Abstract
Paired immunoglobulin-like receptor B (PirB) is a functional receptor of myelin-associated inhibitors for axonal regeneration and synaptic plasticity in the central nervous system, and thus suppresses nerve regeneration. The regulatory effect of PirB on injured nerves has received a lot of attention. To better understand nerve regeneration inability after spinal cord injury, this study aimed to investigate the distribution of PirB (via immunofluorescence) in the central nervous system and peripheral nervous system 10 days after injury. Immunoreactivity for PirB increased in the dorsal root ganglia, sciatic nerves, and spinal cord segments. In the dorsal root ganglia and sciatic nerves, PirB was mainly distributed along neuronal and axonal membranes. PirB was found to exhibit a diffuse, intricate distribution in the dorsal and ventral regions. Immunoreactivity for PirB was enhanced in some cortical neurons located in the bilateral precentral gyri. Overall, the findings suggest a pattern of PirB immunoreactivity in the nervous system after unilateral spinal transection injury, and also indicate that PirB may suppress repair after injury.
Keywords: nerve regeneration, paired immunoglobulin-like receptor B, myelin inhibitory factor, spinal cord injury, peripheral nervous system, central nervous system, cerebral cortex, dorsal root ganglion, neural regeneration
Introduction
Repair of the central nervous system (CNS) after injury is known to be difficult (Yiu and He, 2006; Filbin, 2008), and regeneration occurs after peripheral nervous system (PNS) damage (Faroni et al., 2015). Myelin-associated inhibitors exist in the nerve cell membrane, and many of these inhibitors cannot be promptly removed after CNS damage (Akbik et al., 2013; Gou et al., 2014). Myelin-associated inhibitors bind to their respective receptors on neuronal membranes (Llorens et al, 2011). Suppressing or removing these receptors does not restore the regenerative ability of nerve cells in the CNS (Llorens et al., 2011). The paired immunoglobulin-like receptor B (PirB) is an inhibitory receptor involved in immunomodulation (Takai, 2005; Filbin, 2008). Both the immune and nervous systems are important regulators of the body. The regulatory effect of PirB has therefore gradually attracted attention and thus, PirB has been shown to be an important functional receptor in axonal regeneration and a regulator of synapse (Bléry et al., 1998; Kim et al., 2004; Syken et al., 2006; Filbin, 2008; Boulanger, 2009; Darian-Smith, 2009; Nakamura et al., 2011; Wang et al., 2012). This study sought to determine the distribution and expression of PirB in injured sites of the CNS and PNS in a rat model of unilateral mechanical lumbar spinal cord injury (SCI).
Materials and Methods
Animals
Six clean male Wistar rats, aged 4–6 weeks and weighing 220–240 g were provided by the Experimental Animal Resources, Shanxi Medical University, China (license No. SCXK (Jin) 2009-0001). Rats were fed normal food and housed in a standard cage in a quiet room (prevented from sunlight and noise) under 12-hour light/dark cycles. This study has been approved by the ethics committee at Shanxi Medical University, China. All rats were equally and randomly divided into the model group (left unilateral spinal cord injury group) or sham group (uninjured group). The uninjured right spinal cord and peripheral nerve in the model group served as normal control.
Unilateral SCI
Rats were anesthetized with 1% sterile sodium pentobarbital intraperitoneally. They were then placed face down and the whole spine was exposed. After the animals were shaved (with a hair clipper) and disinfected (with iodophor and 75% alcohol), a 2–3 cm incision was made along the vertebral column longitudinally between the left upper and lower limbs. The fascia and muscle that covered the vertebral column was torn off. The intervertebral space of L3 and L4 vertebra was then located, and this depended on the left crest of ilium. The intervertebral space of T9 and T10 was then subsequently found. The sharp tips of two 1-mL syringe needles were then cut off and polished (with sandpaper). One of the needles was kept straight and the other was angled at approximately 90°, 2–3 mm from the needle tip. The needles were disinfected with 75% alcohol. Two modified needles (Figure 1) were connected to sterile syringes, separately. The straight needle was inserted into the intra-cerebrospinal space, and then vertically into the spinal cord through the intervertebral space between T9 and T10, against the left of cristae (Qi et al., 2015).
Figure 1.

One-milliliter syringe needles used in establishing the model of unilateral spinal cord injury.
The needle was pulled and the entire bent part of the needle was gently inserted into the spinal cord (toward the left). The needle was gently pulled up and down 4–5 times, and the bent needle tip was pushed to swing to the left 4–5 times and then pulled out. The muscle and skin were subsuently sutured. The rat was kept in a warm environment and the wound was cleaned everyday by inserting a straight needle through the hole with iodophor and 75% alcohol. The model of unilateral spinal cord injury was established based on the left ipsilateral loss of sensory and motor function (Yezierski, 2005; Arishima et al., 2006; Ronsyn et al., 2008). For the sham group, the operation was the same except for the syringe needle injury to the spinal cord.
