Abstract
Treatment for optic nerve injury by brain-derived neurotrophic factor or the transplantation of human umbilical cord blood stem cells has gained progress, but analysis by biomechanical indicators is rare. Rabbit models of optic nerve injury were established by a clamp. At 7 days after injury, the vitreous body received a one-time injection of 50 μg brain-derived neurotrophic factor or 1 × 106 human umbilical cord blood stem cells. After 30 days, the maximum load, maximum stress, maximum strain, elastic limit load, elastic limit stress, and elastic limit strain had clearly improved in rabbit models of optical nerve injury after treatment with brain-derived neurotrophic factor or human umbilical cord blood stem cells. The damage to the ultrastructure of the optic nerve had also been reduced. These findings suggest that human umbilical cord blood stem cells and brain-derived neurotrophic factor effectively repair the injured optical nerve, improve biomechanical properties, and contribute to the recovery after injury.
Keywords: nerve regeneration, optic nerve injury, human umbilical cord blood stem cells, brain-derived neurotrophic factor, biomechanical properties, neural regeneration
Introduction
The optic nerve is composed of retinal ganglion cell axons and glial cells. Optic nerve injury often leads to severe visual disorder, even blindness, but the effects of current therapies are not ideal (Johnson et al., 2010; Liu et al., 2010; Tsai et al., 2010; Wu et al., 2010; Yao et al., 2010; Zhong et al., 2010; McClenaghan et al., 2011; Zaverucha-do-Valle et al., 2011; Zhao et al., 2011; Zrenner et al., 2011; Dagnelie, 2012; Hollander et al., 2012; Jiang et al., 2012; Munemasa et al., 2012; Pascolini and Mariotti, 2012; Sui et al., 2013). Recent work on human umbilical cord blood stem cell (hUCBSC) transplantation as a treatment for optic nerve injury has shown encouraging progress. Intravitreal injection of hUCBSCs can repair optic nerve damage to some extent (Zhao et al., 2011; Jiang et al., 2013). Li et al. (2015) considered that intravitreal injection of hUCBSCs slowed down retinal ganglion cell (RGC) apoptosis in rats with traumatic optic nerve injury.
Brain-derived neurotrophic factor (BDNF) has specificity for RGC. It is a target-derived neurotrophic factor and an important axonal guidance molecule for the development of the optic nerve (Lv et al., 2009). BDNF can protect RGCs after optical nerve injury (Lv et al., 2009).
Jiang et al. (2012) believed that recombinant human ciliary neurotrophic factor combined with Chinese medicine Fuguang particles effectively lessened histological and biomechanical damage in rabbit models of optic nerve injury. It is rare to use biomechanical indicators to assess the effects of BDNF or hUCBSCs after optic nerve injury in animals. We presumed that the tensile properties of the optic nerve would alter after injury. This study investigated the neuroprotective effects of BDNF and hUCBSCs on the optic nerve by using biomechanical indicators in a rabbit model of optic nerve injury.
Materials and methods
Experimental animals
A total of 48 healthy clean male Japanese rabbits aged 5 months and weighing 2.5–2.8 kg were provided by the Changchun High-tech Medical Animal Research Center in China (license No. SCXK (Ji) 2003-0004). Animals were housed at 23–24°C and at a relative humidity of 55–70%. The house is light and airy. They were allowed free access to food and water. The experiment was approved by the Animal Ethics Committee, China-Japan Friendship Hospital, Jilin University, China.
Preparation and intervention of animal models of optic nerve injury
Animals were intraperitoneally anesthetized with 10% chloral hydrate (35 mg/kg) before experiments. Rabbits without pathological changes were included in this study after external examination of the eye and ophthalmascopic examination of the fundus. A total of 48 rabbits were randomly divided into a model group, a hUCBSC group and a BDNF group.
