Abstract
In this study, we aimed to determine gastrointestinal problems associated with neurogenic bowel dysfunction in spinal cord injury patients and to assess the efficacy of bowel program on gastrointestinal problems and the severity of neurogenic bowel dysfunction. Fifty-five spinal cord injury patients were included in this study. A bowel program according to the characteristics of neurogenic bowel dysfunction was performed for each patient. Before and after bowel program, gastrointestinal problems (constipation, difficult intestinal evacuation, incontinence, abdominal pain, abdominal distension, loss of appetite, hemorrhoids, rectal bleeding and gastrointestinal induced autonomic dysreflexia) and bowel evacuation methods (digital stimulation, oral medication, suppositories, abdominal massage, Valsalva maneuver and manual evacuation) were determined. Neurogenic bowel dysfunction score was used to assess the severity of neurogenic bowel dysfunction. At least one gastrointestinal problem was identified in 44 (80%) of the 55 patients before bowel program. Constipation (56%, 31/55) and incontinence (42%, 23/55) were the most common gastrointestinal problems. Digital rectal stimulation was the most common method for bowel evacuation, both before (76%, 42/55) and after (73%, 40/55) bowel program. Oral medication, enema and manual evacuation application rates were significantly decreased and constipation, difficult intestinal evacuation, abdominal distention, and abdominal pain rates were significantly reduced after bowel program. In addition, mean neurogenic bowel dysfunction score was decreased after bowel program. An effective bowel program decreases the severity of neurogenic bowel dysfunction and reduces associated gastrointestinal problems in patients with spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, neurogenic bowel, bowel program, gastrointestinal problems, bowel evacuation, neural regeneration
Introduction
Neurogenic bowel dysfunction (NBD) is colon dysfunction due to the absence of nervous control, resulting in constipation, incontinence and discoordination of defecation. Spinal cord injury (SCI) is the most common cause of NBD. NBD is an important cause of morbidity and mortality which has a significant negative effect on the quality of life in SCI patients. Factors such as spending a lot of time for bowel evacuation, obligatory medication usage and digital stimulation for regular bowel movements, fecal incontinence risks and a constant personal help requirement are important problems which affect the quality of life of the patients (Stiens et al., 1997; Lynch et al., 2001; Lynch and Frizelle, 2006; Lisenmeyer et al., 2007). Hanson and Franklin (1976) emphasized that more than 1/3 of SCI patients report bladder and bowel dysfunctions as the primary cause which restrict their daily lives.
The main goals of NBD treatments are to achieve a regular and efficient bowel evacuation as much as possible, to prevent incontinence and complications of NBD and also to increase individual participation in social life (Stiens et al.,1997; Correa and Rotter, 2000; Inanır, 2004). Bowel program is the treatment plan to achieve these goals. Components of the bowel program are diet, physical activity, oral medication, rectal medication and bowel care (Stiens et al.,1997; Correa and Rotter, 2000; Ozel and Erkin, 2006; Stiens and King, 2007). Bowel care is the procedure carried out by the patients or patients’ caregivers to evacuate the stool from the colon periodically. Components of bowel care are the position, equipment, digital stimulation, abdominal massage and Valsalva maneuver which faciliates controllable defecation of the maximal stool volume in the least amount of time.
An individually developed bowel program at the earliest time after injury is very important for prevention of short- and long-term complications of NBD (Stiens et al., 1997; Özel and Erkin, 2006; Lisenmeyer et al., 2007). Most of the studies in the literature about NBD in SCI patients are related with the prevalence of gastrointestinal (GIS) problems. The prevalence of GIS problems in SCI patients is reported to be 27–94.7% (Stone et al., 1990; Glickman and Kamm, 1996; Han et al., 1998; Demirel et al., 1999; Vallès et al., 2006). There are few studies addressing the efficiency of bowel program in functional bowel evacuation and the prevention of GIS complications (Correa and Rotter, 2000).