Observation of general behaviors
Animals regained consciousness 1 hour after the operation and were then placed in individual cages and allowed free movement. Their daily activities and behaviors, such as drinking, eating, excretion, movement capacity, and walking posture, were observed.
Determination of sensory and motor functions
Sensory and motor functions of both hindlimbs in all rats were evaluated 1, 2, 3, and 4 weeks after injury by paw withdrawal thermal latency and holding power measurements. The rats were placed in a square box (that conducted heat) with a glass bottom for the paw withdrawal thermal latency test. Rats were in a quiet state when a light-emitting heat source was initiated to irradiate the foot-pad of rats. The time for rats to lift their foot was counted as the paw withdrawal thermal latency (marked in seconds). Only the paw withdrawal thermal latency of hindlimbs was measured in this study. For the motor function evaluation, the holding power (i.e., grasping force) of hindlimbs was measured by using a rodent animal grip strength instrument (YLS-13A; Beijing Zhishuduobao Biological Technology, Beijing, China). The power was expressed in grams.
Preparation of tissue sections
Animals were deeply anesthetized (with 1% sodium pentobarbital (0.4 mL/kg)) and then fixed with 200 mL 4% paraformaldehyde (by cardiac perfusion after 200 mL ice-cold saline perfusion). The two sides of sciatic nerves, the L3-5 DRG, and injured spinal cord segments (such as the proximal segment (0.3 mm from the center of injury site cranially), the center of injury (0.2–0.3 mm), and the distal segment (0.3 mm from the center of injury site caudally)) were harvested. The frontal cortex, precentral gyri, postcentral gyri, and cerebellum were also collected (Paxinos and Watson, 2005). The precentral and postcentral gyri were located by identifying the central sulcus. The precentral and postcentral gyri tissues were collected 0.5 mm from the central sulcus. After post-fixation (with 4% paraformaldehyde) and immersion with 30% sucrose solution overnight, the tissues were embedded in optimal cutting temperature compound and then sectioned into 12-μm-thick slices by a cryostat (Leica-CM1950, Leica Microsystem GmbH, Wetzlar, Germany).
Immunofluorescence
Sections (n = 9–10) from each sample were placed on polylysine precoated slides and then washed with 0.01 M PBS (3 × 5 minutes) and permeabilized in 0.1% Triton X-100 for 10 minutes at room temperature. Blocking with 5% normal donkey serum for 1 hour at room temperature inhibited non-specific staining. Sections were subjected to the primary antibody goat anti-PirB polyclonal antibody (1:500; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. The sections were then washed with 0.01 M PBS (3 × 10 minutes), followed by incubation with the secondary antibody donkey anti-goat IgG conjugated with Alexa Fluo-488 (1:500; Life Technologies, Shanghai, China) for 1 hour at room temperature (in the dark). Sections were then washed (3 × 5 minutes) with 0.01 M PBS. The sections were subsequently mounted with 25 μL anti-fade gold mounting medium with 4′,6-diamidino-2-phenylindole (Life Technologies). All sections were observed under an upright fluorescent microscope (Olympus, Tokyo, Japan). The presence of PirB was measured based on the optical density value determined by ImageJ software (NIH).
Statistical analysis
All data are expressed as the mean ± SEM and were analyzed by one-way analysis of variance followed by the Tukey's multiple comparison test using Prism graph-pad 5.0 (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
General behavior and motor/sensory function in rats with unilateral SCI
Before rats were subjected to the SCI or sham operation, paw withdrawal thermal latency and motor function (holding power) were both normal. All SCI rats showed paralysis of the left lower limb. The holding power of the ipsilateral limb was totally lost from day 1 up to day 10. The paw withdrawal thermal latency of the ipsilateral limb (left) was significantly (P < 0.05) protracted/lost within the maximal measurement period of 50 seconds compared with the contralateral hindlimb. Furthermore, motor/sensory function in the contralateral limb remained normal (Table 1).
Table 1.
Holding power (g) and paw withdrawal thermal latency (second) test in rat hindlimbs after unilateral spinal cord injury (SCI)
Distribution of PirB in the PNS and CNS
In the PNS, PirB was negative in the DRG neurons of noomal rats. A few PirB-positive cells were identified in the capsule of DRG, and this appeared to be non-neuronal expression. Additionally, PirB immunoreactivity was not found in a transverse section of the sciatic nerve. Compared with the PNS, some positive cells were observed in both the dorsal and ventral horn areas of the spinal cord. PirB-positive cells were mainly distributed along the meninges spinalis, and weakly positive cells were observed in the deep part of dorsal and ventral horns (Figure 2).
