Abstract
Microsurgical suturing is the gold standard of nerve coaptation. Although literature on the usefulness of fibrin glue as an alternative is becoming increasingly available, it remains contradictory. Furthermore, no data exist on how both repair methods might influence the morphological aspects (arborization; branching) of early peripheral nerve regeneration. We used the sciatic nerve transplantation model in thy-1 yellow fluorescent protein mice (YFP; n = 10). Pieces of nerve (1cm) were grafted from YFP-negative mice (n = 10) into those expressing YFP. We performed microsuture coaptations on one side and used fibrin glue for repair on the contralateral side. Seven days after grafting, the regeneration distance, the percentage of regenerating and arborizing axons, the number of branches per axon, the coaptation failure rate, the gap size at the repair site and the time needed for surgical repair were all investigated. Fibrin glue repair resulted in regenerating axons travelling further into the distal nerve. It also increased the percentage of arborizing axons. No coaptation failure was detected. Gap sizes were comparable in both groups. Fibrin glue significantly reduced surgical repair time. The increase in regeneration distance, even after the short period of time, is in line with the results of others that showed faster axonal regeneration after fibrin glue repair. The increase in arborizing axons could be another explanation for better functional and electrophysiological results after fibrin glue repair. Fibrin glue nerve coaptation seems to be a promising alternative to microsuture repair.
Keywords: nerve regeneration, fibrin glue, peripheral nerve regeneration, thy-1-YFP mice, sciatic nerve, branching, arborisation, neural regeneration
Introduction
Microsurgical suturing is the gold standard of nerve coaptation (Terzis et al., 1997). However, evidence also shows that suture materials can result in adverse effects, like tissue damage, inflammation, foreign body reaction and scarring, which have a negative impact on nerve regeneration (Boedts, 1987; Inaloz et al., 1997; Suri et al., 2002). Furthermore, microsurgical coaptation is considered a time-consuming procedure that requires expertise and experience. This can be an issue in cases of extensive nerve damage (Ornelas et al., 2006a, b). On the other hand, some authors consider suture repair to offer important advantages: precision of coaptation, preservation of the tensile strength of repair, and lower risk of gap formation or rupture (Cruz et al., 1986; Temple et al., 2004).
The use of tissue adhesives, like fibrin glue, seems to be a promising alternative. The earliest discussions go as far back as the 1940s (Egloff and Narakas, 1983), and ever since fibrin glue became commercially available in the 1970s, the idea of using it in nerve repair has been becoming increasingly widespread (Isaacs et al., 2008). The advantages of repairing nerves with fibrin glue are said to be less scarring, less local inflammation, and faster surgical and recovery times (Egloff and Narakas, 1983; Boedts, 1987; Smahel et al., 1987; Narakas, 1988; Ornelas et al., 2006a; Nishimura et al., 2008). Furthermore, autologous fibrin is enriched with platelets that can release growth factors and thereby improve nerve regeneration (Choi et al., 2005). It has been reported that fibrin is deposited in the nerve after injury and hinders functional regeneration by inhibiting Schwann cell migration, a crucial event in peripheral nerve regeneration (Akassoglou et al., 2003). However, some animal studies have shown comparable or beneficial functional and electrophysiological results after fibrin glue repair (Inaloz et al., 1997; Menovsky and Beek, 2001; Martins et al., 2005; Ornelas et al., 2006a, b).
As mentioned above, results on the usefulness of fibrin glue in nerve repair are contradictory. Furthermore, scant evidence exists on the potential impact of fibrin glue on the morphology of nerve regeneration and axonal behaviour during early regeneration. The main challenge facing regenerating axons in the first few days after axotomy is crossing the repair site. Among other things, the morphology of regenerating axons is characterized by lateral movement, arborization and pruning (Ramón y Cajal and May, 1928). Given that function follows morphology of regeneration, we used the sciatic nerve transection and transplantation model of the thy-1-yellow fluorescent protein (YFP) mouse line to investigate these aspects (Feng et al., 2000; Witzel et al., 2005). This mouse line expresses YFP in a subset of its axons. With YFP-negative nerve grafts as the distal nerve part, we were able to use fluorescence microscopy to evaluate how many axons regenerated into the distal aspect of the nerve and how these axons behaved. This allowed us to investigate the potential influence of fibrin glue on the number of regenerating axons, the distance of regeneration, and arborization. Furthermore, the coaptation failure rate, gap size and surgical repair time were also quantified.