Models of optic nerve injury were established following the method of Jiang et al. (2012). Rabbits were anesthetized with 0.1% urethane (5 mg/kg) via the auricular vein, and fixed on the table in the prone position. An incision was made on the left orbit of the rabbit to expose the supraorbital notch and the orbital wall. The bone plate of the partial orbital wall on both sides was removed from the supraorbital notch towards the optic canal (7–8-mm depth, 6-mm width). The posterior fascia bulbi was cut, and a blunt dissection was done along the superior rectus muscle towards the posterior part of the eye to expose the optic nerve. Approximately 5 mm of the optic nerve was dissociated, and clamped with a vascular clamp for 5 seconds (equivalent to 98-g constant pressure; model: W40160; Shanghai Medical Equipment Works Co., Ltd., Shanghai, China). The surgical field was washed with gentamicin twice, and sutured with No. 10-0 nylon thread (Qingdao Nesco Medical Co., Ltd., Qingdao, Shandong Province, China). During model induction, it was necessary to avoid damage to the central retinal vessels. After the surgery, the conjunctival sac was coated with erythromycin. The fundus was observed with an ophthalmoscope (model: YZ6F; Suzhou Liuliu Technology Co., Ltd., Suzhou, Jiangsu Province, China). Rabbits without retinal vascular bleeding or infarction were considered as the successful standard. Sixteen normal eyes (right eye) from the 48 rabbits were considered as the control group.
Intervention with hUCBSCs and BDNF
hUCBSCs were incubated with 440 mL of basal medium containing 50 mL of fetal bovine serum, 5 mL of penicillin-streptomycin solution and 5 mL of glutamine (Beike Biotechnology, Shenzhen, China). At 7 days after the surgery, the vitreous body in the hUCBSC group received a one-time 50-μg injection of 1 × 106 hUCBSCs. The BDNF group received a one-time 50-μg injection BDNF into the vitreous body (Beike Biotechnology) using a microsyringe (Shanghai GAOGE Industrial and Trading Co., Ltd., Shanghai, China) (Jiang et al., 2012).
Visual electrophysiological testing
Flash visual evoked potential was measured with a visual electrophysiology instrument (model: REFZ-POZE21sompace; Roland Consult, Wiesbaden, Germany) immediately and 30 days after surgery in each group. The recording electrode was placed 1.5–2.0 cm superior to occipital protuberance, the reference electrode was placed on the center of the frontal region, and the ground electrode was placed on the earlobe. The contact lens electrode was placed on the cornea, the reference electrode was placed on the temporal orbit, and the ground electrode was placed on the earlobe. Stimulus was given with a full-field flash stimulator (model: SLS-3100; Nikon, Tokyo, Japan), two-gear flash light, 60lx, at the frequency of 2 Hz, for 250 ms.
Sample collection
A 6-mL sample of blood was obtained from the rabbit eyeball at 30 days after the surgery, maintained in air for 15 minutes and centrifuged at 2,000 × g for 20 minutes. The supernatant was collected and stored at −40°C until required. The rabbits were sacrificed by injecting gas into the auricular vein, and the optic nerve was exposed. The optic nerve was removed from the orbit, while viewed through an ophthalmoscope, and placed in physiological saline at 4°C for further use.
Determination of serum malondialdehyde (MDA) content and superoxide dismutase (SOD) activity
Serum MDA content was detected by the thiobarbituric acid method and optical density (OD) measured, in accordance with the instructions of the MDA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China).
MDA content = (ODdetected tube − ODdetected blank tube)/ODstandard tube − ODstandard blank tube) × standard concentration (10 nmol/mL) × dilution before test. SOD activity was measured by the xanthine oxidase method, using a spectrophotometer (U3410; Hitachi, Tokyo, Japan), in accordance with the instructions of the SOD kit (Nanjing Jiancheng Bioengineering Institute).
Tensile testing
An automatic control electronic universal testing machine (model: MODEL55100; Changchun Research Institute for Testing Machines, Changchun, Jilin Province, China) was used. The length and diameter of the optic nerve were measured in each group with a reading microscope (model: CCS-3; Third Optical Instrument Factory, Changchun, Jilin Province, China). The lengths of the samples of the optic nerve in each group were controlled within the range 10 ± 0.1 mm. The diameters of the optic nerve samples were 0.99–1.01 mm in the control group, 0.98–0.99 mm in the model group, 0.99–1.01 mm in the hUCBSC group and 0.98–1.02 mm in the BDNF group. To avoid entropy change or loss of mechanical energy during biomaterial deformation, preliminary adjustment was done in samples before testing. According to a previous method (Jiang et al., 2012), the loading and unloading curves overlapped after 10 cycles of preliminary adjustment. The experiment was then conducted at 36.5 ± 1.0°C. Four groups of optic nerve were separately placed in the testing machine and subjected to tension at 2 mm/min. The output by the computer gave stress and strain values and stress-strain curves automatically.
