Abstract
Purpose
To evaluate the efficacy and safety of melatonin for the treatment of chronic central serous chorioretinopathy (CSCR).
Methods
Prospective comparative case series. A total of 13 patients with chronic CSCR were treated for 1 month: 8 patients were treated orally with 3 mg melatonin t.i.d., and 5 with placebo. All patients had 20/40 or worse Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) in the affected eye or presented an incapacitating scotoma. Most of the patients had previous failed treatments for their condition. Observational procedures included ETDRS BCVA, and complete ophthalmic examination. Optical coherence tomography (OCT) was performed at day 1 and week 4. Fluorescein angiography was performed at baseline only for diagnostic purposes. Data were subjected to two-sample t-test statistical analysis. P-values of <0.05 were considered statistically significant.
Results
At 1-month follow-up, BCVA significantly improved in 87.5% of patients treated with melatonin (7 of 8 patients, P<0.05). All patients showed a mean significant reduction (P<0.01) of central macular thickness (CMT) when compared with the baseline, with 3 patients (37.5%) exhibiting complete resolution of subretinal fluid at 1-month follow-up. No significant side effects were observed. No changes in BCVA or CMT were noted in the control group.
Conclusions
These results suggest that melatonin is safe, well tolerated, and effective in the treatment of chronic CSCR, as it significantly improved BCVA and CMT in patients with this pathology. Further evaluations with longer follow-up and a larger patient population are desirable.
Introduction
Central serous chorioretinopathy (CSCR) is a retinal disease characterized by serous detachment of the neurosensory retina and/or the retinal pigment epithelium (RPE) because of altered functions at the RPE level.1 CSCR is a common cause of mild to moderate visual impairment. Visual acuity (VA) can fluctuate from 20/20 to 20/200 (although usually averages 20/30), and patients typically complain of unilateral blurring of vision, micropsia, metamorphopsia, decreased color vision, and abnormalities in contrast sensitivity or central scotoma. Patients are usually mid-life men, with type A personality, though women can also be affected.2 Fundoscopic signs of CSCR vary from discrete isolated leakage point at the level of the RPE to more diffuse RPE dysfunction, multifocal lesions, and bullous retinal detachments.
Although symptoms in patients with CSCR are usually self-limited with 80–90% spontaneous resolution of serous retinal detachment, patients with classic CSCR have a 40–50% risk of recurrence of the disease in the same eye,3 resulting in persistent detachments, often associated with chronic RPE changes and failure to recover high-quality central VA. Moreover, choroidal neovascularization (CNV) may occur as a complication of CSCR, and is associated with progressive and permanent visual loss. The risk of CNV from previous CSCR has been established as <5%, but has an increasing frequency in older patients.4 Despite a return to good central VA, many of these patients still notice residual symptoms as dyschromatopsia, loss of contrast sensitivity, or metamorphopsia.
Melatonin is known to be involved in the regulation of many physiological functions, including the entrainment of seasonal and circadian rhythms. In humans, melatonin participates in the regulation of sleep, seasonal disorders, and aging.5 Moreover, antitumoral properties of melatonin, as well as its involvement in the responsiveness of the immune system, have been described.6, 7 Besides the pineal gland, melatonin is also biosynthesized in the retina, where it behaves as an endogenous neuromodulator.8
Although it has been shown that melatonin may provide neuroprotection in different systems,9 the full range of actions of melatonin is still not completely known. In that context, experimental evidence supports the actions of it and its metabolites as a direct and indirect antioxidant,10, 11 scavenging free radicals,11 stimulating antioxidant enzymes,12 and enhancing the activities of other antioxidants.12 Moreover, several lines of evidence suggest that melatonin may act as a protective agent in ocular conditions such as photokeratitis, cataract, retinopathy of prematurity, and ischemia/reperfusion injury.13, 14 We have previously shown the beneficial effect of melatonin against retinal glaucomatous15 and diabetic16 damage. In addition to its antioxidant effects, several other mechanisms may be involved in neuroprotection induced by melatonin, like the inhibition of the nitridergic pathway,17 decrease in vascular endothelial growth factor (VEGF) levels,6 as well as its inhibitory effect on glucocorticoid actions.18
At present, there are no optimal therapies for CSCR; current treatments have limited effectiveness and a significant number of side effects. It has been demonstrated that even at very high concentrations given during pregnancy, melatonin had no maternal or fetal toxicity.19 In this context, the aim of this report was to analyze the safety and efficacy of melatonin in the treatment of CSCR.