Materials and Methods
Animals
Ten thy-1-YFP transgenic mice (YFP+, Jackson Laboratory, Bar Harbor, Maine, USA) expressing YFP in a subset of their axons (B6.Cg-Tg (thy1-YFPH) 16Jrs/J; Jackson Laboratory, Bar Harbor, Maine, USA) (Feng et al., 2000; Witzel et al., 2005) were used as experimental animals. C57BL/6 mice (n = 10) served as YFP- donors for peripheral nerve grafting. The animals were kept in a temperature-controlled environment, exposed to 12-hour light-dark cycles and given food and water ad libitum. The State Animal Protection Committee approved all procedures.
Surgical procedure and experimental groups
All surgical procedures were performed in mice of both genders at the age of 12–16 weeks. Anaesthesia was induced with isoflurane (ForeneÒ, AbbVie Deutschland, Wiesbaden, Germany) and continued by intraperitoneal injection of a mixture of xylazine (16 mg/kg) and ketamine (100 mg/kg). A dorsal approach was used for the bilateral sciatic nerve exposure in the YFP+ mice. After transection of the sciatic nerve 5 mm proximal to the trifurcation, the distal nerve part was completely removed and partially replaced by a 1-cm sciatic nerve graft harvested from YFP- control mice (Koulaxouzidis et al., 2015). The partially fluorescent axons of the YFP+ mice made it easy for us to quantify regeneration distance and morphology. To exclude interference from fluorescent debris caused by Wallerian degeneration and thus difficulties in quantifying the results, we used fluorescent-free nerve grafts for the distal nerve section (Witzel et al., 2015 in press). On one side, proximal coaptation of the nerve graft to the transected sciatic nerve of the YFP+ mice was performed using two single-stitch sutures (11-0 nylon). On the contralateral side, the nerve ends were fixed with a 0.25 mL two-component fibrin glue (Tissue Col Duo S Immuno 0.5 ml, Baxter SA, Germany). One component, fibrin sealant, is made of pooled human plasma and contains fibrinogen and aprotinine. The other component contains thrombin and calcium chloride.
A randomization list was used to assign the sides, thus creating two experimental groups. One group contained the nerves coapted by sutures (Suture), and the other had the nerves coapted by fibrin glue (Fibrin). Intraindividual comparisons were possible because both types of coaptation were performed in each experimental animal. The distal end of the nerve graft was not coapted, which ruled out any influence from the target organ (Frey et al., 1996). After surgery, the animals were able to move around freely. The follow-up period was limited by the length of the nerve graft (1 cm). Seven days after nerve repair, the fastest axons had exceeded the distal end of the graft and therefore could not be used for evaluation. The mice received a daily subcutaneous injection of 0.1 mg/kg buprenorphine at the abdomen as analgesic during the repair.
Nerve harvesting and fixation
After 7 days, the sciatic nerves and their grafts were harvested and the animals were sacrificed by CO2 inhalation. Each nerve was carefully stretched out on a piece of wood and sutured with 9-0 nylon at both ends. The nerves were fixed for 24 hours in 4% paraformaldehyde. We used a freezing microtome to produce 100-μm longitudinal sections. For the imaging of Schwann cell tubes, we used antibodies against laminin (Sigma, Munich, Germany) and Alexa Fluor 568 (Invitrogen, Darmstadt, Germany) as a secondary antibody.