The main aim of the present study was to assess the efficacy of bowel program on GIS problems and on reducing the severity of NBD.
Subjects and Methods
Patients
55 traumatic SCI patients out of spinal shock who received rehabilitation program in our clinic between March 2006 and September 2008 were included in our study. Patients with known organic GIS problems or systemic disordes affecting GIS function were not included in this study. Informed consent was obtained from all patients and the study protocol was approved by the local ethics committee of our institution. Patients’age, sex, and date of injury were recorded. Neuromuscular system examination of patients was performed to determine the severity of injury according to ASIA (American Spinal Injury Association) Impairment Scale (Marino et al., 2003). Patients were divided into two groups according to their injury severity as motor complete (ASIA A, B) and motor incomplete (ASIA C, D) (Valles et al, 2006). Anal examinations (anal sensation, anal tone, anal view, hemorrhoids, anal bleeding) of patients were performed.
Assessment of GIS problems
Chronic GIS problems after SCI (constipation, difficult intestinal evacuation (DIE), incontinence, abdominal pain, abdominal distension, loss of appetite, hemorrhoids, rectal bleeding and GIS induced autonomic dysreflexia) were questioned before bowel program. Significant chronic gastrointestinal symptoms were defined as the GIS induced symptoms that affect the quality of life and need chronic treatment (Stone et al., 1990). Fecal incontinence was defined as unplanned and unwanted defecation happening outside of bowel maintenance periods (Inanır et al., 1999). Rome II criteria were used to define constipation and having at least 2 of the following symptoms was considered as constipation: (a) straining in more than one fourth of defecations, (b) lumpy or hard stools in more than one fourth of defecations, (c) sensation of incomplete evacuation in more than one fourth of defecations, (d) sensation of anorectal obstruction/blockage in more than one fourth of defecations, (e) manual maneuvers to facilitate defecation in more than one fourth of defecations, and (f) less than three defecations per week (Rasquin et al., 2006). DIE was defined as the presence of two or more of the following conditions: (a) defecation frequency less than three times a week, (b) hard stools, (c) prolonged intestinal management time more than 45 minutes (Correa and Rotter, 2000). GIS induced autonomic dysreflexia was defined as having symptoms such as sweating in patients following rectal distension or anal manipulations, headache, flushing, nasal congestion, blurred visions or sudden blood pressure increase (Furusawa et al., 2007).
Bowel program and evaluation of NBD
Patients were grouped as lower motor neuron (LMN) and upper motor neuron (UMN) type bowel dysfunction according to their neurological levels, anal examinations, and gastrointestinal problems. Two different bowel program protocols were performed in LMN and UMN bowel dysfunction. Patients were evaluated daily. The bowel program was finally to achieve an effective and efficient evacuation and socially acceptable period of time with an appropriate stool consistency and defecation frequency. Patients’ diet and fiber intakes were planned in accordance with their stool consistency and GIS problems by our clinic's dietician. Fluid intake of patients was increased as much as possible considering their neurogenic bladder treatment programs as well. At the beginning and end of bowel program, medications (oral laxatives, suppositories, enemas) and bowel evacuation methods (digital stimulation, abdominal massage, enema, Valsalva maneuver and manual evacuation) for bowel care used by patients were recorded. Bowel care was defined as scheduled technique for stool evacuation (Lynch et al., 2001). NBD scores of patiens were calculated. NBD score includes 10 items: (a) frequency of defecation (0–6 points), (b) time used for each defecation (0–3 points), (c) presence of headache, uneasiness or perspiration during defecation (0–2 points), (d) regular use of tablets against constipation (0–2 points), (e) regular use of drops against constipation (0–2 points), (f) digital stimulation or evacuation of the anorectum (0–6 points), (g) frequency of fecal incontinence (0–13 points), (h) medication against fecal incontinence (0–4 points), (i) flatus incontinence (0–2 points), and (j) perianal skin problems (0–3 points). Total score is between 0–47. NBD levels were defined as a score of 0-6 very minor, 7–9 minor, 10–13 moderate and >14 severe NBD (Krogh et al., 2006).