Materials and methods
Patients
This work received institutional review board approval from the Oulton-Romagosa Joint Committee on Clinical Investigation (C.I.E.I.S OULTON-Romagosa). Patients of any gender and >18 years old with diagnosis of chronic CSCR, as demonstrated by complete eye examination, fluorescein angiography (FA), and optical coherence tomography (OCT), were included in the study. Patients must have had a best corrected Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity worse than 20/40, a central scotoma, or incapacitating metamorphopsia in the affected eye, and the ability to comply with the study protocol.
Exclusion criteria were as follows: patients with significantly compromised VA in the studied eye due to concomitant ocular conditions, history of vitrectomy in the studied eye, history of prior laser treatment or anti-VEGF therapy in the past 3 months, any suspicion of CNV, patients participating in any other investigational drug study, pregnant or nursing patients, inability to obtain photographs (including difficulties with venous access), or patients with known adverse reaction to fluorescein. All participants were informed about the scope and purpose of the study, and told that it was voluntary to participate, without compensation, and that their medical care would not be compromised if they refused to participate in the study. A full and detailed explanation of the proposed interventions was given to all patients and informed consent was obtained in every case.
Patients were randomly selected for receiving melatonin or placebo. A regimen of 3 mg three times a day (9 mg/day) was administrated for a period of 1 month to 8 patients (denoted numbers 1 to 8) with chronic, relapsing CSCR, defined as persistence of the detachment for >6 months, or chronic recurrent acute detachments with widespread compromise of the RPE. This dose of melatonin was chosen because it is well tolerated in children, adolescents, and young adults without reporting any side effects.20 Five patients (denoted numbers 9 to 13) with similar clinical characteristics served as control. Sugar pills were administered as placebo.
The primary end point was 4 weeks and afterwards patients were followed up for 1 year. The VA was converted to decimal equivalents and then to a logarithm of the minimum angle of resolution (logMAR) scale before being averaged. The FA images of the eyes were obtained using VISUCAMlite Digital Camera (Carl Zeiss Meditec, Dublin, CA, USA) and the OCT images were obtained using Cirrus high-resolution spectral domain-OCT (SD-OCT) system (Carl Zeiss Meditec). OCT scanning was carried out by a retinal specialist who was blind with respect to the treatment applied to each patient. Cirrus high-resolution SD-OCT scans were performed using the Macular Cube 512 × 128 scanning protocol and the HD 5 line raster protocol. Scan inclusion criteria allow only images with signal strength of ≥7. Percent variation was calculated to compare the baseline best-corrected visual acuity (BCVA; logMAR) with the final BCVA.
Data were subjected to two-sample t-test statistical analysis using SPSS version (Chicago, IL, USA) 11.5 statistics program. P-values of <0.05 were considered statistically significant.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Clinical and ophthalmologic characteristics of patients with chronic CSCR treated with melatonin or placebo are listed in Table 1. Eight patients were treated with melatonin. The mean age of melatonin-treated patients was 46.6 years (range, 40–56), with the woman/man ratio being 1 : 7. The number of right and left eyes studied was similar. The average number of episodes was 3.87 per patient, with a range of 1 to 8. Five patients with chronic CSCR served as a control group. The mean age of control patients was 44 years old (range, 34–53), with the woman/man ratio being 1 : 4. The number of right and left eyes studied was also similar (3 right eyes and 2 left eyes). The average number of episodes was 2 per patient, with a range of 1 to 3.
Table 1. Clinical and ophthalmologic characteristics of melatonin-treated and control patients.
| Patient no. | Age (years) | Gender | Eye | Symptom/s | Duration of symptoms | No. of episodes | Mean duration of symptoms within each episode | Previous treatment/s |
|---|---|---|---|---|---|---|---|---|
| 1 | 48 | Male | OD | Central scotoma | 9 Days | 3rd | The patient failed to recall | NSAIDs; steroids |
| 2 | 45 | Female | OS | Central scotoma; dischromatopsia | 1 Month | 2nd | 4 Months | None |
| 3 | 42 | Male | OS | Decreased VA | 9 Months | 4th | The patient failed to recall | IVB |
| 4 | 56 | Male | OD | Decreased VA; metamorphopsia | Almost never symptoms free since 1983 | 8th | Patient could only state that each episode lasted longer | None |
| 5 | 40 | Male | OD | Central scotoma; metamorphopsia | 3 Years | 2nd | About 1 year and a half | Topical drug (does not know which) |
| 6 | 42 | Male | OS | Decreased VA; metamorphopsia | 2 Years | 1st | NA | IVB |
| 7 | 48 | Male | OS | Decreased VA; metamorphopsia; micropsia | Almost never symptom free since 1992 | 6th | Patient could only state that each episode lasted longer | Argon/thermal laser |
| 8 | 52 | Male | OD | Decreased VA | 10 Months | 5th | The patient failed to recall | IVB |
| 9 | 40 | Male | OS | Central scotoma | 3 Months | 2nd | The patient failed to recall | None |
| 10 | 44 | Female | OS | Central scotoma; decreased VA | 4 Months | 2nd | ∼6 months | None |
| 11 | 53 | Male | OD | Decreased VA; metamorphopsia | >20 Months | 1st | NA | IVB |
| 12 | 49 | Male | OD | Central scotoma | 6 Months | 2nd | ∼9 Months | Anti-VEGF |
| 13 | 34 | Male | OD | Central scotoma; decreased VA | 9 Months | 3rd | The patient failed to recall | IVB |
Abbreviations: IVB, intravitreal bevacizumab; NA, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; OD, right eye; OS, left eye; VA, visual acuity.
