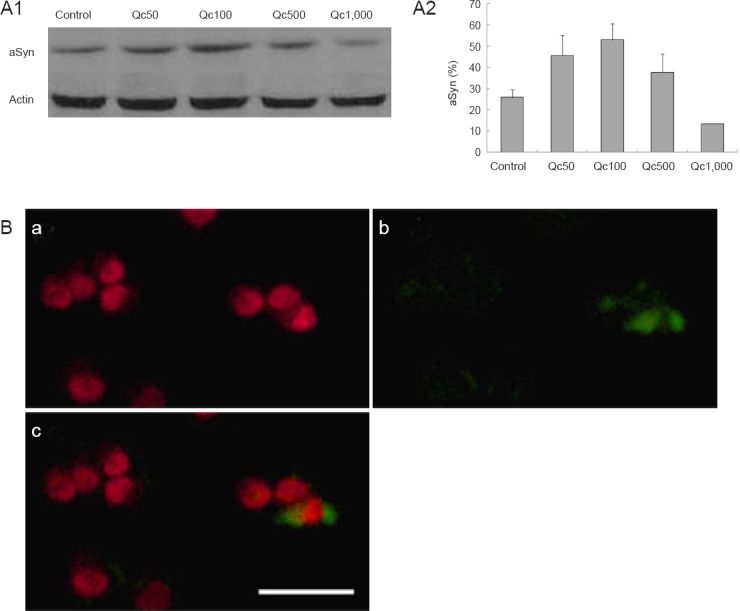
α-Synuclein (aSyn) expression in PC12 cells by quercetin (Qc).
(A) Western blot shows a constitutional expression of aSyn of control cells (A1), in which the ratio of aSyn to actin increases up to 500 μM of Qc (A2). Each experiment was performed in triplicate. (B) Cells treated with 100 μM of Qc are stained with aSyn (a) and ubiquitin (b) antibody to show rare co-localization (c). Red = aSyn; green = ubiquitin. Scale bar: 50 μm.
Figure 5.
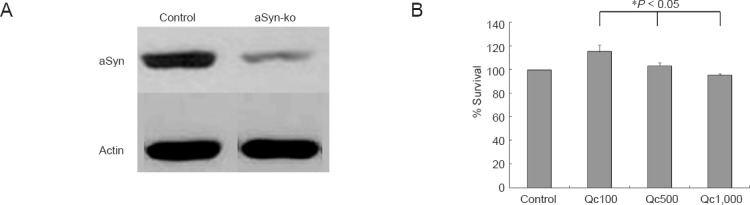
Effect of RNA silencing of α-synuclein (aSyn) on PC12 cell survival following quercetin (Qc) treatment.
(A) Western blot confirms the knockout of aSyn (aSyn-ko). (B) Cell survival after Qc treatment in aSyn-ko cell line shows similar pattern to that in the wild type cells (see Figure 1; * indicates analysis of variance). Data represent the mean ± SD of five independent experiments.
Discussion
Qc played a dual role on the survival of PC12 cells, while most previous studies only focused on either positive or negative effects of cell survival (Ansari et al., 2009; Galluzzo et al., 2009).
Cell viability was enhanced at 100 μM of Qc (Figure 1). Because apoptotic fraction decreased in the presence of 100 μM of Qc, decreased apoptosis could be a mechanism of protective role of Qc, which was suggested in the previous studies (Figure 3) (Chen et al., 2005; Zanini et al., 2007). However, increased apoptosis at higher concentration of Qc could limit the usefulness of Qc to boost cell survival (Figure 3). The neurotoxic effect of 1,000 μM Qc was abolished by a pan-caspase inhibitor zVf (Figure 1). In the previous studies, caspase-3 activity increased after long-term exposure to higher concentration of Qc while it decreased after short-term exposure (Ossola et al., 2008). Thus, caspase-dependent cascade would be critical in the toxicity of Qc.
Qc was also effective for the cell death induced by autophagy and aggresome inhibitor, with maximal effect at 100 μM of Qc (Figure 2). The advantage of Qc on autophagy has been controversial in the previous studies, whereas no study was done on the role of Qc on aggresome (Psahoulia et al., 2007; Pan et al., 2008). Our results suggested that Qc could modulate key molecular mechanisms associated with formation of aggregates (LB; Sulzer et al., 2010).