Figure 2.
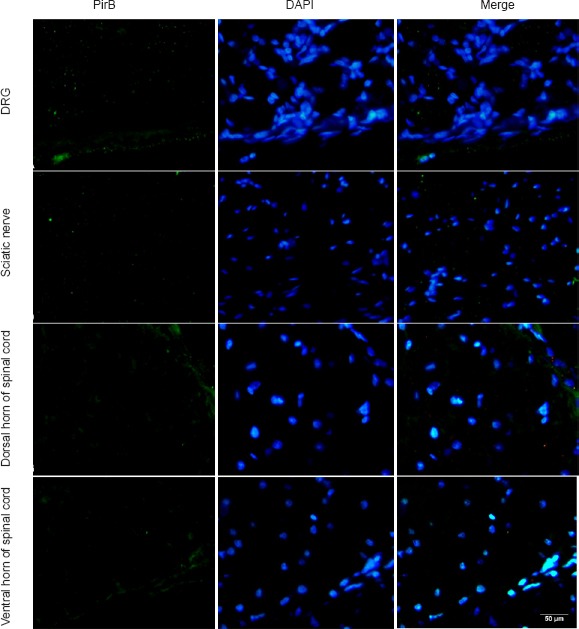
Immunofluorescence staining for PirB in the peripheral nervous system and spinal cord of normal rats.
PirB (green staining) is weakly present and scattered in the DRG and spinal cord of normal rats. Additionally, PirB immunoreactivity was not found in a transverse section of the sciatic nerve. Scale bar: 50 μm. DRG: Dorsal root ganglion; PirB: paired immunoglobulin-like receptor B; DAPI: 4′,6-diamidino-2-phenylindole.
In uninjured rats, PirB immunoreactivity was differentially distributed in the cerebellum, cortex of the frontal cerebrum, and cortex of the precentral and postcentral gyri. Compared with other brain areas, PirB immunoreactivity was distinct in the cerebellum. PirB was heavily distributed in neuronal processes instead of the cytoplasm. Moreover, PirB was diffusely distributed in both cerebellar gray and white matter. In the cortices of the precentral and postcentral gyri of the two cerebral hemispheres, PirB staining was observed in only a couple of neuronal processes. The distribution pattern of PirB staining in the cortices of both frontal cerebrum was found to be cytoplasmic and mainly distributed along the cell membrane (Figure 3).
Figure 3.
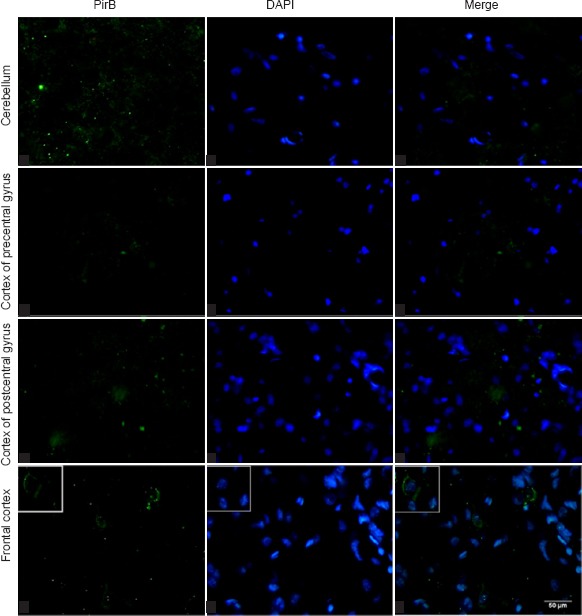
Immunofluorescence staining for PirB in different brain regions of normal rats.
PirB (green staining) is weakly present and scattered in the peripheral nervous system and spinal cord of normal rats. Scale bar: 50 μm. PirB: Paired immunoglobulin-like receptor B; DAPI: 4′,6-diamidino-2-phenylindole.
Unilateral SCI altered the expression of PirB in the spinal cord
Ten days after left unilateral SCI, cells of the ipsilateral (left) dorsal and ventral horns were positive for PirB. This was also observed in neuronal processes at the proximal, middle and distal segments of the injury site. PirB immunoreactivity on the left side of the spinal cord was observed to be stronger compared with the respective locations of the right contralateral spinal cord. Quantification of PirB immunoreactivity in the proximal, middle, and distal segments of the left (ipsilateral) dorsal horn was 2.15, 5.70, and 2.20 times stronger, respectively, than those respective contralateral segments. Compared with the contralateral segments in the ventral horns, PirB immunoreactivity was 2.94, 2.60 and 5.73 times stronger on the ipsilateral side. PirB immunoreactivity in the ventral horn was relatively stronger than that in the dorsal horn on the ipsilateral side. PirB immunoreactivity in the ipsilateral spinal cord was higher than that in the contralateral spinal cord (P < 0.05 or P < 0.001; Figure 4).