Melatonin-treated patients: 1–8; control patients: 9–13.
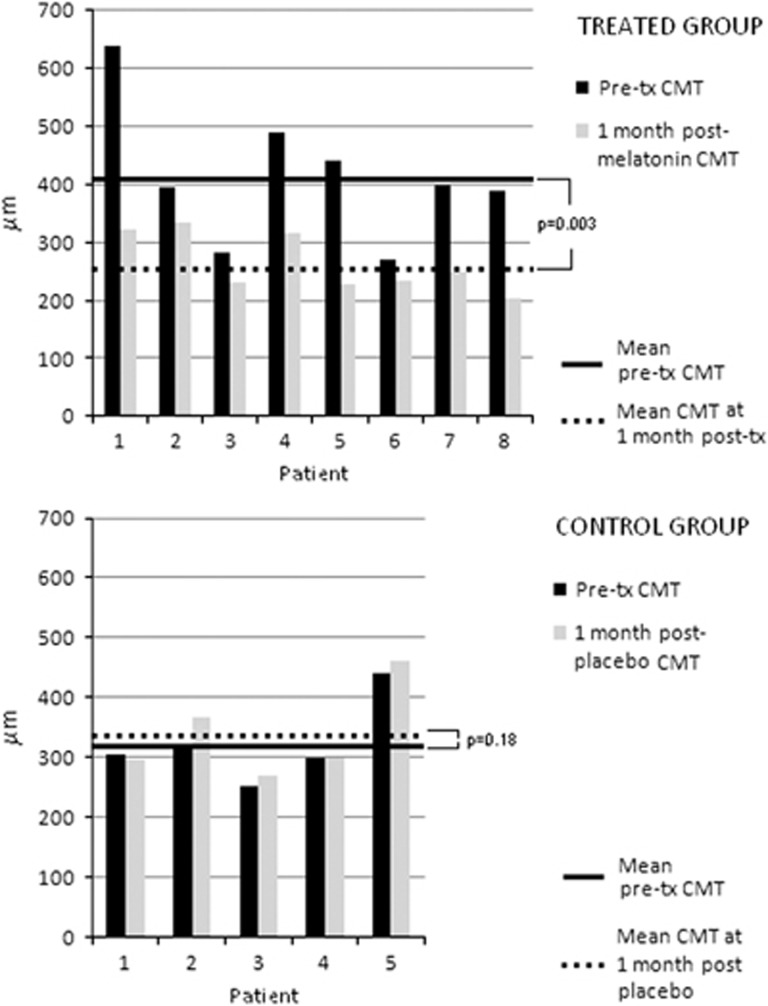
In the melatonin group, pretreatment ETDRS BCVA ranged from 20/20 to 20/63 and all patients were experiencing new visual changes before starting the treatment. Mean pretreatment logMAR BCVA improved from 0.29 at baseline to 0.12 at 1-month follow-up, with the change in VA being statistically significant (P<0.05; interval coefficient: 95%, Figure 1). All patients treated with melatonin showed a reduction in central macular thickness (CMT) within the same period, with complete resolution of subretinal fluid (SRF) in 3 patients (37.5%, patient nos. 5, 6, and 7). When compared with baseline CMT, a mean decrease of 34.7% was achieved at 1 month of treatment. Mean CMT at baseline was 413.3±117.8 μm (range, 315–512) and it was reduced to 264.5±57.8 μm (range, 202–335) at the end of the follow-up time (Table 2). As shown in Figure 2, this reduction was statistically significant (P=0.003; interval coefficient: 95%). Representative OCT images of a patient (patient no. 7) before and after 1 month of melatonin treatment are shown in Figure 3. In the control group, mean pretreatment logMAR BCVA changed from 0.28 at baseline to 0.36 at 1-month follow-up, with the change in VA being nonstatistically significant (average percentage change 6.12%, P=0.391; interval coefficient: 95%). Regarding retinal thickness, mean CMT at baseline was 323.6±71.4 μm (range, 252–442) and it increased to 338.2±76.8 μm (range, 270–460) at the end of the follow-up time (Table 2). The mean change in CMT of control patients did not differ along the study (14.60±20.02, P=0.178; interval coefficient: 95%). In contrast, the average difference between the initial and the final CMT when comparing melatonin-treated patients with controls (−148.75 y +14.60, respectively) was statistically significant (P=0.002).