Statistical analysis
SPSS 10.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis of data. A level of P < 0.05 was considered statistically significant. Descriptive statistics were made. The chi-square test was used to compare the GIS problem rates according to the injury level. The Mc-Nemar test was used to research the difference in GIS problem rates between treatment methods used in bowel program. The Wilcoxon test was used to determine NBD score changes. The Mann-Whitney U test was used to compare the NBD score differences between patients with motor complete and motor incomplete SCI and the Spearman's correlation analysis was used to analyze the relationship between disease duration and NBD scores.
Results
Patient characteristics
42 (76%) male and 13 (24%) female patients were included in this study. The mean age of the patients was 33.01 ± 12.25 years. The mean interval since injury was 162.0 ± 110.1 (21–360) days and the mean follow-up duration was 42.3 ± 17.0 days. 37 (67%) patients had motor complete SCI and 18 (33%) patients had motor incomplete SCI. There were no significant differences in the mean of age, injury time and follow-up duration between patients with motor complete SCI and motor incomplete SCI (P > 0.005).
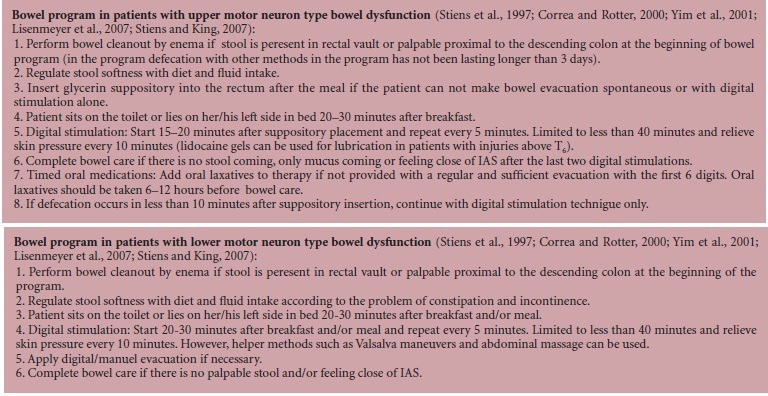
Prevalance of GIS problems
Before bowel program, 44 patients (80%) had at least one GIS complaint. Constipation (56%) and incontinence (42%) were the most common problems (Table 1). 97% of motor complete SCI patients and 44% of motor incomplete SCI patients had at least one GIS problem. Incontinence rate in motor complete SCI patients was significantly higher than in motor incomplete SCI patients both before and after the program (P < 0.05; Table 1).
Table 1.
Gastrointestinal problems in patients with motor complete versus motor incomplete spinal cord injury (SCI) before bowel program
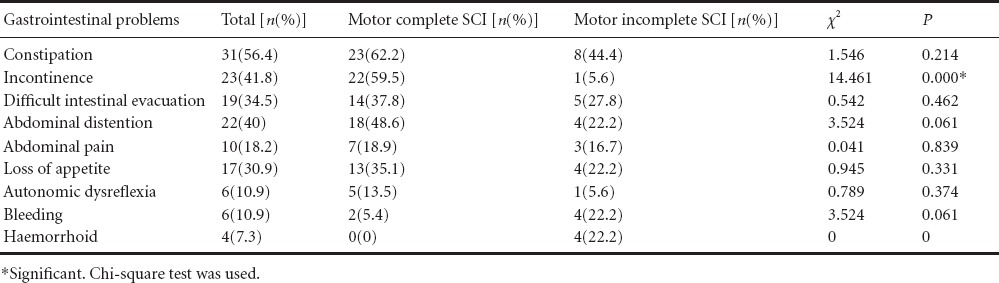
Evaluation of changes in bowel program
Digital stimulation and Valsalva maneuvers were the most common methods used by patients at the beginning (76%, 67%) and at the end (73%, 67%) of bowel program. Oral medication (P = 0.016), enema (P = 0.001) and manual evacuation (P = 0.008) application rates significantly decreased at the end of bowel program when compared to the begining of bowel program. However, no difference was found in suppository, digital stimulation, abdominal massage and valsalva maneuver application rates (P > 0.05). Digital stimulation (before bowel program) and abdominal massage (no matter before or after the bowel program) applicatioin rates were significantly higher in patients with motor complete SCI than in patients with motor incomplete SCI (P < 0.005). There were no significant differences in other methods used between patients with motor complete and imcomplete SCI (P > 0.05; Table 2). Constipation (P = 0.031), DIE (P = 0.039), abdominal distension (P = 0.004) and abdominal pain (P = 0.008) application rates were significantly decreased after bowel program. The rates of other GIS problems were slightly, but not significantly, decreased after bowel program. After bowel program, signficant decreases in constipation (P = 0.031), abdominal distension (P = 0.004) and abdominal pain rates (P = 0.031) were observed in patients with motor complete SCI, however, no significant decreases were found in patients with motor incomplete SCI (P > 0.05; Table 3).
