Abstract
We previously showed that the repair of bone defects is regulated by neural and vascular signals. In the present study, we examined the effect of topically applied β-nerve growth factor (β-NGF) on neurogenesis and angiogenesis in critical-sized bone defects filled with collagen bone substitute. We created two symmetrical defects, 2.5 mm in diameter, on either side of the parietal bone of the skull, and filled them with bone substitute. Subcutaneously implanted osmotic pumps were used to infuse 10 μg β-NGF in PBS (β-NGF + PBS) into the right-hand side defect, and PBS into the left (control) defect, over the 7 days following surgery. Immunohistochemical staining and hematoxylin-eosin staining were carried out at 3, 7, 14, 21 and 28 days postoperatively. On day 7, expression of β III-tubulin was lower on the β-NGF + PBS side than on the control side, and that of neurofilament 160 was greater. On day 14, β III-tubulin and protein gene product 9.5 were greater on the β-NGF + PBS side than on the control side. Vascular endothelial growth factor expression was greater on the experimental side than the control side at 7 days, and vascular endothelial growth factor receptor 2 expression was elevated on days 14 and 21, but lower than control levels on day 28. However, no difference in the number of blood vessels was observed between sides. Our results indicate that topical application of β-NGF promoted neurogenesis, and may modulate angiogenesis by promoting nerve regeneration in collagen bone substitute-filled defects.
Keywords: nerve regeneration, β-nerve growth factor, collagen, angiogenesis, protein gene product 9.5, vascular endothelial growth factor, β III-tubulin, neural regeneration
Introduction
The poor healing of large or segmental bone defects remains a problem for clinicians. The process of bone repair is complex and involves the regulation of many cell types by biochemical and mechanical signals, such as neural and vascular growth factors governing neurogenesis and angiogenesis. Vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, is essential for normal angiogenesis and formation of appropriate callus architecture (Yonamine et al., 2010). Localized VEGF delivery is beneficial for bone regeneration, since it promotes neovascularization, bone turnover, osteoblast migration and mineralization (Fang et al., 2005; Kent Leach et al., 2006; Geris et al., 2008; Keramaris et al., 2008; Yonamine et al., 2010).
Abnormalities in the vascular or nervous systems will delay bone regeneration (Konttinen et al., 1996; Wang et al., 2009). Previously, our group successfully established models of bone substitute-filled critical-sized defects, and topically introduced β-nerve growth factor (β-NGF) via an osmotic pump. We also showed that application of β-NGF can promote bone regeneration (Chen et al., 2014). The present study was designed to examine the effect of topical β-NGF, applied directly to bone defects filled with collagen bone substitute, on neurogenesis and angiogenesis.
Materials and Methods
Animals
Thirty clean adult male Sprague-Dawley rats, weighing 250–300 g, were purchased from the Shanghai Laboratory Animal Center, China (license No. SCXK 2012-0002). The rats were housed in cages with free access to food and water, under a 12-hour light-dark schedule. All procedures were performed with the approval of the Care and Use of Laboratory Animals Committee at the Union Hospital Affiliated to Fujian Medical University, China.
Bone defect creation and repair
Rats were anesthetized with 10% chloral hydrate (0.3 mL/ 100 g intraperitoneally) and a local injection of 5% lidocaine (0.2 mL subcutaneously) over the cranium, and fixed on an operating table. The surgical area was shaved and the skin cleaned with 0.5% iodophor. A 3 cm incision was made in the scalp along the sagittal suture, and the muscles and periosteum were separated to expose the cranium. Using a trephine drill (external diameter, 5 mm) under constant 4°C saline irrigation, two symmetric critical-sized bone defects were created in the parietal bone, on either side of the sagittal suture. To minimize tissue necrosis, care was taken to avoid excessive heat and damage to the dura mater during drilling. Subcutaneous pockets were created over the forelimbs using a hemostat. The pump containing β-NGF + PBS was inserted into the right pocket, and the pump containing PBS was inserted into the left pocket. The bone defects were then filled with collagen bone substitute (Dentium, Seoul, Korea). Spacers were placed over the bone substitute-filled defects using glass ionomer cement (3M, Saint Paul, MN, USA) (Brentegani et al., 1997; Giannoudis et al., 2005) (Figure 1). The day of surgery was defined as day 0.
Figure 1.