Figure 1.
Effect of melatonin on logMAR BCVA in patients with CSCR. Melatonin (3 mg/day t.i.d.) significantly increased logMAR BCVA at 1-month follow-up, whereas no significant changes in this parameter were noted in the control group (*P<0.05 vs pretreatment values, by two-sample t-test (n=9, for melatonin group; n=5, for control group)).
Table 2. Effect of melatonin on mean BCVA and CMT.
| Patient no. | Pre-tx BCVA | Pre-tx CMT (μm) | BCVA at 1-month post tx | CMT at 1-month post tx (μm) | Changes in BCVA (letters) | Changes in CMT (μm) |
|---|---|---|---|---|---|---|
| 1 | 20/63 | 640 | 20/20 | 323 | +25 | −317 |
| 2 | 20/32− | 396 | 20/25 | 335 | +6 | −61 |
| 3 | 20/50−2 | 281 | 20/70 | 230 | −13 | −51 |
| 4 | 20/50−2 | 490 | 20/20 | 316 | +22 | −174 |
| 5 | 20/20 | 441 | 20/20 | 227a | 0 | −214 |
| 6 | 20/50 | 270 | 20/32− | 233a | +9 | −37 |
| 7 | 20/40−2 | 399 | 20/20 | 250a | +17 | −149 |
| 8 | 20/32+1 | 389 | 20/32+2 | 202 | +1 | −187 |
| 9 | 20/20−1 | 304 | 20/40 | 295 | −14 | −9 |
| 10 | 20/50+1 | 322 | 20/50−1 | 366 | −2 | +44 |
| 11 | 20/50 | 252 | 20/50+1 | 270 | +1 | +18 |
| 12 | 20/32 | 298 | 20/32−1 | 300 | −1 | +2 |
| 13 | 20/50−2 | 442 | 20/63−1 | 460 | −4 | +18 |
Abbreviations: BCVA, best-corrected visual acuity; CMT, central macular thickness; tx, treatment.
Melatonin-treated patients: 1–8; control group: patients 9–13.
Complete resolution of subretinal fluid and/or pigment epithelial detachment (PED).
Figure 2.
Effect of melatonin on CMT. The treatment with melatonin (3 mg/day t.i.d.) induced a significant decrease in this parameter at 1-month follow-up, whereas no significant changes in CMT were observed in the control group (*P=0.003 vs pretreatment values, by two-sample t-test (n=9, for melatonin group; n=5, for control group)).
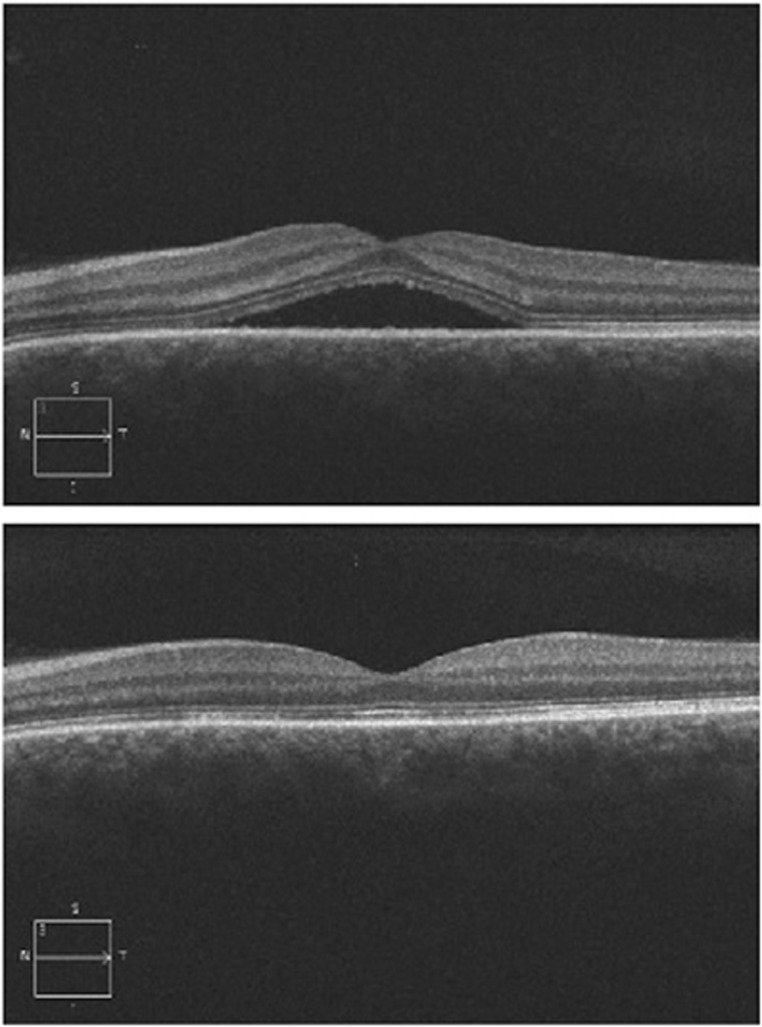
Figure 3.