Observation under the scanning electron microscope
One optic nerve from each group was collected and trimmed into a 5-mm segment. Each of these nerve segments was pre-fixed in 4% glutaraldehyde, post-fixed in 1% osmic acid, dehydrated through a graded acetone series, dried at the critical point, and coated by vacuum spraying. The morphology of the optic nerve was observed by using the field emission scanning electron microscope (Carl Zeiss, Schwerte, Germany).
Statistical analysis
Data are expressed as the mean ± SD, and were analyzed by using SPSS 16.0 software (SPSS, Chicago, IL, USA). Intergroup difference was compared using one-way analysis of variance and Scheffe's method. A value of P < 0.05 was considered statistically significant. Unary linear regression analysis was applied to establish stress-strain function in each group.
Results
BDNF or hUCBSCs improved electrophysiological properties of injured optic nerve
Flash visual evoked potential detection results revealed that peak voltages of optic nerve were greater and peak latencies were shorter in the hUCBSC group and BDNF group than in the model group (both P < 0.05). The peak voltage of rabbit optic nerve was higher and the peak latency was shorter in the hUCBSC group than in the BDNF group (both P < 0.05; Figure 1).
Figure 1.
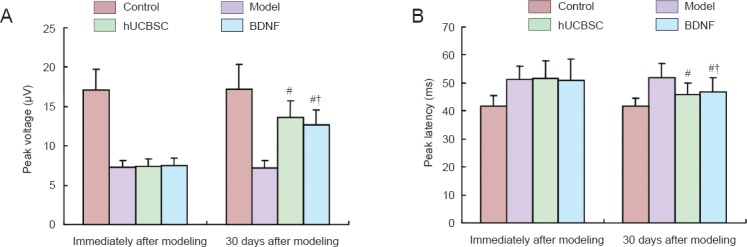
Effects of BDNF or hUCBSCs on electrophysiological properties of rabbit optic nerve.
(A) Peak voltage (μV); (B) peak latency (ms). Data are expressed as the mean ± SD, with 16 rabbits in each group. Intergroup comparison was done using the Scheffe's methods. #P < 0.05, vs. model group; †P < 0.05, vs. hUCBSC group. BDNF: Brain-derived neurotrophic factor; hUCBSC(s): human umbilical cord blood stem cell(s).
Effects of BDNF or hUCBSCs on serum SOD activity and MDA content in rabbits with optic nerve injury
Colorimetric test results demonstrated that serum SOD activity was higher, but MDA content was lower in the hUCBSC and BDNF groups than in the model group (both P < 0.05; Figure 2).
Figure 2.
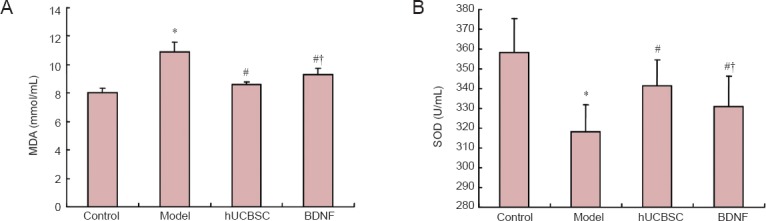
Effects of BDNF or hUCBSCs on serum MDA content (A) and SOD activity (B) in rabbits with optic nerve injury.
Data are expressed as the mean ± SD, with 16 rabbits in each group. Intergroup comparison was done using Scheffe's method. *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. hUCBSC group. BDNF: Brain-derived neurotrophic factor; hUCBSC(s): human umbilical cord blood stem cell(s); MDA: malondialdehyde; SOD: superoxide dismutase.