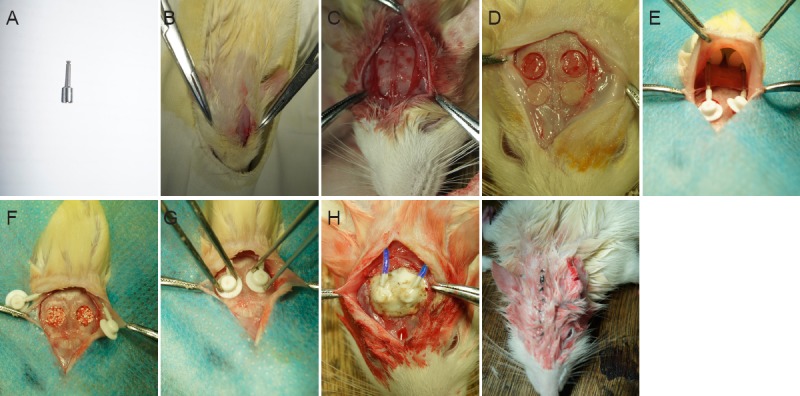
Creation and repair of bone defects.
(A) Trephine drill; (B) incision; (C) exposed cranium; (D) two critical-sized bone defects in the parietal bone; (E) placement of osmotic pumps into subcutaneous pockets; (F) defects filled with bone substitute; (G) fixation of pumps; (H) cementation of spacers; (I) sutured incision.
Infusion of β-NGF
Sixty osmotic pumps (Alzet model 2001, Durect Corp., Cupertino, CA, USA) were used with the Alzet brain infusion kit 2 (Durect), according to the manufacturer's instructions. In brief, one end of the catheter tubing (1.85 cm in length) was attached to a cannula spacer, and the other end was attached to the pump flow moderator (Figure 2). Rat β-NGF (Sigma, St. Louis, MO, USA) was dissolved in 0.01 M PBS (Mammoto et al., 2008), which contained 1% Evans blue to confirm that the drug had been delivered correctly (Marko and Damon, 2008). The pumps were filled with either β-NGF in PBS, or PBS alone, so that the experimental side (the right-hand side parietal bone defect) received β-NGF + PBS, and the control (left) side received PBS, immediately upon implantation. The method delivered a total of 10 μg β-NGF to the injury site over the first 7 days after surgery.
Figure 2.
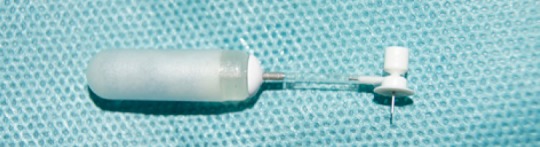
Prepared (empty) osmotic pump used for drug infusion into the bone defect.
Tissue preparation
The process of bone regeneration encompasses four overlapping phases: inflammation, soft and hard callus formation, and remodeling (Geris et al., 2008). To determine the effects of β-NGF across the entire process, we used six rats at each of five key time points, representing each stage: day 3, inflammation; day 7, soft callus formation; days 14 and 21, hard callus formation; and day 28, remodeling.
The rats were anesthetized with 10% chloral hydrate. A median sternotomy was performed, and an 18-gauge catheter was inserted into the left ventricle of the heart. Saline (0.9% w/v) containing 5,000 U/mL heparin was perfused transcardially until the liver appeared white, followed by 4% paraformaldehyde in 0.01 M PBS for 5–10 minutes. The skull and dura were immediately dissected out and immersed overnight in the same fixative at 4°C. The fixed specimens were decalcified in 10% EDTA in 0.01 M PBS (pH 7.0) for 8 weeks at 25°C. After decalcification, the specimens were embedded in paraffin and cut into sections 4 μm thick for histological processing and immunohistochemical staining.