Representative OCT images of a patient (patient no. 7), before (upper panel) and after (lower panel) 1-month treatment with melatonin. Note the complete resolution of subretinal fluid after 1-month treatment. It is it is worth noting that, as stated in Table 1, the patient could not the recall last time that he was symptom free.
Only one case of recurrence was observed in the melatonin-treated group at 1-year follow-up. Patient no. 2 showed an initial reduction in CMT with improvement in BCVA and decreased dischromatopsia, but increased macular elevation with drop in BCVA developed yet again at ∼5 months after treatment. No significant drug-related side effects were observed at 1-year follow-up. Two patients referred some drowsiness at the first day on treatment, with normalization after ∼2 days.
Discussion
The present results suggest that melatonin is safe, well tolerated, and effective in the treatment of chronic CSCR. The uses of the conventional forms of treatment for CSCR are restricted by their limited success and significant side effects. Focal laser photocoagulation therapy has been described as the first therapeutic option for the treatment of long-lasting CSCR.21 However, this approach is not usually beneficial in the handling of chronic CSCR as there is not an easily identifiable leakage point, but rather a diffuse dysfunction of the RPE. Moreover, laser treatment can induce a permanent scotoma and the development of choroidal neovascular membranes.22 More recently, several authors have attempted photodynamic therapy (PDT) as a new therapeutic option, with acceptably good results in VA recovery.23, 24 Nevertheless, this alternative management is also not devoid of ocular adverse effects, as PDT has been associated to choroidal atrophy with retinal toxicity,25, 26 as well as with the development of CNV.27 Even though changes in PDT parameters are currently explored in order to reduce the possible side effects of PDT, controversy still exists regarding timing and fluence of administration.1
Other treatment options for CSCR include the use of intravitreal bevacizumab (IVB),28 oral propanolol,29 low-dose aspirin,30 mifepristone,31 and systemic administration of ketoconazole.32 However, further evidence is needed to substantiate these potential treatments, as inconsistent results regarding the efficacy of these interventions have been reported in the literature.32, 33
At present, the pathophysiology of CSCR remains unclear. Several factors have been implicated in the initiation and development of this retinal disorder, including immunological reactions, toxins, infections, and neuronal, hormonal, or circulatory processes.34, 35, 36, 37, 38 One theory suggests that there is a focal increase in the choriocapillaris permeability that causes damage to the overlying RPE, and it is suspected that the disturbance would originate in an alteration of the mechanisms of choroidal blood flow autoregulation.39 An alternative hypothesis postulates that CSCR is provoked by a dysfunction of the RPE that, in turn, causes a reverse in fluid movement in a chorioretinal direction, leading to subretinal space leakage and retinal detachment.40 Furthermore, autonomic function is impaired in patients with CSCR.41 As autonomic supply modulates the choroidal blood flow, there might be a correlation between measures of autonomic function and the occurrence of CSCR. In experimental studies in monkeys, findings resembling CSCR were produced by repeated intravenous administration of adrenaline.42 In addition, exogenous corticosteroid use or elevated endogenous corticosteroid levels (eg, Cushing syndrome) have been found to be significant risk factors for CSCR.43 Moreover, a case report showing improvement of CSCR following MR antagonist eplerenone administration has been published.44
The present results indicate that all melatonin-treated patients showed a reduction in CMT and that mean pretreatment logMAR BCVA improved from 0.29 to 0.12 at 1-month follow-up, even when it is well known that still after complete resolution of SRF, BCVA does not improve in some patients because of previous damage at the RPE inner and outer segment photoreceptor level, alterations that were present in some of our patients. Although the natural course of CSCR is mostly of spontaneous resolution, it should be noted that no changes on VA or CMT were observed in the control group, that the clinical conditions of our patients were stable for months/years before being included in these case series, that melatonin-treated patients yet improved even at 1 week of treatment with melatonin (data not shown), and that this improvement continued through week 4.