Table 2.
Treatment methods in patients with motor complete versus motor incomplete spinal cord injury (SCI) before and after bowel program
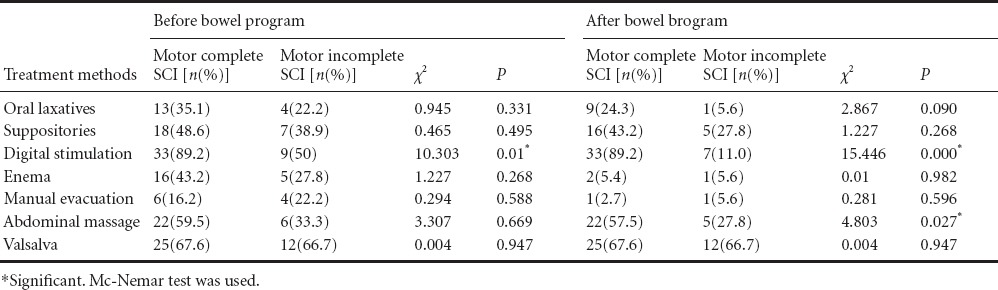
Table 3.
Changes in gastrointestinal problems in patints with motor complete and motor incomplete spinal cord injury (SCI) after bowel program
The mean NBD score in patients with motor complete SCI was significantly higher than in patients with motor incomplete SCI before (17.45 ± 6.37 vs. 8.44 ± 9.39, P = 0.001) and after (11.40 ± 3.58 vs. 5.22 ± 6.38; P = 0.000) bowel program. After bowel program, the mean NBD score was significantly decreased in both patients with motor complete (P = 0.000) and incomplete (P = 0.018) SCI patients. After bowel program, the NBD score was signficiantly reduced in patients with motor complete SCI than in patients with motor incomplate SCI (6.05 ± 4.66 vs. 3.27 ± 4.65; P = 0.017). A weak negative correlation was detected between disease duration and NBD score before (r = –0.311; P = 0.021) and after (r = –0.315; P = 0.019) bowel program. This suggests that as the disease duration increased, NBD score was signficantly decreased in patients with motor complete and motor incomplete SCI.
Discussion
Han et al. (1998) emphasized that chronic GIS problems caused by bowel dysfunction in SCI patients are quite common. However, Correa et al. (2000) reported that following SCI, an effective bowel program created by giving priority to the physiological methods can lead to better results and will also help inhibit the insufficient bowel evacuation in later term. The prevalence of NBD induced GIS problems in SCI patients was 80% in our study. The definition differences of GIS problems in SCI patients and presence of different views on planned goals of bowel programs can cause very different prevalence rates ranging from 27% to 94.7% (Stone et al., 1990; Glickman and Kamm, 1996; Demirel et al.,1999; Valles et al., 2006).