Immunohistochemistry and hematoxylin-eosin staining
Immunohistochemistry was used to detect the expression of β III-tubulin, protein gene product 9.5 (PGP9.5), and neurofilament 160 (NF160) at each time point, to determine how topical application of β-NGF affects neurogenesis. All slices were deparaffinized in xylene and rehydrated through a graded alcohol series. Antigen retrieval was performed, and the sections were rinsed three times in PBS for 3 minutes each time. Endogenous peroxidase was neutralized with 0.3% hydrogen peroxide. After three more washes in PBS, the slices were blocked with normal serum for 20 minutes and incubated at 37°C for 1 hour in a humid chamber with streptavidin and rabbit anti-rat antibodies against β III-tubulin (1:300; Epitomics, Cambridge, MA, USA), PGP9.5 (1:100; Epitomics), NF160 (1:200; Abcam, Cambridge, MA, USA), VEGF (1:250; Abcam), and VEGF receptor 2 (VEGFR-2) (1:20; Abcam). The sections were washed in PBS as before, reacted with peroxidase-conjugated streptavidin goat anti-rabbit IgG (ready-to-use, ZSGB-Bio, Beijing, China) at 37°C for 15 minutes in a humid atmosphere, and immersed in diaminobenzidine hydrochloride. Three areas of bone defect region were selected for analysis: C1 and C3, corresponding to regions close to the defect wall, and C2, corresponding to the central region of the defect.
To determine whether the stimulation of nerve regeneration by β-NGF also increased angiogenesis, blood vessels were counted in hematoxylin and eosin-stained tissue in each of the three bone defect regions under a digital microscope camera (400×, Olympus, Tokyo, Japan), and the mean calculated.
To further understand the effect of β-NGF on neurogenesis and VEGF/VEGFR-2 overexpression on angiogenesis, the average optical densities of β III-tubulin, PGP9.5, NF160, VEGF, and VEGFR-2 were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Washington, USA).
Statistical analysis
All data are expressed as the mean ± SD. Paired t-tests were used to compare experimental and control sides (SPSS 19.0 for Windows; IBM Corporation, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Topical application of β-NGF promoted neurogenesis in collagen bone substitute-filled defects
Significant differences were found between the experimental (β-NGF + PBS) side and the control (PBS only) side at 7, 14 and 28 days postoperatively. On day 7, β III-tubulin expression was lower on the experimental side than the control side, whereas NF160 expression was greater; on day 14, β III-tubulin and PGP9.5 expression levels were greater on the experimental side than the control side; and at 28 days, PGP9.5 expression was lower on the experimental side than the control side (P < 0.05; Figure 3).
Figure 3.
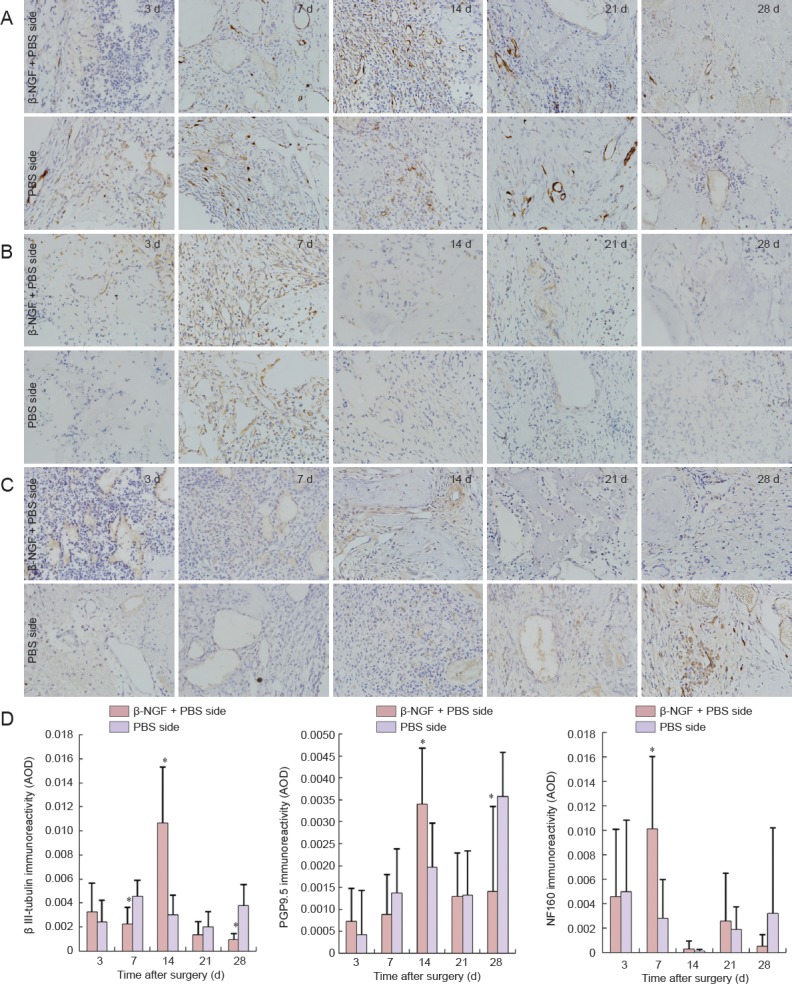
Effect of topical application of β-NGF neurogenesis in collagen bone substitute-filled defects.