When attempting new therapies for CSCR, physicians usually include patients without prior treatments.45, 46 In contrast, in our study, only one patient was naive of medical care, whereas the rest of the patients failed to respond to other treatment modalities before being included in this trial. Three of our patients had a past medical history of IVB, and patient no. 7 had a previous laser treatment. Notably, in the melatonin-treated group, we did not notice a high recurrence rate as reported with other handling modalities.47 As already mentioned, one of the patients had a new reactivation after 5 months, and we treated her with melatonin without achieving improvement on her BCVA or a reduction on CMT in this second opportunity, and hence a new attempt was to carry on doubling the dose. With 18 mg/day, she achieved a complete resolution of the pigment epithelium detachment (PED) and 20/20 vision (data not show). This patient is still using melatonin at a lower dose, once a day, at her own discretion, and she has been disease free for 2 years. This result could suggest that higher doses should be considered particularly in those cases unresponsive to lower doses. In this vein, Nordlund et al48 have shown that high doses of melatonin (ie, 1 g per day for 30 days) show no evidence of eye, liver, kidneys, or bone marrow toxicity.
Current data remain incapable of addressing how melatonin acts to benefit CSCR patients. It is well known that VEGF contribute to the breakdown of the blood–retinal barrier (BRB) and subsequent macular edema in various retinal pathologies. We have previously shown that melatonin decreases retinal levels of VEGF in an experimental model of type II diabetes in rats.16 Moreover, we showed that melatonin significantly attenuates biochemical, clinical, histological, ultrastructural, and functional alterations induced by experimental uveitis, and it preserves the BRB integrity.49 As already mentioned, glucocorticoids induce and aggravate CSCR. The antagonism exerted by melatonin on the glucocorticoid response has been well established. In fact, it was shown that the inhibitory effect on glucocorticoid actions is involved in melatonin modulation of the immune system.7 Although the molecular mechanisms regarding the antagonism between melatonin and glucocorticoids are still unclear, it was shown that melatonin inhibits glucocorticoid receptor mRNA expression50 and modulates glucocorticoids receptor ligand interaction.51 Furthermore, we have demonstrated that melatonin inhibits apoptosis of rat thymocytes induced by glucocorticoid.18
Melatonin and its metabolites have a potent protective action against oxidative stress in neurons through direct and indirect mechanisms. In retina, this antioxidant activity is achieved by its ability to scavenge light-induced free radicals, reducing lipid peroxidation, increasing the activity of antioxidant enzymes, and inhibiting the nitridergic pathway.13, 17 Moreover, melatonin has a protective effect on the RPE against oxidative damage. Based on these lines of evidence, it seems likely that melatonin can behave as an antioxidant, anti-inflammatory, anti-VEGF, and antiglucocorticoid therapy, among other mechanisms, in the context of CSCR. In addition, melatonin might decrease norepinephrine plasmatic levels, as previously described,52 or it may have antiadrenergic effects through the choroid-melatonin receptor.53
One of the limitations of this study is the relatively small number of patients; however, the prevalence of CSCR has been estimated as low as 5.8 per 100 000 population, with chronic CSCR reported to be as infrequent as 5% of those cases, making very difficult to obtain larger recruitment groups. Another limitation of this study is the difficulty in obtaining reliable data about previous episodes of the patient's disease as most of them cannot recall the prior events with precision. Notwithstanding, as the administration of melatonin is easy, cheap, safe (even at high doses), and may benefit patients with chronic CSCR, decreasing the rate of other therapy-induced complications, the therapeutic use of melatonin for CSCR is particularly worthy of further examination.
| Activity evaluation | ||||
| 1. The activity supported the learning objectives. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly disagree | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 |
The authors declare no conflict of interest.
Footnotes
This submission has not been previously published anywhere and it is not simultaneously being considered for any other publication. It was partially presented at the VII National Meeting of the Asociación de Investigacion en Visión y Oftalmología (AIVO), Córdoba, Argentina, November 2010, and at ARVO 2014 Annual Meeting, 5–9 May 2013. Seattle, WA (Program Number: 6374 Poster Board Number: C0151).