Figure 4.
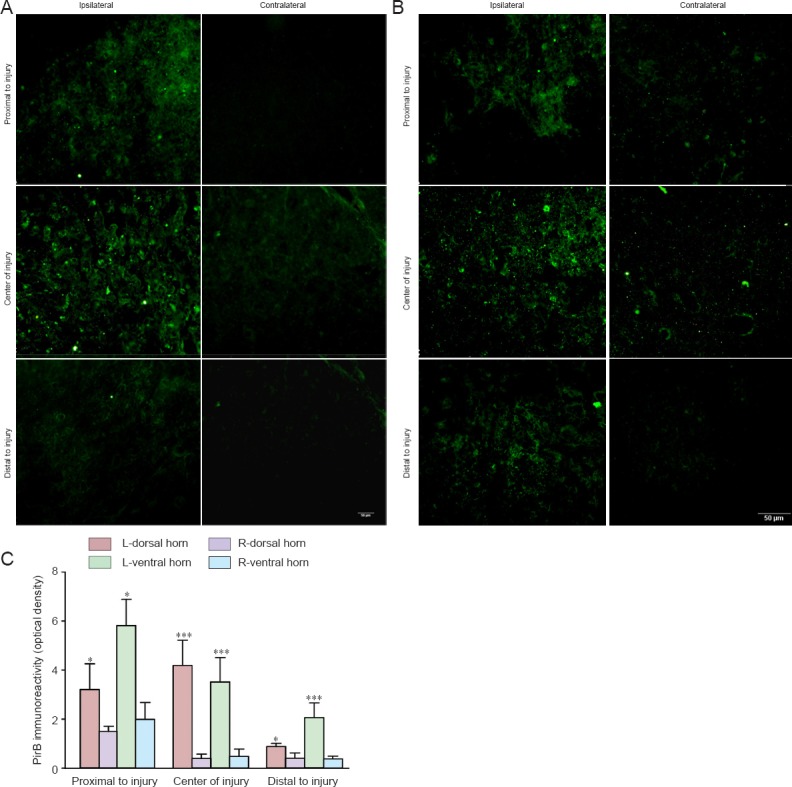
PirB immunoreactivity in the spinal cord following unilateral mechanical dissociative injury.
(A, B) PirB immunoreactivity (green staining) in the ipsilateral and contralateral dorsal and ventral horns at three selected segments (proximal side, center, and distal side). Scale bars: 50 μm. (C) Quantification of PirB immunoreactivity in the ipsilateral and contralateral dorsal horn and ventral horn of the spinal cord. Data are expressed as the mean ± SEM (3 rats per time point) and were analyzed by one-way analysis of variance followed by the Tukey's multiple comparison test. *P < 0.05, ***P < 0.001, vs. right side. L: Left, injury side; R: right, non-injured side; PirB: paired immunoglobulin-like receptor B.
PirB immunoreactivity in the dorsal or ventral horn of the contralateral spinal cord in injured rats was stronger than in sham surgery rats 10 days after injury (Figure 5). The number of PirB-positive neurons in both cortices of the left and right precentral gyri in the model group was not statistically different compared with sham surgery rats. PirB expression in the cerebellum after injury was not altered compared with sham surgery rats (data not shown).
Figure 5.
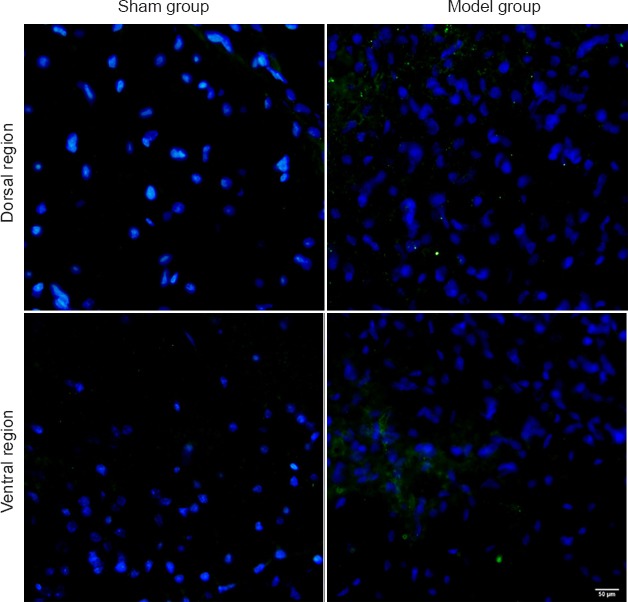
PirB immunoreactivity in the right (non-injured side) dorsal and ventral horn of rat spinal cord after injury.