(A–C) Immunohistochemistry for β III-tubulin (A), NF160 (B), and PGP9.5 (C) at bone defect site (× 400; positive immunoreactivity appears brown). (D) Quantitation of β III-tubulin, PGP9.5 and NF160 immunoreactivity in collagen bone substitute-filled defects. All data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. PBS side (paired t-test). β-NGF: β-Nerve growth factor; PGP9.5: protein gene product 9.5; NF160: neurofilament 160; AOD: average optical density; d: days.
Topical application of β-NGF promoted angiogenesis in collagen bone substitute-filled defects
The number of blood vessels at both defect sites increased after injury, and decreased again at the time corresponding to mineralization of the bone defect area (days 14–21). No significant differences were observed between the number of blood vessels on each side (P > 0.05; Figure 4).
Figure 4.
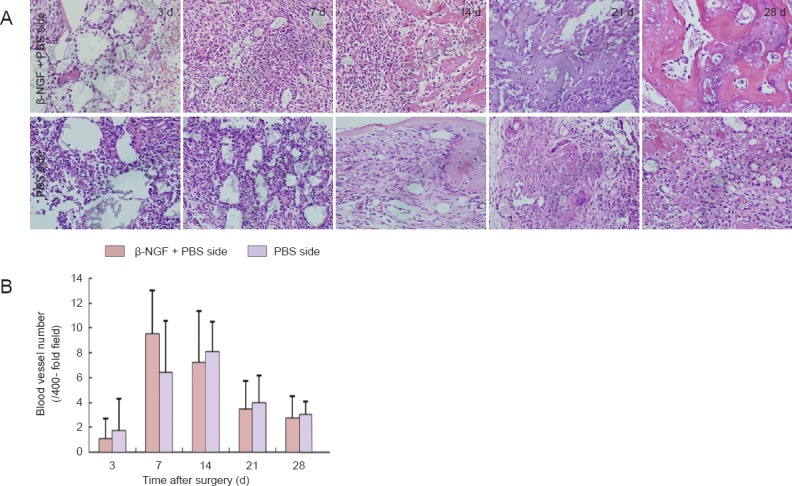
Effect of topical application of β-NGF on angiogenesis in collagen bone substitute-filled defects.
(A) Blood vessels in the bone defect during repair (hematoxylin-eosin staining, × 400). (B) Number of blood vessels at the bone defect site increased on both sides until days 7–14, and diminished thereafter. All data are expressed as the mean ± SD (n = 6). β-NGF: β-Nerve growth factor; d: days.
Topical application of β-NGF promoted VEGF/VEGFR-2 expression in collagen bone substitute-filled defects
On day 7, VEGF expression in the bone defect was greater on the β-NGF + PBS side than the control side (P < 0.05). On days 14 and 21, VEGFR-2 expression was greater on the experimental side than the control side, but by day 28 had reduced to below control levels (P < 0.05; Figure 5).
Figure 5.