BDNF or hUCBSCs improved the ultrastructure of injured optic nerves
Scanning electron microscopy demonstrated uniform nuclei of optic nerve cells and distinct axons without apparent edema, swelling or inflammatory cells in the control group. In the model group, there were sparse optic nerve fibers, indistinct structures, and nuclear fragmentation. In the BDNF group, some nerve fibers on the optic cross section were distributed regularly; axonal morphology was almost normal; some glial nuclei were disordered. A small number of vacuoles, karyolysis, and optic nerve tortuosity were all visible. Nuclear fragmentation could be seen occasionally, but optic nerve axons did not become thin. In the hUCBSC group, optic nerve fibers in the cross section were densely packed; most axons were normal, and glial nuclei appeared more normal (Figure 3).
Figure 3.
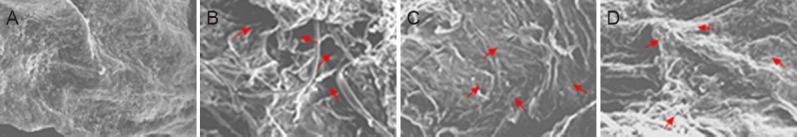
Effects of BDNF or hUCBSCs on ultrastructure of injured optic nerve (scanning electron microscope, × 100).
(A) Normal morphology of optic nerve cells in rabbits of the control group; (B) evident injury to optic nerve of rabbits in the model group; some nerve fibers were ordered on the cross section in the hUCBSC group (C) and BDNF group (D). Arrows show nerve fibers. BDNF: Brain-derived neurotrophic factor; hUCBSC(s): human umbilical cord blood stem cell(s).
BDNF or hUCBSCs improved tensile properties of injured optic nerve
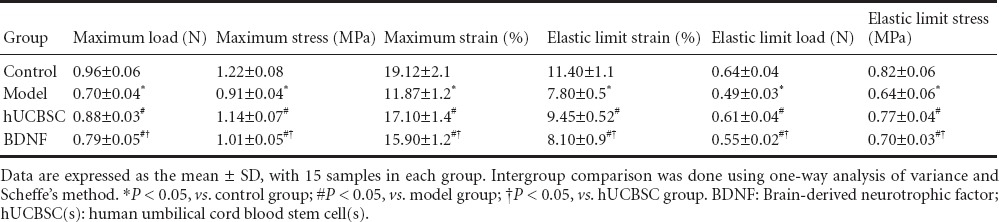
The tensile test results showed that the maximum load, maximum stress, maximum strain, elastic limit load and elastic limit stress were greater in the hUCBSC group and BDNF group than in the model group (P < 0.05). These measures were greater in the hUCBSC group than in the BDNF group (P < 0.05; Table 1).
Table 1.
Effects of BDNF or hUCBSCs on tensile properties of injured optic nerve
Curve fitting with tensile data was carried out in each group, and stress-strain curves were obtained (Figure 4). Under tensile loading, the stress-strain curves exhibited an exponential relationship, and then a non-linear relationship in each group. With increased stress, the slope of stress-strain curves increased, and the sample extended until it reached a breaking point.
Figure 4.
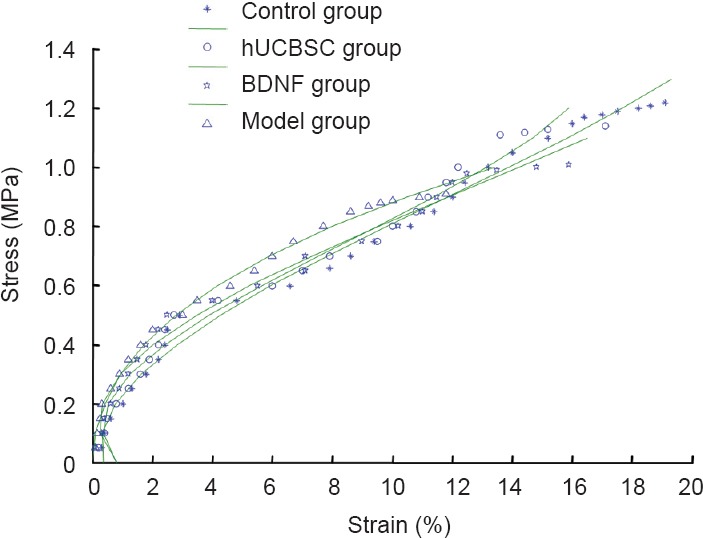
Effects of BDNF or hUCBSCs on stress-strain curves of injured optic nerve.