PirB immunoreactivity (green) in dorsal and ventral horn of injured spinal cord is stronger than that of sham surgery rats 10 days after surgery. Scale bar: 50 μm. PirB: Paired immunoglobulin-like receptor B.
The altered expression of PirB in the PNS and CNS occurred away from the injury site
After unilateral mechanical injury, the variation in PirB immunoreactivity in ipsilateral L3–5 DRG and sciatic nerve was similar to that of the respective segments on the contralateral side (i.e., increased presence of PirB in the ipsilateral DRG and sciatic nerve). More specifically, PirB immunoreactivity in ipsilateral DRG was mainly distributed along the neuronal or axonal membrane (Figure 6). PirB immunoreactivity in the sciatic nerve was distributed along the axonal membrane. Quantification revealed that PirB immunoreactivity in the ipsilateral DRG and transverse sciatic nerve was sigificantly (P < 0.01) greater than that of the contralateral side. In the injured brain, the increase in PirB immunoreactivity and its cytoplasmic and membrane distribution pattern were observed in the cortex of the precentral gyri (Figure 6C). Interestingly, in the uninjured brain, PirB was only distributed along neuronal processes in this region (Figure 2).
Figure 6.
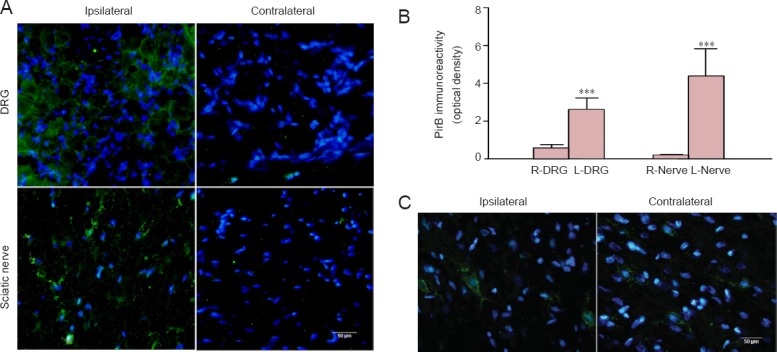
Immunoreactivity for PirB in the PNS and CNS is localized away from the injury site.
(A) PirB immunoreactivity (green) in DRG neurons and transverse sciatic nerve is mainly localized on the cellular membrane. (B) Quantification of PirB immunoreactivity in the DRG and sciatic nerve. Data are expressed as the mean ± SEM (3 rats per time point) and were analyzed by one-way analysis of variance followed by the Tukey's multiple comparison test. ***P < 0.001, vs. contralateral (right). (C) PirB immunoreactivity (green) in the cortex of the bilateral precentral gyri. Scale bars: 50 μm. DRG: Dorsal root ganglion; PirB: paired immunoglobulin-like receptor B.
Discussion
Myelin-associated inhibitors may play an inhibitory role in axonal regrowth after CNS injury because of myelin-associated inhibitor receptors on the neuronal membrane (Lopez et al., 2011). In addition to the Nogo receptor-p75 neurotrophin receptor Lingo/Troy receptor complex, PirB has been identified as the functional receptor for myelin-associated inhibitors (Kim et al., 2004; Syken et al., 2006; Filbin, 2008; Adelson et al., 2012). Increased expression of PirB at the injury site inhibits nerve regeneration in the brain after hypoxic-ischemic cerebral damage (Adelson et al., 2012; Wang et al., 2012; Wang and Zhi, 2014). However, the total expression pattern of PirB after injury in specific brain regions has not been clearly described. We therefore investigated the distribution and level of expression of PirB in the rat PNS and CNS after 10 days of left SCI. The results showed that PirB expression at the injury site was increased, and this finding corroborates with previous studies (Zhou et al., 2010; Wang et al., 2012; Gou et al., 2014; Israelsson et al., 2014). The present study also showed that the increased expression of PirB occurred remotely from the injury site, but its expression was still relevant to the site of injury based on the anatomical structure or physiological co-relationship. Our findings revealed that the altered expression occurred in many regions except the injury site. The modulation of PirB expression in remote locations after unilateral injury may be indicative of intracellular signaling occurring antegradely and/or retrogradely. These pathways may interfere with the functional status of the CNS after injury.