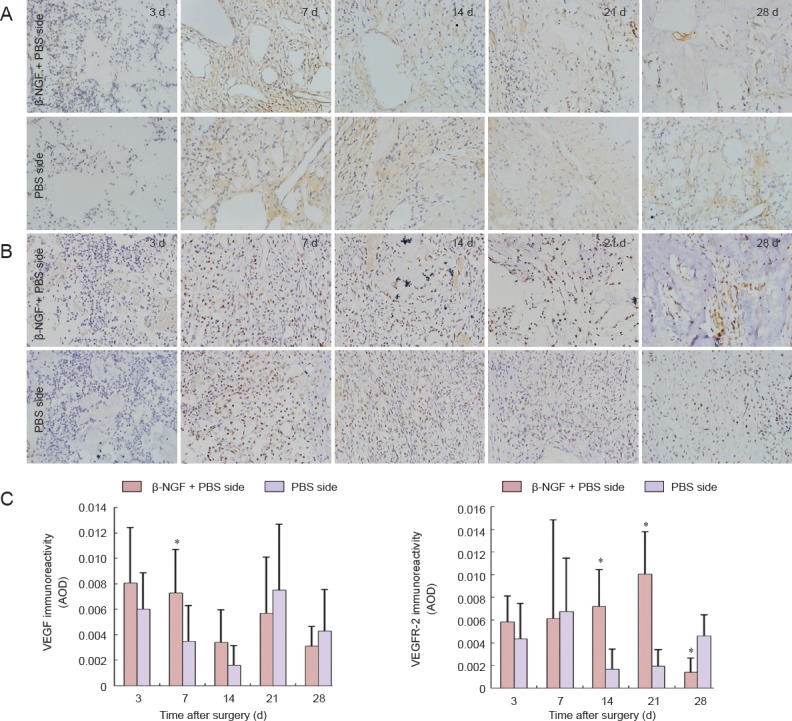
Effect of topical application of β-NGF on VEGF and VEGFR-2 immunoreactivity in collagen bone substitute-filled defects.
(A, B) Immunohistochemistry for VEGF (A) and VEGFR-2 (B) at the bone defect site (× 400; positive immunoreactivity appears brown). (C) Quantitation of VEGF and VEGFR-2 immunoreactivity in collagen bone substitute-filled bone defects. All data are expressed as the mean ± SD (n = 6). *P < 0.05, vs. PBS side (paired t-test). β-NGF: β-Nerve growth factor; VEGF: vascular endothelial growth factor; VEGFR-2: VEGF receptor 2; AOD: average optical density; d: days.
Discussion
Our results suggest that local application of β-NGF promoted neurofilament expression during bone regeneration in the defects filled with collagen bone substitute. However, the measurement of β III-tubulin also indicated that β-NGF inhibited neuronal regeneration. There are three key steps in the pathway of cytoskeletal specialization during neuronal differentiation (Fanarraga et al., 1999). The first of these begins with the upregulation of class-III β-tubulin expression by neuroepithelial cells, indicating microtubular reorganization towards neurons. The second involves cytoplasmic accumulation of unphosphorylated class-III β-tubulin in neuroblasts, whereas the third key step occurs when most of the class-III β-tubulin that is being incorporated into microtubules is phosphorylated, presumably beginning microtubular reorganization in mature neurons. Since neuron-specific class-III β-tubulin is most unstable in microtubules, its phosphorylation in differentiated neurons results in the appearance of highly stable colchicine-resistant microtubules, which are required for neurite extension (Fanarraga et al., 1999). In the present study, we looked at phosphorylated β III-tubulin; the mature nervous system possesses very little of the unphosphorylated form (Fanarraga et al., 1999). The statistical difference in β III-tubulin expression observed at 7 days may therefore be due to the fact that most of the class-III β-tubulin was unphosphorylated. On day 14, significantly higher β III-tubulin and PGP9.5 expression levels were observed on the β-NGF + PBS side than on the control side; this suggests that the presence of β-NGF promoted neuronal regeneration during bone regeneration in the collagen-filled defects, as well as verifying the hypothesis that the class-III β-tubulin at 7 days was unphosphorylated. At 21 and 28 days, bone formation was significantly more pronounced in the β-NGF + PBS group than in the PBS group (data not shown), and this observation was in agreement with reports by other investigators (Eppley et al., 1992; Letic-Gavrilovic et al., 2003). Although β III-tubulin and PGP9.5 expression levels were not significantly different between the two sides on day 21, significant differences in both proteins were observed between the two sides by day 28. Since β-NGF enhances bone regeneration in the collagen bone substitute-filled defect, one possible explanation for the lack of significant difference between the two sides at 21 days may be that the regenerated bone was undergoing remodeling, and so there was a decrease in nerve fiber regeneration (Suzuki et al., 2010).
Angiogenesis is the formation of new blood vessels from preexisting vessels, which creates the primary vascular plexus (Raab and Plate, 2007). Considerable evidence indicates that angiogenesis is a fundamental process in bone formation and repair, and that impaired angiogenesis plays an important role in nonunion (failure to establish bony bridging of the fracture gap) and in delayed union (Geris et al., 2008; Yonamine et al., 2010). Therefore, one of the critical parameters for successful bone tissue regeneration is the formation of the microvascular network needed to supply oxygen and nutrients for cellular growth and differentiation (Kent Leach et al., 2006).