In our study, constipation and incontinence in SCI patients were the most common gastrointestinal problems both before and after bowel program (56.4%, 41.8% respectively) and 34.5% of patients had DIE at the beginning of bowel program. Han et al. (1998) also reported constipation (43.1%) as the most common GIS problem and DIE rate was 33.3% which is similar to our study results. Glickman and Kamm (1996) and Demirel et al. (1999) also reported constipation as the most commonly diagnosed GIS complaint (30% and 52% respectively). Stone et al. (1990) reported DIE as the most important complaint in SCI patients and symptoms such as abdominal bloating, abdominal distension, hemorrhoidal bleeding and autonomic dysreflexia were linked to DIE. Incontinence rates between 41–61% were reported in the previous studies (Stone et al., 1990; Glickman and Kamm, 1996; De Looze et al., 1998; Correa and Rotter, 2000; Ng et al., 2005). But Stone et al. (1990) only reported two serious incontinence cases in LMN type bowel dysfunction patients, and other patients sometimes had incontinence which did not cause too much discomfort. 97.3% of motor complete SCI patients and 54.2% of motor incomplete SCI patients had chronic GIS complaints in our study. We detected that incontinence rates were significantly higher in patients with motor complete SCI than in patients with motor incomplete SCI (Table 1). Injury level has been shown not to be related to GIS complaints in SCI patients (Han et al., 1998; Kirshblum et al., 1998). However, many studies showed a certain relationship between injury level and GIS complaints. Previous studies have shown that bowel evacuation was more irregular, bowel evacuation frequency and periods were greater and longer, and incontinence was more common in patients with complete motor SCI than in patients with motor incomplete SCI (Demirel et al., 1999; Inanır et al., 1999). Valles et al. (2006) reported that GIS complaints caused by neurogenic bowel were more often seen in patients with motor complete SCI and 67% of those patients suffered from constipation as well as 85% had some level of incontinence. Stone et al. (1990) detected no significant relation between GIS symptom prevalence and SCI level in their study. Compared to patients with motor incomplete SCI, Patients with motor complete SCI had more symptoms, 76% of DIE patients were motor complete SCI patients and 80% of them were injured at T5 and higher.
In our study, we found that the most common method used by patients was digital stimulation before (76%) and after (73%) bowel program. Both before and after bowel program, patients with motor complete SCI needed all the components of bowel care (except for manual evacuation and enema administration after rehabilitation program) more often than patients with motor incomplete SCI. There were significant differences in digital stimulation before bowel program and in digital stimulation and abdominal massage after bowel program between patients with motor complete and motor incomplete SCI. Inanir et al. (1999) reported that patients with motor complete SCI used higher rate of digital stimulation and more laxatives whereas patients with motor incomplate SCI used Valsalva maneuver more often. Lynch et al. (2000) reported higher rates of enema and manual evacuation usage in patients with motor complete SCI than in patients with motor incomplate SCI. In another study, patients with LMN lesions mostly used Valsalva maneuver and manual evacuation methods while patients with UMN lesions used suppositories (Yim et al., 2001).
Most studies on this issue are ususally performed to define the pervalence of GIS problems in SCI patients with NBD, but there are few studies which determine the efficiency of bowel program in functional bowel discharge and preventing GIS complications. In the present study, the prevalences of constipation, DIE, abdominal distension, and abdominal pain were significantly reduced in all patients after bowel program. In addition, we found that long-term use of oral laxatives, enema and manual evacuation is not desirable in bowel dysfunction treatment. Correa and Rotter (2000) detected significant decreases in manual evacuation, oral laxative usage, DIE, abdominal distension, rectal bleeding and fecal incontinence following bowel program administration. In addition, hard or lumpy stools were reduced and evacuation period was shortened in patients after bowel program.