PirB has also been described and identified on cells of the immune system (Kubagawa et al., 1999; Takai and Ono, 2001; Uehara et al., 2001; Masuda et al., 2005; Nakayama et al., 2012). Importantly, PirB is a dual functional molecule for brain and immunity (Boulanger et al., 2001; Nakamura et al., 2004; Syken et al., 2006). PirB is involved in normal brain development, synaptic plasticity, and neurodegeneration (Llorens et al., 2011; Mironova and Giger, 2013). The intracellular mechanism of PirB has been shown to be similar in both immune and nervous systems. Neon light signaling for T cells has helped to understand the intracellular effects when major histocompatibility complex proteins (such as PirB) communicate with specific peptides/proteins on its cell surface (Takai et al., 2011). Major histocompatibility complex expression on the cell membrane initiates T-cell binding for immune signaling activation (Imada et al., 2009). In the nervous system, PirB mediates inhibitory effects mainly on neutrophils and macrophages (cells that infiltrate the nervous tissue) (Pereira et al., 2004), and also initiates an inhibitory effect by binding to myelin-associated inhibitors on neuronal membranes (Atwal et al., 2008; Filbin, 2008). However, the expression and spatial and developmental regulation of PirB in some subsets of neurons strongly suggests that PirB is involved in neuronal functions under both physiological and pathophysiological conditions (Boulanger, 2009; VanGuilder Starkey et al., 2012). In addition to its inhibitory role in axonal regeneration after CNS injury, PirB has multiple effects on the structure and function of the CNS by interacting with myelin-associated inhibitors. These effects include the restriction development and neuroprotection after stroke (Llorens et al., 2011; Adelson et al., 2012), neuronal degeneration, and changes in synaptic structures in Alzheimer's disease (Djurisic et al., 2013; Kempf and Schwab, 2013; Kim et al., 2013).
A structural or functional relationship is likely to exist between the NgR-P75NTR-Lingo/Troy complex and PirB (Filbin, 2003). PirB-mediated inhibition of axonal re-growth has been thought to be due to the inhibition of Trk by the tyrosine phosphatase SHP-2 (Maeda et al., 1998; Fujita et al., 2011b). Binding of myelin-associated inhibitors to the NgR-P75NTR-Lingo/TROY receptor complex initiates the axonal regrowth inhibitory pathways, and has recently been shown to be directly or indirectly related to the Rho-associated coiled-coil containing protein kinase and myosin light chain signaling pathways (Peng et al., 2011; Rolando et al., 2012). SHP-2 is a novel regulator of RhoA isoenzyme RhoGAP in cultured vascular smooth muscle cells (Bregeon et al., 2009; Kimura and Eguchi, 2009). RhoA is activated by SHP-2 via RhoA phosphorylation, and therefore, PirB interacts synergistically with NgR-P75NTR-Lingo/TROY (Llorens et al., 2011; Mironova and Giger, 2013). Moreover, PirB itself inhibits the Rho-ROCK signaling pathway (Wang et al., 2012). Three myelin-associated inhibitors have been shown to lose their efficacy for inhibiting axonal regeneration when their respective receptor complex is neutralized or eliminated (Zheng et al., 2005; Fujita et al., 2011a). In the current study, immunoreactivity for PirB was found to be weak in some specific regions in the PNS and CNS of the sham group. The distribution pattern of PirB in the DRG and sciatic nerve appeared to be non-neuronal, but there may have been a neuronal distribution in the cerebral cortex. However, the results did not provide information about the PirB-positive component. Therefore, more details about the distribution pattern of PirB in the nervous system under normal conditions need further verification. After unilateral mechanical lumbar injury in our study, the expression of PirB was activated in selective regions of the PNS and CNS. Remarkably, immunofluorescence staining for PirB was enhanced at the ipsilateral spinal cord 10 days after left SCI. PirB staining in all of the proximal, central, and distal segments of the left spinal cord, including the dorsal horn and ventral horn, was more diffuse and stronger compared with the contralateral segments. PirB immunoreactivity was strongest in the center of the injury. Moreover, the morphology of PirB-positive cells appeared neuron-like in the PNS and CNS. However, the temporal and spatial pattern of PirB immunoreactivity at the injury site in the relevant locations to injury could not be concluded from this study. The degradation of myelin may have occurred during SCI. Therefore, the high level of PirB immunoreactivity at the injury site may have been a cellular response to the local accumulation of myelin-associated inhibitors, and it may have also been activated by these inhibitors.
Myelin-associated inhibitors originate from local injury in the PNS and are quickly engulfed by phagocytes that infiltrate the injury area (Ma et al., 2011). Therefore, the high immunoreactivity level of PirB in the PNS after unilateral SCI in this study should be interpreted differently. Therefore, the stronger expression of PirB in the ipsilateral DRG and sciatic nerve after left SCI in this study represents a signaling pathway from the CNS to the PNS. This can be hypothesized because of the afferent terminals of DGR neurons that extend into the dorsal horn of the spinal cord, and the efferent processes of ventral motors in the spinal cord that are part of the sciatic nerve.