BDNF: Brain-derived neurotrophic factor; hUCBSC(s): human umbilical cord blood stem cell(s).
Using regression analysis, stress-strain functions in each group are as follows:
Control group:
σ(ε) = − 9.7447e5 + 25.9838e4 − 2.7340e3 + 0.3636e2
Model group:
σ(ε) = 4.9716e5 + 8.1990e4 + 0.2606e3 − 0.0094e2
hUCBSC group:
σ(ε) = −15.9661e5 + 36.2587e4 − 7.954e3 + 0.8066e2
BDNF group:
σ(ε) = −12.4019e5 + 34.7472e4 − 8.9528e3 + 0.8239e2
Discussion
Pedowitz et al. (1992) considered that electrophysiological testing is the most sensitive indicator to judge nerve injury. In this study, peak voltage was higher and peak latency was shorter in the hUCBSC and BDNF groups than in the model group. The hUCBSC group recovered peak voltage and latency better than in the BDNF group. These data indicate that optical nerve function was restored, in animal models with optic nerve injury, after treatment with hUCBSCs or BDNF, especially hUCBSCs.
SOD and MDA are important indicators reflecting free radical metabolism. A decrease in SOD activity reduces the ability to scavenge for free radicals and increased MDA content reflects enhanced lipid peroxidation. Bar-Ilan et al. (1991) confirmed that after optic nerve injury, changes to arachidonic acid metabolism can produce thromboxane A2 and prostacyclin, cause vasoconstriction, spasm and occlusion, secondary tissue ischemia and anoxia. Injury-generated free radical release induces biofilm damage and destroys biological barriers. SOD is a key antioxidase in the body and changes superoxide anion radicals into hydrogen peroxide and oxygen ions, diminishes lipid peroxidation, and protects body tissues and cells from damage. Results from the present study suggest that the ability to metabolize free radicals is enhanced in animal models with optic nerve injury after intervention with hUCBSC or BDNF.
In the BDNF group, some nerve fibers in cross sections of the optic nerves were distributed regularly; axonal morphology returned to a degree of normality; some glial nuclei were disordered. A small number of vacuoles, karyolysis and optic nerve tortuosity were visible. Nuclear fragmentation could be seen occasionally, but optic nerve axons did not become thin. In the hUCBSC group, optic nerves on the cross section were dense; most axons were normal, and glial nuclei had become more normal. The results from this study demonstrated that BDNF or hUCBSCs have some beneficial effects on the recovery of optic nerve morphology. Moreover, the effects of hUCBSCs were better than BDNF.
The tensile test results verified that structural properties of the optic nerves were weakened in the animal model of optic nerve injury but partially recovered after treatment with hUCBSCs or BDNF. All these findings support our hypothesis that hUCBSCs or BDNF promote recovery from optic nerve injury.
Footnotes
Funding: This study was supported by a grant from Science and Technology Development Program of Jilin Province of China, No. 20110492.
Conflicts of interest: None declared.