References
- Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86:126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115:169–173. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Yap EY, Robertson DM. The long-term outcome of central serous chorioretinopathy. Arch Ophthalmol. 1996;114:689–692. doi: 10.1001/archopht.1996.01100130681007. [DOI] [PubMed] [Google Scholar]
- Gomolin JE. Choroidal neovascularization and central serous chorioretinopathy. Can J Ophthalmol. 1989;24:20–23. [PubMed] [Google Scholar]
- Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol. 2008;4:436–447. doi: 10.1038/ncpneuro0847. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett. 2001;22:45–47. [PubMed] [Google Scholar]
- Nelson RJ, Drazen DL. Melatonin mediates seasonal adjustments in immune function. Reprod Nutr Dev. 1999;39:383–398. doi: 10.1051/rnd:19990310. [DOI] [PubMed] [Google Scholar]
- Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 2010;8:228–242. doi: 10.2174/157015910792246155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla P, Sun C, O'rourke ST. Melatonin inhibits nitric oxide signaling by increasing PDE5 phosphorylation in coronary arteries. Am J Physiol Heart Circ Physiol. 2012;303:H1418–H1425. doi: 10.1152/ajpheart.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Xiao X, Wang J, Liu L, Chen W, Fu L, et al. Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-κB, c/EBPβ, and p300 signaling. J Pineal Res. 2012;53:154–165. doi: 10.1111/j.1600-079X.2012.00982.x. [DOI] [PubMed] [Google Scholar]
- Siu AW, Maldonado M, Sanchez-Hidalgo M, Tan DX, Reiter RJ. Protective effects of melatonin in experimental free radical-related ocular diseases. J Pineal Res. 2006;40:101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- Alio JL, Ayala MJ, Mulet E, Artola A, Bellot JL, Ruiz-Moreno JM. Treatment of experimental acute corneal inflammation with inhibitors of the oxidative metabolism. Ophthalmic Res. 1993;25:331–336. doi: 10.1159/000267333. [DOI] [PubMed] [Google Scholar]
- Belforte NA, Moreno MC, de Zavalía N, Sande PH, Chianelli MS, Keller Sarmiento MI, et al. Melatonin: a novel neuroprotectant for the treatment of glaucoma. J Pineal Res. 2010;48:353–364. doi: 10.1111/j.1600-079X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- Salido EM, Bordone M, De Laurentiis A, Chianelli M, Keller Sarmiento MI, Dorfman D, et al. Therapeutic efficacy of melatonin in reducing retinal damage in an experimental model of early type 2 diabetes in rats. J Pineal Res. 2013;54:179–189. doi: 10.1111/jpi.12008. [DOI] [PubMed] [Google Scholar]
- Sáenz DA, Turjanski AG, Sacca GB, Marti M, Doctorovich F, Sarmiento MI, et al. Physiological concentrations of melatonin inhibit the nitridergic pathway in the Syrian hamster retina. J Pineal Res. 2002;33:31–36. doi: 10.1034/j.1600-079x.2002.01880.x. [DOI] [PubMed] [Google Scholar]
- Hoijman E, Rocha Viegas L, Keller Sarmiento MI, Rosenstein RE, Pecci A. Involvement of Bax protein in the prevention of glucocorticoid-induced thymocytes apoptosis by melatonin. Endocrinology. 2004;145:418–425. doi: 10.1210/en.2003-0764. [DOI] [PubMed] [Google Scholar]
- Giles W, Bisits A. Preterm labour. The present and future of tocolysis. Best Pract Res Clin Obstet Gynaecol. 2007;21:857–868. doi: 10.1016/j.bpobgyn.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Coppola G, Iervolino G, Mastrosimone M, La Torre G, Ruiu F, Pascotto A. Melatonin in wake-sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: a double-blind, cross-over, placebo-controlled trial. Brain Dev. 2004;26:373–376. doi: 10.1016/S0387-7604(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979;63:674–677. doi: 10.1136/bjo.63.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997;104:616–622. doi: 10.1016/s0161-6420(97)30262-0. [DOI] [PubMed] [Google Scholar]
- Wali UK, Al-Kharousi N, Hamood H. Photodynamic therapy with verteporfin for chronic central serous choroidoretinopathy and idiopathic choroidal neovascularization-first report from the sultanate of oman. Oman Med J. 2008;23:282–286. [PMC free article] [PubMed] [Google Scholar]
- Tarantola RM, Law JC, Recchia FM, Sternberg P, Jr, Agarwal A. Photodynamic therapy as treatment of chronic idiopathic central serous chorioretinopathy. Lasers Surg Med. 2008;40:671–675. doi: 10.1002/lsm.20720. [DOI] [PubMed] [Google Scholar]
- Rouvas AA, Papakostas TD, Ladas ID, Vergados I. Enlargement of the hypofluorescent post photodynamic therapy treatment spot after a combination of photodynamic therapy with an intravitreal injection of bevacizumab for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2008;246:315–318. doi: 10.1007/s00417-007-0669-3. [DOI] [PubMed] [Google Scholar]