In the present study, the sensory and motor center in the cortex of the bilateral precentral and postcentral gyri showed different expression patterns of PirB after left unilateral SCI. The increased expression of PirB on some neuronal membranes was observed in the cortex of the bilateral precentral gyri but not in postcentral gyri. This finding may suggest that the signal for PirB activation can spread to the bilateral locomotor centers through information exchange at different neural ascending pathways by synapses. The message may then reach both sides of the motor center. Nevertheless, the negative reactivity of PirB in the sensory center that we observed was not expected. The accuracy of the anatomical location, temporal transport of PirB from the spinal cord to the center, and the severity of spinal dorsal horn injury may be the reasons for the lack of immunoreactivity. The expression of PirB at different locations in the CNS at different time periods of injury deserves further investigation.
The study was unable to provide more detailed information about how local PirB-related signals at the injury site are involved in retrograde and anterograde transport to the remote and relevant areas of the PNS and CNS. Furthermore, intracellular signal modulation of PirB and its effect on modulating the local environment after injury to the CNS needs further investigation.
In summary, the increased expression of PirB at the injury site and at specific neural afferent and efferent pathways suggests the involvement of myelin-associated inhibitors and their receptors for the CNS during injury.
Footnotes
Funding: This project was supported by the National Natural Science Foundation of China, No. 81171178; the Natural Science Foundation of Shanxi Province in China, No. 2012011036-3; the Research Project of Shanxi Scholarship Council of China, No. 2012-047.
Conflicts of interest: None declared.
Copyedited by Mark F, Raye W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giffard RG. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arishima Y, Setoguchi T, Yamaura I, Yone K, Komiya S. Preventive effect of erythropoietin on spinal cord cell apoptosis following acute traumatic injury in rats. Spine. 2006;31:2432–2438. doi: 10.1097/01.brs.0000239124.41410.7a. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Bléry M, Kubagawa H, Chen CC, Vély F, Cooper MD, Vivier E. The paired Ig-like receptor PIR-B is an inhibitory receptor that recruits the protein-tyrosine phosphatase SHP-1. Proc Natl Acad Sci U S A. 1998;95:2446–2451. doi: 10.1073/pnas.95.5.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Huh GS, Shatz CJ. Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol. 2001;11:568–578. doi: 10.1016/s0959-4388(00)00251-8. [DOI] [PubMed] [Google Scholar]
- Bregeon J, Loirand G, Pacaud P, Rolli-Derkinderen M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2009;297:C1062–1070. doi: 10.1152/ajpcell.00174.2009. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hübener M, Shatz CJ. PirB regulates a structural substrate for cortical plasticity. Proc Natl Acad Sci U S A. 2013;110:20771–20776. doi: 10.1073/pnas.1321092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliver Rev. 2015;82-83C:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Filbin MT. PirB, a second receptor for the myelin inhibitors of axonal regeneration Nogo66, MAG, and OMgp: implications for regeneration in vivo. Neuron. 2008;60:740–742. doi: 10.1016/j.neuron.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011a;30:1389–1401. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Takashima R, Endo S, Takai T, Yamashita T. The p75 receptor mediates axon growth inhibition through an association with PIR-B. Cell Death Dis. 2011b;2:e198. doi: 10.1038/cddis.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Mi Y, Jiang F, Deng B, Yang J, Gou X. PirB is a novel potential therapeutic target for enhancing axonal regeneration and synaptic plasticity following CNS injury in mammals. J Drug Target. 2014;22:365–371. doi: 10.3109/1061186X.2013.878939. [DOI] [PubMed] [Google Scholar]
- Imada M, Masuda K, Satoh R, Ito Y, Goto Y, Matsuoka T, Endo S, Nakamura A, Kawamoto H, Takai T. Ectopically expressed PIR-B on T cells constitutively binds to MHC class I and attenuates T helper type 1 responses. Int Immunol. 2009;21:1151–1161. doi: 10.1093/intimm/dxp081. [DOI] [PubMed] [Google Scholar]
- Israelsson C, Flygt J, Åstrand E, Kiwanuka O, Bengtsson H, Marklund N. Altered expression of myelin-associated inhibitors and their receptors after traumatic brain injury in the mouse. Restor Neurol Neurosci. 2014;32:717–731. doi: 10.3233/RNN-140419. [DOI] [PubMed] [Google Scholar]