Evaluation of tissue sections
Regeneration distance
Sections were analyzed with standard fluorescence microscopy (Carl Zeiss Microscopy GmbH, Berlin, Germany) at a wavelength of 568 nm and 466 nm for the yellow autofluorescence of the nerve. The coaptation site was set as the zero reference point. We counted the number of fluorescent axons proximal to the coaptation site. The number of axons exactly 1 mm proximal to the coaptation was defined as the maximum number of axons able to regenerate. Furthermore, the regenerating axons were counted at intervals of precisely 1 mm distal to the coaptation site. Retrograde regenerating axons were excluded from the assessment. Each axon branch was counted as one axon at each millimetre interval (Figure 1) (Witzel et al., 2015).
Figure 1.
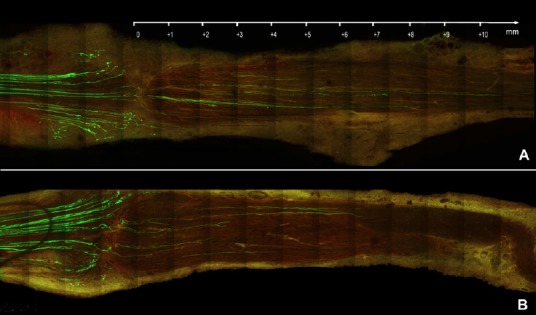
Overview of nerve repairs; 100 μm sections assessed with fluorescence microscopy.
The proximal stump is on the left, the repair site runs vertically between the first and second quarters on the left of each plate, and the recipient graft is on the right. Sensory and motor axons that express yellow fluorescent protein are greenish-yellow, while basement membrane that contains laminin is light red. The samples are harvested 7 days after nerve repair. (A) Fluorescent proximal stump on the left, non-fluorescent distal nerve on the right. The nerve section shows an example of fibrin glue repair. (B) Example of a suture repair using single button sutures (11-0 nylon). Scale bar: 1 mm.
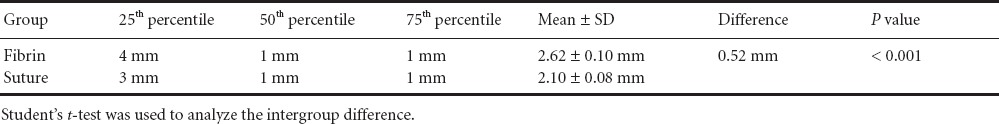
The number of regenerating axons counted at each millimetre distal to the coaptation site was set against the maximum number of axons able to regenerate and displayed as Kaplan-Meier curves. The mean and standard deviation of the distance, and the distance of the 25th, 50th and 75th percentiles (representing the fastest 25%, the middle and the slowest 25% of regenerating axons) were determined for each experimental group.
Percentage of regenerating axons
The number of fluorescent axons 1 mm proximal to and 1 mm distal to the coaptation site were counted in order to calculate the percentage of regenerating axons for each group. All counts performed were done manually during fluorescent microscopy without using professional software.
Branches and arborization
The total number of regenerating axons (RA), arborizing axons (AA) and branches (B) were counted in the zone running from the transection to 2 mm inside the nerve graft. We used these values to calculate the ratio of AA to RA. Furthermore, we determined the branches per regenerating axon as a ratio of B to RA and the branches per arborizing axon as a ratio of B to AA.
Based on the ratio of branches to arborizing axons, it is possible to say whether there has been a homogeneous increase or decrease in branching, or an excessive sprouting of single regenerating axons.
Failure rate
Macroscopic rupture of the nerve coaptation was defined as complete coaptation failure. This was assessed when harvesting the nerve 7 days after grafting.
Gap size
During the microscopic evaluation, the distance between the proximal and distal stumps in each section was measured in μm. The maximum distance between the proximal and distal nerve stumps at the repair site of a single nerve was defined as the gap size of the nerve.
Surgical repair times
The time (in seconds) needed for end-to-end nerve coaptation using either two single-stitch sutures or fibrin glue fixation was recorded for each nerve repair. The clock started at the beginning of suturing or fibrin glue application and finished when the last suture was complete or the fibrin clot had formed and was stable.