Copyedited by Dawes EA, Stow A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Bar-Ilan A, Naveh N, Weissman C, Belkin M, Schwartz M. Prostaglandin E2 changes in the retina and optic nerve of an eye with injured optic nerve. Neuroscience. 1991;45:221–225. doi: 10.1016/0306-4522(91)90118-8. [DOI] [PubMed] [Google Scholar]
- Dagnelie G. Retinal implants: emergence of a multidisciplinary field. Curr Opin Neurol. 2012;25:67–75. doi: 10.1097/WCO.0b013e32834f02c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander A, D’Onofrio PM, Magharious MM, Lysko MD, Koeberle PD. Quantitative retinal protein analysis after optic nerve transection reveals a neuroprotective role for hepatoma-derived growth factor on injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2012;53:3973–3989. doi: 10.1167/iovs.11-8350. [DOI] [PubMed] [Google Scholar]
- Jiang B, Zhang P, Zhou D, Zhang J, Xu X, Tang L. Intravitreal transplantation of human umbilical cord blood stem cells protects rats from traumatic optic neuropathy. PLoS One. 2013;8:e69938. doi: 10.1371/journal.pone.0069938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YY, Xu HT, Liu JX, Li P, Wu YZ. Biomechanical analysis of optic nerve injury treated by compound light granules and ciliary neurotrophic factor. Neural Regen Res. 2012;7:2889–2900. doi: 10.3969/j.issn.1673-5374.2012.36.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Zou Y, Hu ZL, Xiao LB, Wu M, Liu M, Chen YY, Zhang Y. Protective effects of human umbilical cord blood stem cells vitreous transplantation on function restoration after traumatic optic nerve injury. Yanke Xinjinzhan. 2015;35:125–131. [Google Scholar]
- Liu Y, Gong Z, Liu L, Sun H. Combined effect of olfactory ensheathing cell (OEC) transplantation and glial cell line-derived neurotrophic factor (GDNF) intravitreal injection on optic nerve injury in rats. Mol Vis. 2010;16:2903–2910. [PMC free article] [PubMed] [Google Scholar]
- Lv YC, Lv LQ, Lu YC. Research progress of retinal ganglion cells after optic nerve injury. Xiandai Shiyong Yixue. 2009;21:1147–1149. [Google Scholar]
- McClenaghan FC, Ezra DG, Holmes SB. Mechanisms and management of vision loss following orbital and facial trauma. Curr Opin Ophthalmol. 2011;22:426–431. doi: 10.1097/ICU.0b013e3283499420. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Chang CS, Kwong JM, Kyung H, Kitaoka Y, Caprioli J, Piri N. The neuronal EGF-related gene Nell2 interacts with Macf1 and supports survival of retinal ganglion cells after optic nerve injury. PLoS One. 2012;7:e34810. doi: 10.1371/journal.pone.0034810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Pedowitz RA, Gershuni DH, Fridén J, Garfin SR, Rydevik BL, Hargens AR. Effects of reperfusion intervals on skeletal muscle injury beneath and distal to a pneumatic tourniquet. J Hand Surg Am. 1992;17:245–255. doi: 10.1016/0363-5023(92)90400-j. [DOI] [PubMed] [Google Scholar]
- Sui X, Sun J, Li L, Zhou C, Luo X, Xia N, Yan Y, Chen Y, Ren Q, Chai X. Evaluation of a MEMS-based dual metal-layer thin-film microelectrode array for suprachoroidal electrical stimulation. IEEE Trans Neural Syst Rehabil Eng. 2013;21:524–531. doi: 10.1109/TNSRE.2012.2188042. [DOI] [PubMed] [Google Scholar]
- Tsai RK, Chang CH, Sheu MM, Huang ZL. Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res. 2010;90:537–445. doi: 10.1016/j.exer.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Wu MM, Fan DG, Tadmori I, Yang H, Furman M, Jiao XY, Young W, Sun D, You SW. Death of axotomized retinal ganglion cells delayed after intraoptic nerve transplantation of olfactory ensheathing cells in adult rats. Cell Transplant. 2010;19:159–166. doi: 10.3727/096368910X492625. [DOI] [PubMed] [Google Scholar]
- Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31:5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- Zaverucha-do-Valle C, Gubert F, Bargas-Rega M, Coronel JL, Mesentier-Louro LA, Mencalha A, Abdelhay E, Santiago MF, Mendez-Otero R. Bone marrow mononuclear cells increase retinal ganglion cell survival and axon regeneration in the adult rat. Cell Transplant. 2011;20:391–406. doi: 10.3727/096368910X524764. [DOI] [PubMed] [Google Scholar]
- Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol. 2011;249:1021–1028. doi: 10.1007/s00417-011-1635-7. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Shen X, Liu X, Cheng Y. The early effect of nerve growth factor in the management of serious optic nerve contusion. Clin Exp Optom. 2010;93:466–470. doi: 10.1111/j.1444-0938.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011;278:1489–1497. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]