- Kempf A, Schwab ME. Nogo-A represses anatomical and synaptic plasticity in the central nervous system. Physiology (Bethesda) 2013;28:151–163. doi: 10.1152/physiol.00052.2012. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Eguchi S. Angiotensin II type-1 receptor regulates RhoA and Rho-kinase/ROCK activation via multiple mechanisms. Focus on “Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells”. Am J Physiol Cell Physiol. 2009;297:C1059–1061. doi: 10.1152/ajpcell.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa H, Chen CC, Le Hong H, Shimada T, Gartland L, Mashburn C, Uehara T, Ravetch JV, Cooper MD. Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B. J Exp Med. 1999;189:309–318. doi: 10.1084/jem.189.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens F, Gil V, del Río JA. Emerging functions of myelin-associated proteins during development, neuronal plasticity, and neurodegeneration. FASEB J. 2011;25:463–475. doi: 10.1096/fj.10-162792. [DOI] [PubMed] [Google Scholar]
- Lopez PH, Ahmad AS, Mehta NR, Toner M, Rowland EA, Zhang J, Doré S, Schnaar RL. Myelin-associated glycoprotein protects neurons from excitotoxicity. J Neurochem. 2011;116:900–908. doi: 10.1111/j.1471-4159.2010.07069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Pan PY, Eisenstein S, Divino CM, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J Exp Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Kubagawa H, Ikawa T, Chen CC, Kakugawa K, Hattori M, Kageyama R, Cooper MD, Minato N, Katsura Y, Kawamoto H. Prethymic T-cell development defined by the expression of paired immunoglobulin-like receptors. EMBO J. 2005;24:4052–4060. doi: 10.1038/sj.emboj.7600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YA, Giger RJ. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci. 2013;36:363–373. doi: 10.1016/j.tins.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kobayashi E, Takai T. Exacerbated graft-versus-host disease in Pirb -/- mice. Nat Immunol. 2004;5:623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Kurokawa K, Nakamura K, Lee BL, Sekimizu K, Kubagawa H, Hiramatsu K, Yagita H, Okumura K, Takai T, Underhill DM, Aderem A, Ogasawara K. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J Immunol. 2012;189:5903–5911. doi: 10.4049/jimmunol.1201940. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Peng X, Kim J, Zhou Z, Fink DJ, Mata M. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J Neurochem. 2011;119:1183–1193. doi: 10.1111/j.1471-4159.2011.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S, Zhang H, Takai T, Lowell CA. The inhibitory receptor PIR-B negatively regulates neutrophil and macrophage integrin signaling. J Immunol. 2004;173:5757–5765. doi: 10.4049/jimmunol.173.9.5757. [DOI] [PubMed] [Google Scholar]
- Qi C, Li XQ, Zhang H. New model for lateral lumbar spinal cord injury in rats. Zhongguo Yixue Chuangxin. 2015;12:14–17. [Google Scholar]
- Rolando C, Parolisi R, Boda E, Schwab ME, Rossi F, Buffo A. Distinct roles of Nogo-a and Nogo receptor 1 in the homeostatic regulation of adult neural stem cell function and neuroblast migration. J Neurosci. 2012;32:17788–17799. doi: 10.1523/JNEUROSCI.3142-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsyn MW, Berneman ZN, Van Tendeloo VFI, Jorens PG, Ponsaerts P. Can cell therapy heal a spinal cord injury? Spinal Cord. 2008;46:532–539. doi: 10.1038/sc.2008.13. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takai T, Ono M. Activating and inhibitory nature of the murine paired immunoglobulin-like receptor family. Immunol Rev. 2001;181:215–222. doi: 10.1034/j.1600-065x.2001.1810118.x. [DOI] [PubMed] [Google Scholar]
- Takai T, Nakamura A, Endo S. Role of PIR-B in autoimmune glomerulonephritis. J Biomed Biotechnol 2011. 2011 doi: 10.1155/2011/275302. 275302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Bléry M, Kang DW, Chen CC, Ho LH, Gartland GL, Liu FT, Vivier E, Cooper MD, Kubagawa H. Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B. J Clin Invest. 2001;108:1041–1050. doi: 10.1172/JCI12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder Starkey HD, Van Kirk CA, Bixler GV, Imperio CG, Kale VP, Serfass JM, Farley JA, Yan H, Warrington JP, Han S, Mitschelen M, Sonntag WE, Freeman WM. Neuroglial expression of the MHCI pathway and PirB receptor is upregulated in the hippocampus with advanced aging. J Mol Neurosci. 2012;48:111–126. doi: 10.1007/s12031-012-9783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhi MD. Neuroregeneration of newborn rats with hypoxic-ischemic brain damage following antibody-mediated neutralization of paired-immunoglobulin-like receptor B. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:67–72. [PubMed] [Google Scholar]
- Wang H, Xiong Y, Mu D. PirB restricts neuronal regeneration in developing rat brain following hypoxia-ischemia. Mol Med Rep. 2012;6:339–344. doi: 10.3892/mmr.2012.907. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Spinal cord injury: a model of central neuropathic pain. Neurosignals. 2005;14:182–193. doi: 10.1159/000087657. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qian R, Rao J, Weng M, Yi X. Expression of PirB in normal and injured spinal cord of rats. J Huazhong Univ Sci Technolog Med Sci. 2010;30:482–485. doi: 10.1007/s11596-010-0453-1. [DOI] [PubMed] [Google Scholar]