Statistical analysis
Statistical significance of the compared Kaplan-Meier curves was determined by the log-rank test. Statistical analysis of the percentage of regenerating axons, the percentage of arborizing axons, the number of branches per regenerating axon and per arborizing axon, the gap size and the surgical repair time was performed by Student's t-test after testing for normal distribution. Statistical comparison of the failure rate was performed by the chi-square test. The significance level was set at P < 0.05. IBM SPSS Statistics 22 software (Ehningen, Germany) was used. Data are expressed as the mean ± SD.
Results
Regeneration distance
Fibrin glue coaptation was associated with longer regeneration distances after 7 days (Figures 1, 2) compared with suture coaptation (P < 0.001) (Table 1).
Figure 2.
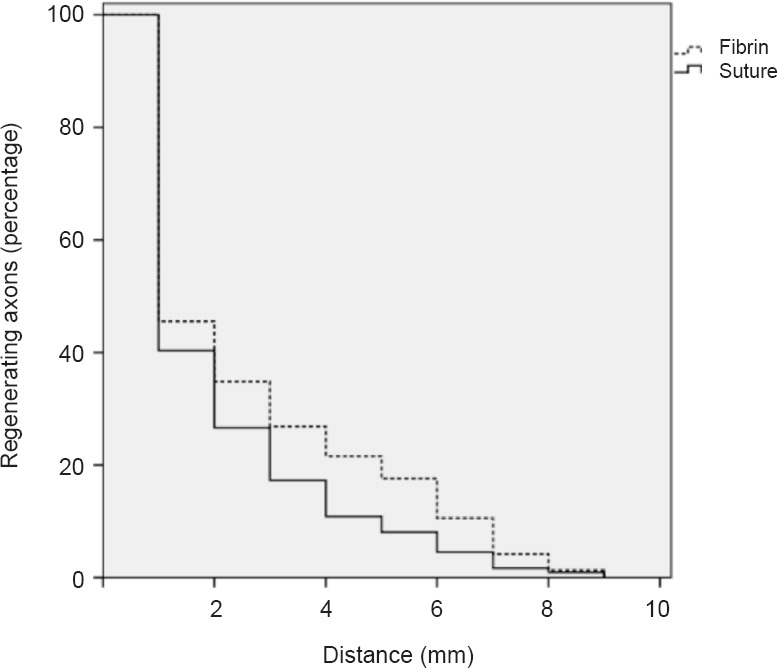
Using fibrin glue for peripheral nerve coaptation increases regeneration distance 7 days after surgery (percentage and travelling distance of regenerating axons).
Peripheral nerve coaptation using fibrin glue increased regeneration distance compared with suture repair (P < 0.001; Student's t-test).
Table 1.
Description of the sciatic nerve regeneration distances following fibrin repair and suture coaptation
Percentage of regeneration and arborizing axons
With regard to the percentage of regenerating axons, the figures were 36.1 ± 4.63% in the fibrin glue group and 33.5 ± 3.33% in the suture group without significant difference (P = 0.65; Figure 3A).
Figure 3.
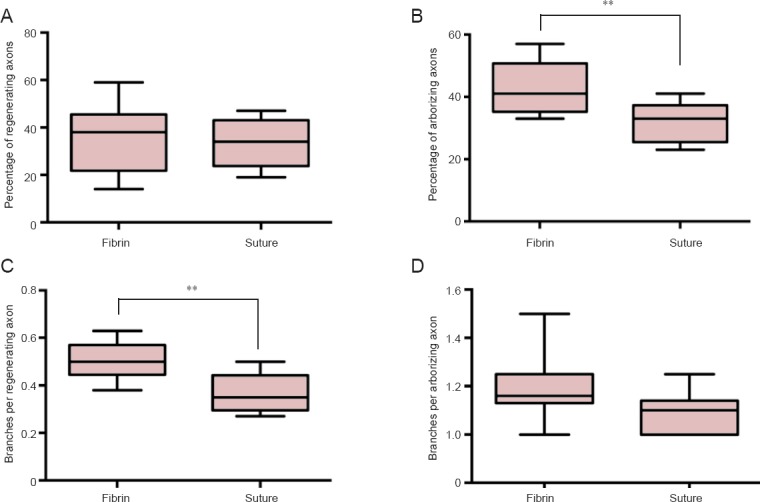
Percentage of regenerating axons and branching behaviour 7 days after surgery.