Angiogenesis is essential for wound healing and remodeling, embryogenic development, organ formation, and tissue regeneration (Seo et al., 2001). Non-tumorous conditions can trigger neoangiogenesis in nervous tissue by shifting the balance between angiogenic and angiostatic factors towards activation (Harik et al., 1995; Patt et al., 1997). Angiogenesis also occurs in nerve cell grafts and at spinal cord lesions during peripheral nerve regeneration, and it can be triggered by physiological tasks that increase neural function and synaptic activity (Calzà et al., 2001). Overall, these previous findings support the hypothesis that nerve regeneration and increased neural activity can affect angiogenesis. In the present study, we observed that the mean nerve density and number of blood vessels correlated with each other across time points. Additionally, in agreement with previous findings that nerve growth factor promotes angiogenesis (Emanueli et al., 2002; Asanome et al., 2014), we observed that the number of blood vessels on the β-NGF + PBS side was 1.5 times greater than on the control side at 7 days in collagen bone substitute-filled defects. In contrast to nerve regeneration, the number of blood vessels at 14 days was slightly lower than that at 7 days. The reason for this may be that the blood vessels are remodeling from day 7 to 14, resulting in larger-diameter blood vessels and fewer capillaries. However, the lack of statistically significant difference between the two sides in the number of blood vessels observed at other time periods contradicts observations by other researchers. These conflicting results may be due to the use of different quantification methods.
The biological effects of NGF are also known to be dependent on two specific cell surface receptors, namely the tropomyosin-related kinase receptor TrkA, which is involved in mediating growth and survival, and p75, which forms a trimolecular complex with TrkA during NGF stimulation to increase the ligand specificity and affinity of TrkA (Rahbek et al., 2005). TrkA is a transmembrane tyrosine kinase of 140 kDa, which is phosphorylated at tyrosine residues after binding to its ligand. Once phosphorylated, a cascade of events follows, via extracellular signal-regulated kinase (ERK) 1/2 and VEGF/phosphatidyl-3′kinase/Akt-B/endothelial nitric oxide synthase pathway activation, leading to the stimulation of cell proliferation (Nico et al., 2008). Julio-Pieper et al. (2006, 2009) reported that NGF promoted ovarian angiogenesis by enhancing the synthesis and secretion of VEGF via a trkA- and ERK2-dependent mechanism. Similarly, Emanueli et al. (2002) suggested that NGF acted directly as an angiogenic factor or indirectly by stimulating VEGF expression in the ischemic hind limb. In the present study, we observed significantly higher expression levels of VEGF at 7 days in the β-NGF + PBS side compared to the PBS side, suggesting that β-NGF stimulated VEGF expression in the collagen bone substitute-filled defect. In addition, NGF-induced endothelial cell migration was also reported to be completely blocked by the NGF/TrkA receptor antagonist K252a, a tyrosine kinase inhibitor specific for VEGFR-2 (Nico et al., 2008). Consistent with this study, we observed a significantly greater expression of VEGFR-2 on days 14 and 21 in the defects treated with β-NGF + PBS compared to the control defects.
In summary, during bone regeneration in defects filled with collagen bone substitute, topical application of β-NGF may modulate angiogenesis by promoting nerve regeneration.