In contrast to the previous study on the role of Qc on 6-OHDA treated cells, Qc failed to increase the survival of PC12 cells treated with 6-OHDA (Figure 2) (Ossola et al, 2008). Although previous studies suggested that 6-OHDA toxicity was more closely related to apoptosis than MPP+ toxicity, FACS analysis showed that cells treated with MPP+ were more apoptotic than those with 6-OHDA in the baseline without Qc (Figure 3) (Choi et al., 1999). The apoptotic fraction associated with MPP+ treatment was successfully rescued by 100 μM of Qc, which was not observed in the cells treated with 6-OHDA. Thus, the neuroprotection by 100 μM of Qc on the cells treated with MPP+ can be closely related to reduced apoptosis (Figure 2, 3). However, the role of Qc on deceasing apoptotic fraction was lost even in the cells treated with MPP+ at higher concentration of Qc (1,000 μM), which reminded the dualistic role of Qc (Ossola et al., 2008). Aggravated apoptosis at higher concentration of Qc may be critical (Figures 1, 3A).
As the formation of aggregation of aSyn is a core feature of PD, the relationship between aSyn and Qc was studied (Polymeropoulos et al., 1997; Spillantini et al, 1997). Western blot assay showed increased aSyn expression by Qc treatment (Figure 4). Qc was previously reported to prevent fibrillation of aSyn, but up-regulation of aSyn by Qc was not reported (Zhu et al., 2013). The normal function of aSyn is not fully understood except few constitutional roles such as helping the formation of SNARE or vesicle trafficking (Chandra et al., 2005; Cooper et al., 2006). Increased expression of aSyn with beneficial role was demonstrated in the cells treated by drugs or mutant cells with duplicated aSyn gene (SNCA) (Leng et al., 2006; Kim et al., 2008). However, SNCA duplication or triplication resulted in PD and increased aSyn expression was more commonly considered as being detrimental to the cells (Singleton et al., 2003; Chartier-Harlin et al., 2004; Ibáñez et al., 2004; Sala et al., 2013). In some studies, aSyn exerted neuroprotection at nanomolar concentration but became toxic or lost its beneficial role at micromolar concentration (Seo et al., 2002; Kim et al., 2013).
In this study, the expression of aSyn remarkably increased at lower concentration of Qc (50–500 μM) but was even below the level of constitutional expression at 1,000 μM of Qc, which was a dualistic role of Qc on aSyn expression (Figure 1). As the pattern of aSyn expression after Qc treatment was similar to that of cell survival, we performed aSyn knockout by RNA interference to investigate the contribution of aSyn in cell survival (Figure 5). Down-regulation of aSyn did not affect cell survival in our experiments. These results may suggest a non-critical role of aSyn on the survival of cells treated with Qc.
Pathologic aSyn associated with LB pathology is usually modified by posttranslational mechanisms such as phosphorylation, ubiquitination or nitration (Beyer and Ariza, 2013). In ubiquitination, ubiquitin is attached to aberrant or denatured proteins and ubiquitinated proteins are degraded by proteasome (Ristic et al., 2014). To explore ubiquitination of aSyn, we performed immunofluorescent staining. The results showed only rare co-localization of aSyn and ubiquitin (Figure 4). Thus, aSyn over-expressed by Qc treatment was not ubiquitinated, which was also suggestive of non-fatal nature of increased aSyn.
Qc is ubiquitous in common diets and its bioavailability is better than previously assumed (Bischoff, 2008; Ossola, 2009). In our experiment, Qc was beneficial at acceptable concentration. Thus, although the therapeutic efficacy of Qc was questioned in some experimental models for neurodegenerative diseases, as it could touch various cellular machineries such as apoptosis, autophagy, aggresome, and aSyn expression, it is necessary to further study Qc as a significant dietary factor related to the pathogenesis of PD (Bischoff, 2008; Ossola, 2009).
Footnotes
Funding: This research was supported by a grant (03-2010-0240) from the Seoul National University Hospital Research Fund (BSJ) and Yuhan Cooperation (Seoul, Republic of Korea; TBA).
Conflicts of interest: None declared.