(A) The percentage of regenerating axons did not differ significantly between the two groups (P = 0.65). (B) Fibrin glue coaptation significantly increased the number of arborizing axons (**P < 0.01). (C) The fibrin glue group had significantly more branches per regenerating axon within the first 2 mm inside the distal nerve (**P < 0.01). (D) The number of branches per arborizing axon did not differ significantly between the groups (P = 0.052). Data are expressed as the mean ± SD. Student's t-test was used to analyze the intergroup difference.
The percentage of arborizing regenerating axons was 42.5 ± 2.7% in the fibrin glue group and 32.13 ± 2.26% in the suture group (P < 0.01; Figure 3B).
Branches per regenerating and arborizing axon
The branches per regenerating axon were significantly increased in the fibrin glue group (0.51 ± 0.02) compared with the suture group (0.37 ± 0.03; P < 0.01; Figure 3C). Fibrin glue produced 1.20 ± 0.05 branches per arborizing axon, while the sutures achieved 1.08 ± 0.03 (P = 0.052; Figure 3D).
Coaptation failure, coaptation gap size, surgical repair time
Coaptation failure did not occur in either of groups. Both groups exhibited a comparable gap size (P = 0.25), which measured 106.2 ± 9.57 μm in the fibrin group and 90.6 ± 9.08 μm in the suture group (Figure 4). Finally, the time needed to perform a nerve repair with fibrin glue (63.9 ± 4.75 seconds) was significantly less than the time needed to perform the same procedure with sutures (187.5 ± 4.71 seconds; Figure 5).
Figure 4.
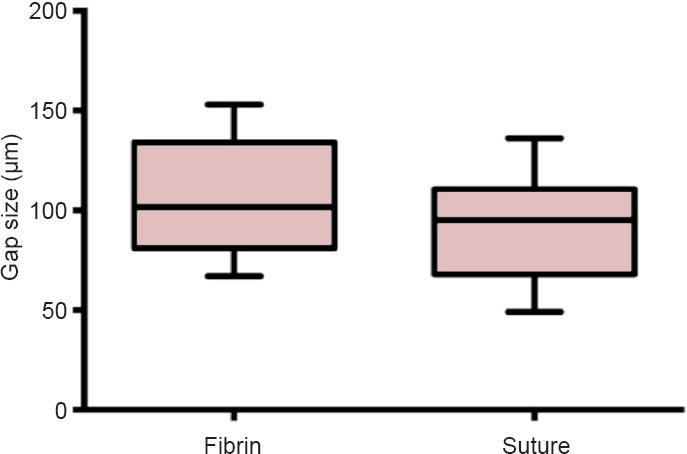
Gap size at the repair site at 7 days after surgery.
Gap size was 106.2 ± 9.57 μm in the fibrin glue group, and 90.6 ± 9.08 μm (P = 0.25) in the microsuture group. Student's t-test was used for statistical comparisons. Data are expressed as the mean ± SD.
Figure 5.
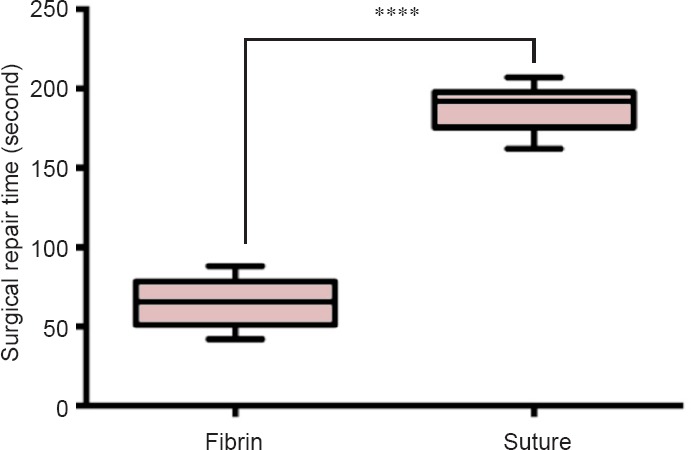
Surgical repair with fibrin glue versus microsutures for sciatic nerve regeneration at 7 days after surgery.