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752–763. doi: 10.1097/00006982-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006;26:239–242. doi: 10.1097/00006982-200602000-00027. [DOI] [PubMed] [Google Scholar]
- Inoue M, Kadonosono K, Watanabe Y, Kobayashi S, Yamane S, Arakawa A. Results of one-year follow-up examinations after intravitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica. 2011;225:37–40. doi: 10.1159/000314709. [DOI] [PubMed] [Google Scholar]
- Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J Ocul Pharmacol Ther. 2006;22:145–149. doi: 10.1089/jop.2006.22.145. [DOI] [PubMed] [Google Scholar]
- Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903. doi: 10.2147/opth.s12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011;31:1928–1936. doi: 10.1097/IAE.0b013e31821c3ef6. [DOI] [PubMed] [Google Scholar]
- Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27:943–946. doi: 10.1097/IAE.0b013e318050ca69. [DOI] [PubMed] [Google Scholar]
- Lim JW, Ryu SJ, Shin MC. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol. 2010;24:155–158. doi: 10.3341/kjo.2010.24.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SI-BOEN-LIAN The etiological agent of serous central chorioretinitis. Ophthalmologica. 1964;148:263–267. doi: 10.1159/000304784. [DOI] [PubMed] [Google Scholar]
- Cotticelli L, Borrelli M, D'alessio AC, Menzione M, Villani A, Piccolo G, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274–278. doi: 10.1177/112067210601600213. [DOI] [PubMed] [Google Scholar]
- Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath. 2007;11:253–257. doi: 10.1007/s11325-007-0112-3. [DOI] [PubMed] [Google Scholar]
- Carvalho-Recchia CA, Yannuzzi LA, Negrão S, Spaide RF, Freund KB, Rodriguez-Coleman H, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834–1837. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- Tittl M, Maar N, Polska E, Weigert G, Stur M, Schmetterer L. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2005;46:4717–4721. doi: 10.1167/iovs.05-0268. [DOI] [PubMed] [Google Scholar]
- Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986;224:321–324. doi: 10.1007/BF02150023. [DOI] [PubMed] [Google Scholar]
- Tewari HK, Gadia R, Kumar D, Venkatesh P, Garg SP. Sympathetic-parasympathetic activity and reactivity in central serous chorioretinopathy: a case-control study. Invest Ophthalmol Vis Sci. 2006;47:3474–3478. doi: 10.1167/iovs.05-1246. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Katsume Y, Akune H. Experimental central serous chorioretinopathy in monkey eyes: fluorescein angiographic findings. Ophthalmologica. 1982;185:168–178. doi: 10.1159/000309239. [DOI] [PubMed] [Google Scholar]
- Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110:698–703. doi: 10.1016/S0161-6420(02)01975-9. [DOI] [PubMed] [Google Scholar]
- Gruszka A. Potential involvement of mineralocorticoid receptor activation in the pathogenesis of central serous chorioretinopathy: case report. Eur Rev Med Pharmacol Sci. 2013;17 (10:1369–1373. [PubMed] [Google Scholar]
- Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:100–106. doi: 10.1097/IAE.0b013e3181bcf0b4. [DOI] [PubMed] [Google Scholar]
- Rouvas A, Stavrakas P, Theodossiadis PG, Stamatiou P, Milia M, Giannakaki E, et al. Long-term results of half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Eur J Ophthalmol. 2012;22:417–422. doi: 10.5301/ejo.5000051. [DOI] [PubMed] [Google Scholar]
- Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010;149:441–446. doi: 10.1016/j.ajo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. 1977;45:768–774. doi: 10.1210/jcem-45-4-768. [DOI] [PubMed] [Google Scholar]
- Sande PH, Fernandez DC, Aldana Marcos HJ, Chianelli MS, Aisemberg J, Silberman DM, et al. Therapeutic effect of melatonin in experimental uveitis. Am J Pathol. 2008;173:1702–1713. doi: 10.2353/ajpath.2008.080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Tan DX, Reiter RJ, Kim SJ, Manchester LC, Cabrera J, et al. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci. 1999;44:2257–2262. doi: 10.1023/a:1026656720868. [DOI] [PubMed] [Google Scholar]
- Blackhurst G, Mcelroy PK, Fraser R, Swan RL, Connell JM. Seasonal variation in glucocorticoid receptor binding characteristics in human mononuclear leucocytes. Clin Endocrinol (Oxf) 2001;55:683–688. doi: 10.1046/j.1365-2265.2001.01383.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Yasue H, Moriyama Y, Tsunoda R, Ogawa H, Yoshimura M, et al. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am Heart J. 2001;141:E9. doi: 10.1067/mhj.2001.114368. [DOI] [PubMed] [Google Scholar]
- Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J Pineal Res. 2008;45:449–458. doi: 10.1111/j.1600-079X.2008.00615.x. [DOI] [PubMed] [Google Scholar]