Footnotes
Funding: This work was supported by the Fujian Foundation for Distinguished Young Scientists in China, No. Grant#2060203; the National Natural Science Foundation of China, No. 31070838.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Asanome A, Kawabe JI, Matsuki M, Kabara M, Hira Y, Bochimoto H, Yamauchi A, Aonuma T, Takehara N, Watanabe T, Hasebe N. Nerve growth factor stimulates regeneration of perivascular nerve, and induces the maturation of microvessels around the injured artery. Biochem Biophys Res Commun. 2014;443:150–155. doi: 10.1016/j.bbrc.2013.11.070. [DOI] [PubMed] [Google Scholar]
- Brentegani LG, Bombonato KF, Carvalho TL. Histological evaluation of the biocompatibility of a glass-ionomer cement in rat alveolus. Biomaterials. 1997;18:137–140. doi: 10.1016/s0142-9612(96)00110-x. [DOI] [PubMed] [Google Scholar]
- Calzà L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Zhuo LL, Mao CQ, Wang J, Lu M, Wang CY. Establishment of a model for β-NGF administration in rat skull bone defect. Fujian Yike Daxue Xuebao. 2014;48:303–307. [Google Scholar]
- Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- Eppley BL, Snyders RV, Windelmann T, Delfino JJ, Sadove AM. Effects of nerve growth factor on craniofacial onlay bone graft survival: preliminary findings. J Craniofac Surg. 1992;2:174–180. doi: 10.1097/00001665-199203000-00003. [DOI] [PubMed] [Google Scholar]
- Fanarraga ML, Avila J, Zabala JC. Expression of unphosphorylated class III beta-tubulin isotype in neuroepithelial cells demonstrates neuroblast commitment and differentiation. Eur J Neurosci. 1999;11:517–527. [PubMed] [Google Scholar]
- Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20:1114–1124. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- Geris L, Gerisch A, Sloten JV, Weiner R, Oosterwyck HV. Angiogenesis in bone fracture healing: A bioregulatory model. J Theor Biol. 2008;251:137–158. doi: 10.1016/j.jtbi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36 Suppl 3:S20–27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Harik SI, Hritz MA, LaManna JC. Hypoxia-induced brain angiogenesis in the adult rat. J Physiol. 1995;485:525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio-Pieper M, Lara HE, Bravo JA, Romero C. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta1 in the rat ovary. Reprod Biol Endocrinol. 2006;4:57. doi: 10.1186/1477-7827-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio-Pieper M, Lozada P, Tapia V, Vega M, Miranda C, Vantman D, Ojeda SR, Romero C. Nerve growth factor induces vascular endothelial growth factor expression in granulosa cells via a trkA receptor/mitogen-activated protein kinase-extracellularly regulated kinase 2-dependent pathway. J Clin Endocr Metab. 2009;94:3065–3071. doi: 10.1210/jc.2009-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent Leach J, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- Konttinen Y, Imai S, Suda A. Neuropeptides and the puzzle of bone remodeling. State of the art. Acta Orthop Scand. 1996;67:632–639. doi: 10.3109/17453679608997772. [DOI] [PubMed] [Google Scholar]
- Letic-Gavrilovic A, Piattelli A, Abe K. Nerve growth factor beta(NGF beta) delivery via a collagen/hydroxyapatite (Col/HAp) composite and its effects on new bone ingrowth. J Mater Sci Mater Med. 2003;14:95–102. doi: 10.1023/a:1022099208535. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Seerattan RA, Paulson KD, Leonard CA, Bray RC, Salo PT. Nerve growth factor improves ligament healing. J Orthopaed Res. 2008;26:957–964. doi: 10.1002/jor.20615. [DOI] [PubMed] [Google Scholar]
- Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008;294:H2646–2652. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75:135–141. doi: 10.1016/j.mvr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Patt S, Sampaolo S, Theallier-Janko A, Tschairkin I, Cervos-Navarro J. Cerebral angiogenesis triggered by severe chronic hypoxia displays regional differences. J Cereb Blood Flow Metab. 1997;17:801–806. doi: 10.1097/00004647-199707000-00010. [DOI] [PubMed] [Google Scholar]
- Raab S, Plate K. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113:607–626. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- Rahbek U, Dissing S, Thomassen C, Hansen A, Tritsaris K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflugers Arch. 2005;450:355–361. doi: 10.1007/s00424-005-1436-0. [DOI] [PubMed] [Google Scholar]
- Seo K, Choi J, Park M, Rhee C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci. 2001;2:125–130. [PubMed] [Google Scholar]
- Suzuki A, Uemura T, Nakamura H. Control of bone remodeling by nervous system. Neural involvement in fracture healing and bone regeneration. Clin Calcium. 2010;20:1820–1827. [PubMed] [Google Scholar]
- Wang L, Zhao R, Shi X, Wei T, Halloran BP, Clark DJ, Jacobs CR, Kingery WS. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309–320. doi: 10.1016/j.bone.2009.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine Y, Matsuyama T, Sonomura T, Takeuchi H, Furuichi Y, Uemura M, Izumi Y, Noguchi K. Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:225–231. doi: 10.1016/j.tripleo.2009.09.010. [DOI] [PubMed] [Google Scholar]