In our study, NBD scores were decreased in all patients including motor complete and motor incomplete SCI patients after administration of bowel program. In addition, patients with motor complete SCI who had higher mean NBD scores had greater reductions in abovementioned indices after bowel program than patients with motor incomplate SCI. Before bowel program, 83.8% of motor complete SCI patients were serious according to NBD scores. This proportion was decreased to 16.2% after bowel program. This suggests that SCI patients, even suffering from serious NBD consequence, might benefit from necessary attention and care and positive outcomes can be achieved. There is evidence that 56% of patients who receive bowel program considered the program as “good and successful” and the conclusion has been shown not to be linked with disease duration or injury type (Correa and Rotter, 2000).
In our study, we detected a weak negative correlation between NBD scores and disease duration. NBD scores decreased as the disease duration increased. Some studies have shown that there is no correlation between disease duration and GIS complaints (Yim et al., 2001; Furusawa et al., 2007). Yet Stone et al. (1990) reported that chronic GIS complaints in the first 5 years of SCI were quite rare and as the disease duration increased, the frequency of GIS complaints also increased. However, the mean duration was 12.1 ± 10.1 years which is quite long when compared to that in our study. This difference might affect the results we got from our research.
Therefore, an effective bowel program is to reduce the severity of bowel dysfunction, GIS problems associated NBD score, uses of oral laxatives, enemas, and manual evaculations in SCI patients. Patients with motor complete SCI patients who have severe NBD greatly benefit from bowel program.
The rehabilitation team usually focuses on patient's mobilization function loss during the early rehabilitation stage, and symptoms connected with bowel dysfunction are not adequately questioned. Interdisciplinary rehabilitation team (physiatrist, nurse, occupational therapist, etc.) is necessary for NBD rehabilitation, but patients must take a leadership role in constructing a bowel program that incorporates a life-compatible bowel care schedule. In early rehabilitation program, our aim must be to educate patients and encourage them to construct a bowel care regimen. After creating a bowel care schedule, it should be continued without interruption. But bowel care scheme should be revised according to the needs, and arrangements should be made. A regular and effective bowel program will reduce the risks of long-term complications such as hemorrhoids and colorectal carcinoma. At the same time, regular bowel care will improve the individual's participation in social life and also quality of life. Longer-term prospective studies involving larger patient groups and a sex- and age-matched healthy control group are useful for demonstrating longer-term results of bowel program.
Footnotes
Conflicts of interest: None declared.
Copyedited by Huang MH, Huang MH, Li CH, Song LP, Zhao M
References
- Correa GI, Rotter KP. Clinical evaluation and management of neurogenic bowel after spinal cord injury. Spinal Cord. 2000;38:301–308. doi: 10.1038/sj.sc.3100851. [DOI] [PubMed] [Google Scholar]
- De Looze D, Van Laere M, De Muynck M, Beke R. Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36:63–66. doi: 10.1038/sj.sc.3100531. [DOI] [PubMed] [Google Scholar]
- Demirel G, Soy D, Öztürk Y, Başoğlu I, Yılmaz H. Spinal kord yaralanmalı hastalarda gastrointestinal sistem problemleri ve barsak fonksiyon bozuklukları. Romatol Tıp Rehab. 1999;10:186–189. [Google Scholar]
- Furusawa K, Sugiyama H, Ikeda A, Tokuhiro A, Koyoshi H, Takahashi M, Tajima F. Autonomic dysreflexia during a bowel program in patients with cervical spinal cord injury. Acta Med Okayama. 2007;61:221–227. doi: 10.18926/AMO/32867. [DOI] [PubMed] [Google Scholar]
- Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347:1651–1653. doi: 10.1016/s0140-6736(96)91487-7. [DOI] [PubMed] [Google Scholar]
- Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36:485–490. doi: 10.1038/sj.sc.3100616. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Franklin MR. Sexual loss in relation to other functional loses for spinal cord injured males. Arsh Phys Med Reahbil. 1976;57:291–293. [PubMed] [Google Scholar]
- İnanır M. Nörojenik barsak fonksiyon bozuklukları. In: Oğuz H, editor. In: Tıbbi Rehabilitasyon. Ankara, Nobel tıp kitabevi: 2004. [Google Scholar]
- İnanır M, Akyüz M, Çakcı A. Kronik medulla spinalis yaralnamalı hastalrda barsak fonsksiyon bozukluları ve barsak bakım programları. Romatol Tıp Rehab. 1999;10:190–195. [Google Scholar]
- Kirshblum SC, Gulati M, O’Connor KC, Voorman SJ. Bowel care practices in chronic spinal cord injury patients. Arch Phys Med Rehabil. 1998;79:20–23. doi: 10.1016/s0003-9993(98)90201-5. [DOI] [PubMed] [Google Scholar]
- Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- Lisenmeyer TA, Stone JM, Steins SA. Nörojenik mesane ve barsak fonksiyon bozuklukları. In: De Lisa JA, editor. In: Fiziksel Tıp Ve Rehabilitasyon: İlkeler Ve Uygulamalar. Ankara, Güneş kitabevi: 2007. [Google Scholar]
- Lynch AC, Frizelle FA. Colorectal motility and defecation after spinal cord injury in humans. Prog Brain Res. 2006;152:335–343. doi: 10.1016/S0079-6123(05)52022-3. [DOI] [PubMed] [Google Scholar]
- Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39:193–203. doi: 10.1038/sj.sc.3101119. [DOI] [PubMed] [Google Scholar]
- Lynch AC, Wong C, Anthony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury: a description of bowel function in a spinal cord-injured population and comparison with age and gender matched controls. Spinal Cord. 2000;38:717–723. doi: 10.1038/sj.sc.3101058. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. ASIA Neurological Standards Committee 2002 (2003) International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 26(Suppl 1):S50–56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Ng C, Prott G, Rutkowski S, Li Y, Hansen R, Kellow J, Malcolm A. Gastrointestinal symptoms in spinal cord injury: relationships with level of injury and psychologic factors. Dis Colon Rectum. 2005;48:1562–1568. doi: 10.1007/s10350-005-0061-5. [DOI] [PubMed] [Google Scholar]
- Ozel S, Erkin G. Nörojenik barsak fonksiyon bozukluğunda medikal ve rehabilitatif tedavi. Romatol Tıp Rehab. 2006;17:64–72. [Google Scholar]
- Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiens SA, King JC. Neurogenic bowel: Dusfuncyion and rehabilitation. In: Braddom RL, editor. In: Physical medicine & rehabilitation. Philadelpia: W.B Saunders Company; 2007. pp. 637–650. [Google Scholar]
- Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:86–102. doi: 10.1016/s0003-9993(97)90416-0. [DOI] [PubMed] [Google Scholar]
- Stone JM, Nino-Murcia M, Wolfe VA, Perkash I. Chronic gastrointestinal problems in spinal cord injury patients: a prospective analysis. Am J Gastroenterol. 1990;85:1114–1119. [PubMed] [Google Scholar]
- Vallès M, Vidal J, Clavé P, Mearin F. Bowel dysfunction in patients with motor complete spinal cord injury: clinical, neurological, and pathophysiological associations. Am J Gastroenterol. 2006;101:2290–2299. doi: 10.1111/j.1572-0241.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Vallès M, Terré R, Guevara D, Portell E, Vidal J, Mearin F. Bowel dysfunction in patients with spinal cord injury: relation with neurological patterns. Med Clin. 2007;129:171–173. doi: 10.1157/13107793. [DOI] [PubMed] [Google Scholar]
- Yim SY, Yoon SH, Lee IY, Rah EW, Moon HW. A comparison of bowel care patterns in patients with spinal cord injury: upper motor neuron bowel vs lower motor neuron bowel. Spinal Cord. 2001;39:204–207. doi: 10.1038/sj.sc.3101131. [DOI] [PubMed] [Google Scholar]