The mean surgical repair time with fibrin glue was significantly shorter than with microsutures (****P < 0.0001). Student's t-test was used for statistical comparisons. Data are expressed as the mean ± SD.
Discussion
Ever since fibrin glue was introduced in the 1970s, its use in peripheral nerve coaptation has been increasing (Bertelli and Mira, 1993). However, evidence of its benefit compared with microsutures (the gold standard of nerve repair) is scant and conflicting (Isaacs et al., 2008). Investigations have focused on comparisons of the following parameters: histopathological reaction at the repair site, time of regeneration, functional outcome, electrophysiological parameters, tensile strength, gap formation, coaptation failure rate, and repair time (Egloff and Narakas, 1983; Boedts, 1987; Smahel et al., 1987; Narakas, 1988; Inaloz et al., 1997; Menovsky and Beek, 2001; Martins et al., 2005; Ornelas et al., 2006a, b; Nishimura et al., 2008).
Our results reveal no significant difference in coaptation failure rate and gap size at the repair site. The literature on failure rates is contradictory. Maragh et al. (1990) found a failure rate of 13% after fibrin glue repair and zero after microsuture repair in a rat model. These results were confirmed by some scholars (Suri et al., 2002; Martins et al., 2005; Ornelas et al., 2006b; Nishimura et al., 2008). Measurements of these parameters have shown advantages of microsuture over fibrin glue repair in cadaveric studies and rabbit models (Temple et al., 2004; Isaacs et al., 2008). However, Cruz et al. (1986) found that using fibrin alone led to 80% dehiscence in a rat sciatic nerve transection model. This inconsistency in the results may be explained by variations in the animal models.
Differences in the species used, the size and specific location of the nerve, the number of stitches needed, the amount of suture material used, the stability of the epineurium, and the forces acting on the coaptation make it almost impossible to compare the results. Nevertheless, most study groups showed an increased rate of fibrosis, inflammation and granuloma formation several weeks and months after microsuture repair (Maragh et al., 1990; Myles et al., 1992; Povlsen, 1994; Menovsky and Beek, 2001; Martins et al., 2005; Ornelas et al., 2006b). Most of the studies revealed comparable or better functional and electrophysiological results after fibrin glue repair (Boedts, 1987; Maragh et al., 1990; Inaloz et al., 1997; Suri et al., 2002; Martins et al., 2005; Ornelas et al., 2006b). We showed that significantly less time was needed to perform a nerve repair using fibrin glue (Ornelas et al., 2006b). However, it is currently unclear whether this is relevant in routine clinical settings and cases of extensive nerve trauma (Ornelas et al., 2006a; Sameem et al., 2011).
No data exist on how each method of nerve repair might influence the morphological aspects of early peripheral nerve regeneration, such as arborization and branching. We found that fibrin glue repair was associated with an increase in regeneration distance and percentage of arborizing axons compared with suture repair. The two methods produced no differences in the number of branches per arborizing axon and none in the number of regenerating axons crossing the repair site. The scope of this study does not extend to investigating whether or not these results are due to a probable reduction in inflammation/scarring or to fibrin glue components activating pro-regenerative molecular mechanisms. As stated above, no difference was seen in the gap size of the repair site. Previous investigations showed that there was no correlation between gap size at the repair site and degree of arborization (Witzel et al., 2005). However, the increased distance that regenerating axons travelled into the distal aspect of the nerve is in line with other results that showed faster axonal regeneration after fibrin glue repair (Egloff and Narakas, 1983; Boedts, 1987; Smahel et al., 1987; Narakas, 1988; Inaloz et al., 1997; Menovsky and Beek, 2001; Martins et al., 2005; Ornelas et al., 2006a, b; Nishimura et al., 2008). Furthermore, the increased percentage of arborizing axons could be another explanation for better functional and electrophysiological results after fibrin glue repair (Boedts, 1987; Maragh et al., 1990; Inaloz et al., 1997; Suri et al., 2002; Martins et al., 2005; Ornelas et al., 2006b). Balanced branching might in itself be a substrate to increase regeneration specificity. Given the limited length of the nerve graft used in the mouse model, this study could only investigate the effects during the very early stages of regeneration. Predicting the subsequent and final functional results of repair should be the object of further investigations.