Copyedited by Bobrovskaya L, Fujimori K, Khan HA, Li CH, Song LP, Zhao M
References
- Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer's disease. J Nutr Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Ariza A. α-Synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol Neurobiol. 2013;47:509–524. doi: 10.1007/s12035-012-8330-5. [DOI] [PubMed] [Google Scholar]
- Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–740. doi: 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Choi WS, Yoon SY, Oh TH, Choi EJ, O’Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Bandmann O. Parkinson's disease: insights from pathways. Hum Mol Genet. 2010;19:R21–27. doi: 10.1093/hmg/ddq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20 Suppl 1:S221–238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, Marino M. Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol Nutr Food Res. 2009;53:699–708. doi: 10.1002/mnfr.200800239. [DOI] [PubMed] [Google Scholar]
- Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55–66. doi: 10.1007/978-3-7091-6842-4_7. [DOI] [PubMed] [Google Scholar]
- Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Kääriäinen TM, Piltonen M, Ossola B, Kekki H, Lehtonen S, Nenonen T, Lecklin A, Raasmaja A, Männistö PT. Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced parkinsonian models in rats and dopaminergic cell cultures. Brain Res. 2008;1203:149–159. doi: 10.1016/j.brainres.2008.01.089. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jeon BS, Yoon MY, Park SS, Lee KW. Increased expression of α-synuclein by SNCA duplication is associated with resistance to toxic stimuli. J Mol Neurosci. 2012;47:249–255. doi: 10.1007/s12031-012-9732-6. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jeon BS, Kim HJ, Ahn TB. Nanomolar concentration of alpha-synuclein enhances dopaminergic neuronal survival via Akt pathway. Neural Regen Res. 2013;8:3269–3274. doi: 10.3969/j.issn.1673-5374.2013.35.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jeon BS, Heo C, Im PS, Ahn TB, Seo JH, Kim HS, Park CH, Choi SH, Cho SH, Lee WJ, Suh YH. Alpha-synuclein induces apoptosis by altered expression in human peripheral lymphocyte in Parkinson's disease. FASEB J. 2004;18:1615–1617. doi: 10.1096/fj.04-1917fje. [DOI] [PubMed] [Google Scholar]
- Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 1988;75:345–353. doi: 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Maor G, Youdim MB. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (-)-epigallocatechin-3-gallate. J Mol Neurosci. 2004;24:401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Estimation of daily human intake of food flavonoids. Plant Foods Hum Nutr. 2007;62:93–98. doi: 10.1007/s11130-007-0047-7. [DOI] [PubMed] [Google Scholar]
- Mullin S, Schapira AH. Pathogenic mechanisms of neurodegeneration in Parkinson disease. Neurol Clin. 2015;33:1–17. doi: 10.1016/j.ncl.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Ossola B, Kääräinen TM, Raasmaja A, Männistö PT. Time-dependent protective and harmful effects of quercetin on 6-OHDA-induced toxicity in neuronal SH-SY5Y cells. Toxicology. 2008;250:1–8. doi: 10.1016/j.tox.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009;8:397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis. 2007;28:1021–1031. doi: 10.1093/carcin/bgl232. [DOI] [PubMed] [Google Scholar]
- Ristic G, Tsou WL, Todi SV. An optimal ubiquitin-proteasome pathway in the nervous system: the role of deubiquitinating enzymes. Front Mol Neurosci. 2014;7:72. doi: 10.3389/fnmol.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala G, Arosio A, Stefanoni G, Melchionda L, Riva C, Marinig D, Brighina L, Ferrarese C. Rotenone upregulates alpha-synuclein and myocyte enhancer factor 2D independently from lysosomal degradation inhibition. Biomed Res Int 2013. 2013 doi: 10.1155/2013/846725. 846725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH, Lee S, Suh SW, Suh YH. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR. Mechanism of the neurotoxicity of MPTP. An update. FEBS Lett. 1990;274:1–8. doi: 10.1016/0014-5793(90)81315-f. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Clues to how alpha-synuclein damages neurons in Parkinson's disease. Mov Disord. 2010;25 Suppl 1:S27–31. doi: 10.1002/mds.22639. [DOI] [PubMed] [Google Scholar]
- Tan EK, Tan C, Fook-Chong SM, Lum SY, Chai A, Chung H, Shen H, Zhao Y, Teoh ML, Yih Y, Pavanni R, Chandran VR, Wong MC. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese. J Neurol Sci. 2003;216:163–167. doi: 10.1016/j.jns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M. Fukuoka Kinki Parkinson's Disease Study Group (2011) Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Parkinsonism Relat Disord. 17:446–450. doi: 10.1016/j.parkreldis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Terao J. Dietary flavonoids as antioxidants. Forum Nutr. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272–280. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini C, Giribaldi G, Mandili G, Carta F, Crescenzio N, Bisaro B, Doria A, Foglia L, di Montezemolo LC, Timeus F, Turrini F. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing's sarcoma cell lines. J Neurochem. 2007;103:1344–1354. doi: 10.1111/j.1471-4159.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- Zhu M, Han S, Fink AL. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim Biophys Acta. 2013;1830:2872–2881. doi: 10.1016/j.bbagen.2012.12.027. [DOI] [PubMed] [Google Scholar]