Nevertheless, fibrin glue seems to be a promising alternative to microsuture in cases of peripheral nerve coaptation.
Footnotes
Funding: This study was supported by funding from the Charité – Universitätsmedizin Berlin.
Conflicts of interest: None declared.
Copyedited by Heaton JT, Hidebrandt H, Li CH, Song LP, Zhao M
References
- Akassoglou K, Akpinar P, Murray S, Strickland S. Fibrin is a regulator of Schwann cell migration after sciatic nerve injury in mice. Neurosci Lett. 2003;338:185–188. doi: 10.1016/s0304-3940(02)01387-3. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Mira JC. Nerve repair using freezing and fibrin glue: immediate histologic improvement of axonal coaptation. Microsurgery. 1993;14:135–140. doi: 10.1002/micr.1920140210. [DOI] [PubMed] [Google Scholar]
- Boedts D. A comparative experimental study on nerve repair. Arch Otorhinolaryngol. 1987;244:1–6. doi: 10.1007/BF00453481. [DOI] [PubMed] [Google Scholar]
- Choi BH, Han SG, Kim SH, Zhu SJ, Huh JY, Jung JH, Lee SH, Kim BY. Autologous fibrin glue in peripheral nerve regeneration in vivo. Microsurgery. 2005;25:495–499. doi: 10.1002/micr.20154. [DOI] [PubMed] [Google Scholar]
- Cruz NI, Debs N, Fiol RE. Evaluation of fibrin glue in rat sciatic nerve repairs. Plast Reconstr Surg. 1986;78:369–373. doi: 10.1097/00006534-198609000-00015. [DOI] [PubMed] [Google Scholar]
- Egloff DV, Narakas A. Nerve anastomoses with human fibrin. Preliminary clinical report (56 cases) Ann Chir Main. 1983;2:101–115. doi: 10.1016/s0753-9053(83)80087-8. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Frey M, Koller R, Liegl C, Happak W, Gruber H. Role of a muscle target organ on the regeneration of motor nerve fibres in long nerve grafts: a synopsis of experimental and clinical data. Microsurgery. 1996;17:80–88. doi: 10.1002/(SICI)1098-2752(1996)17:2<80::AID-MICR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Inaloz SS, Ak HE, Vayla V, Akin M, Aslan A, Sari I, Celik Y, Ozkan U. Comparison of microsuturing to the use of tissue adhesives in anastomosing sciatic nerve cuts in rats. Neurosurg Rev. 1997;20:250–258. doi: 10.1007/BF01105896. [DOI] [PubMed] [Google Scholar]
- Isaacs JE, McDaniel CO, Owen JR, Wayne JS. Comparative analysis of biomechanical performance of available “nerve glues”. J Hand Surg Am. 2008;33:893–899. doi: 10.1016/j.jhsa.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Koulaxouzidis G, Reutter W, Hildebrandt H, Stark GB, Witzel C. In vivo stimulation of early peripheral axon regeneration by N-propionylmannosamine in the presence of polysialyltransferase ST8SIA2. J Neural Transm. 2015 doi: 10.1007/s00702-015-1397-1. doi:10.1007/s00702-015-1397-1. [DOI] [PubMed] [Google Scholar]
- Maragh H, Meyer BS, Davenport D, Gould JD, Terzis JK. Morphofunctional evaluation of fibrin glue versus microsuture nerve repairs. J Reconstr Microsurg. 1990;6:331–337. doi: 10.1055/s-2007-1006838. [DOI] [PubMed] [Google Scholar]
- Martins RS, Siqueira MG, Silva CF, Godoy BO, Plese JP. Electrophysiologic assessment of regeneration in rat sciatic nerve repair using suture, fibrin glue or a combination of both techniques. Arq Neuropsiquiatr. 2005;63:601–604. doi: 10.1590/s0004-282x2005000400009. [DOI] [PubMed] [Google Scholar]
- Menovsky T, Beek JF. Laser, fibrin glue, or suture repair of peripheral nerves: a comparative functional, histological, and morphometric study in the rat sciatic nerve. J Neurosurg. 2001;95:694–699. doi: 10.3171/jns.2001.95.4.0694. [DOI] [PubMed] [Google Scholar]
- Myles LM, Gilmour JA, Glasby MA. Effects of different methods of peripheral nerve repair on the number and distribution of muscle afferent neurons in rat dorsal root ganglion. J Neurosurg. 1992;77:457–462. doi: 10.3171/jns.1992.77.3.0457. [DOI] [PubMed] [Google Scholar]
- Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am. 1988;19:187–199. [PubMed] [Google Scholar]
- Nishimura MT, Mazzer N, Barbieri CH, Moro CA. Mechanical resistance of peripheral nerve repair with biological glue and with conventional suture at different postoperative times. J Reconstr Microsurg. 2008;24:327–332. doi: 10.1055/s-2008-1080535. [DOI] [PubMed] [Google Scholar]
- Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante RL, Bustamante JC, Avalos P, Varela D, Lopez M. Fibrin glue: an alternative technique for nerve coaptation--Part II. Nerve regeneration and histomorphometric assessment. J Reconstr Microsurg. 2006a;22:123–128. doi: 10.1055/s-2006-932507. [DOI] [PubMed] [Google Scholar]
- Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante PL, Bustamante JC, Avalos P, Varela D, Lopez M. Fibrin glue: an alternative technique for nerve coaptation--Part I. Wave amplitude, conduction velocity, and plantar-length factors. J Reconstr Microsurg. 2006b;22:119–122. doi: 10.1055/s-2006-932506. [DOI] [PubMed] [Google Scholar]
- Povlsen B. A new fibrin seal in primary repair of peripheral nerves. J Hand Surg Br. 1994;19:43–47. doi: 10.1016/0266-7681(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Ramón y, Cajal S, May RM. London: Oxford university press, Humphrey Milford; 1928. Degeneration & regeneration of the nervous system. [Google Scholar]
- Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127:2381–2390. doi: 10.1097/PRS.0b013e3182131cf5. [DOI] [PubMed] [Google Scholar]
- Smahel J, Meyer VE, Bachem U. Glueing of peripheral nerves with fibrin: experimental studies. J Reconstr Microsurg. 1987;3:211–220. doi: 10.1055/s-2007-1006987. [DOI] [PubMed] [Google Scholar]
- Suri A, Mehta VS, Sarkar C. Microneural anastomosis with fibrin glue: an experimental study. Neurol India. 2002;50:23–26. [PubMed] [Google Scholar]
- Temple CL, Ross DC, Dunning CE, Johnson JA. Resistance to disruption and gapping of peripheral nerve repairs: an in vitro biomechanical assessment of techniques. J Reconstr Microsurg. 2004;20:645–650. doi: 10.1055/s-2004-861525. [DOI] [PubMed] [Google Scholar]
- Terzis JK, Sun DD, Thanos PK. Historical and basic science review: past, present, and future of nerve repair. J Reconstr Microsurg. 1997;13:215–225. doi: 10.1055/s-2007-1006407. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Witzel C, Reutter W, Stark G, Koulaxouzidis G. N-Propionylmannosamine stimulates axonal elongation in a murine injury model. Neural Regen Res in press. 2015 doi: 10.4103/1673-5374.150744. [DOI] [PMC free article] [PubMed] [Google Scholar]


