Abstract
FlyVar is a publicly and freely available platform that addresses the increasing need of next generation sequencing data analysis in the Drosophila research community. It is composed of three parts. First, a database that contains 5.94 million DNA polymorphisms found in Drosophila melanogaster derived from whole genome shotgun sequencing of 612 genomes of D. melanogaster. In addition, a list of 1094 dispensable genes has been identified. Second, a graphical user interface (GUI) has been implemented to allow easy and flexible queries of the database. Third, a set of interactive online tools enables filtering and annotation of genomic sequences obtained from individual D. melanogaster strains to identify candidate mutations. FlyVar permits the analysis of next generation sequencing data without the need of extensive computational training or resources.
Database URL: www.iipl.fudan.edu.cn/FlyVar.
INTRODUCTION
With the rapid progress of next generation sequencing (NGS) technology, analysis and especially interpretation of large amounts of sequencing data is complex. One of the most common applications of NGS technology is to identify causative mutations. Unfortunately, the vast majority of genomic variants in any individual are polymorphisms that do not affect gene function in an obvious fashion (1). One of the most effective ways to distinguish a damaging mutation from a polymorphism is by comparing affected and control genomes. In general, variations that show no enrichment in affected individuals, and/or are shared by a large number of controls, are likely to be benign polymorphisms and can often be excluded from further investigation (2, 3). The efficiency of this filtering step largely depends on the size of the control cohort. In humans, several large scale projects have been carried out to categorize variants of the human genome, such as the 1000 genomes project (4). Results obtained from these projects are essential resources for human genetics research. Similar NGS based methods have also been shown to be practical for mutation identification in model organisms, including D. melanogaster (5, 6). However, the key challenge is that the density of polymorphisms is high among fly strains. Recent studies of natural populations indicate the presence of more than 1 polymorphism per 500 base pairs in the fly genome, more than two times higher than that in the human genome (6–8). As a result, identification of a causative mutation among such a large number of polymorphisms is challenging. The best approach to solve this issue is to sequence parental strains from which a mutant is derived and use it as the control to identify variants that are unique to the mutant strain (9). However, this approach cannot be applied to the vast majority of the existing mutants that have been accumulated over the past 100 years as their parental strains do not exist anymore. Alternatively, similar to the human genetics field, a database containing a large number of variants observed from control individuals could be used to filter out polymorphisms. Existing databases usually contain a relatively small number of variants, ranging from several hundreds to tens of thousands (10–13). Recently dbSNP reports 5.2 million sequence variations for D. melanogaster, in which these variants could be queried. While, none of these databases offer variant filtering tools that remove out benign variants from raw sequence data to help narrow down mutation candidates.
To resolve this issue, we have constructed a database, FlyVar (www.iipl.fudan.edu.cn/FlyVar), by collecting data from both our own and public whole genome sequencing (WGS) projects. Currently, FlyVar contains 4.86 million single nucleotide polymorphisms (SNPs) and 1.08 million indels. A set of web based interactive tools has been developed to enable easy filtering of each variant to evaluate and prioritize variants. Through the GUI, users will be able to efficiently analyse individual fruit fly genome data without the requirement of extensive bioinformatics skills or computational resources.
DATA SOURCE AND PROCESSING
Variants were collected from both our internal data and public data. Most of the internal data comes from the sequencing of a collection of X chromosome lethal mutants (9). In brief, EMS mutagenesis was performed and individual fly strains carrying lethal mutations on the X chromosome were established. Since the parental fly strains used for mutagenesis were isogenized for the X chromosome only, WGS of individual mutant strains allowed us to identify autosomal variants existing in the population of parental and balancer stocks. As a result, 0.92 million variants were identified. To further increase the size of the database, we have also deposited variants obtained from public sequencing data. Most of the public data are derived from the Drosophila Genome Research Project (DGRP) (14). For the DGRP, flies were collected at a food market in Raleigh, NC, USA and were used to establish many inbred strains, which were extensively phenotyped. WGS was performed for 205 inbred strains, from which 6.14 million variants were identified (15). As all these inbred strains are viable and free of apparent developmental defects, homozygous variants in these strains are likely to have minimal effects on development. Hence, a total of 4.7 million homozygous variants were identified and added into the FlyVar database. In addition, we have also included variants identified in four studies (7, 14, 16–18). All of these projects consisted of WGS of natural populations of D. melanogaster. From 49 WGS data sets, we were able to include an additional 0.32 million variants into the database. In total, 612 WGS data of D. melanogaster strains was collected into FlyVar, representing the most comprehensive data set.
SUMMARY OF VARIANTS
A summary of variants by chromosome and their annotation is shown in Table 1 for SNPs and Table 2 for small insertion/deletions. To avoid potential mutations generated by EMS on X chromosome, only variants from the autosomes are included in our database for the X chromosome lethal screen project.
Table 1.
Summary of SNPs in fly
| SNPs/MNPs | Chr2L | Chr2R | Chr3L | Chr3R | ChrX | Chr4 |
|---|---|---|---|---|---|---|
| No. sites overall | 1 109 349 | 916 178 | 110 5112 | 1 079 279 | 638 526 | 13552 |
| Variant density(per kb) | 44.2539 | 43.4374 | 45.0892 | 38.7591 | 28.6962 | 10.4878 |
| No. in Splicing | 1013 | 1130 | 962 | 1130 | 403 | 20 |
| No. in UTR3 | 26 354 | 28 092 | 27 875 | 30 862 | 17 759 | 469 |
| No. in UTR5 | 20 774 | 21 906 | 22 029 | 23 413 | 11 741 | 286 |
| No. in upstreama | 74 083 | 70 417 | 73205 | 74 496 | 39 628 | 822 |
| No. in downstreamb | 59 859 | 55128 | 60 261 | 59 652 | 34 243 | 744 |
| No. in intergenic | 351 864 | 238 941 | 349 703 | 302 888 | 206 443 | 3484 |
| No. in intron | 437 754 | 370 587 | 439 610 | 450 121 | 258 369 | 6271 |
| No. in exon | 137 657 | 129 977 | 131 467 | 136 717 | 69 940 | 1456 |
| Synonymous | 89 032 | 83 262 | 83 274 | 84 094 | 46 512 | 604 |
| Nonsynonymous | 48 058 | 46 159 | 47 688 | 52 017 | 23 224 | 842 |
| Stop loss | 65 | 75 | 57 | 83 | 26 | 0 |
| Stop gain | 502 | 481 | 448 | 523 | 178 | 10 |
aUpstream: variant overlaps 1-kb region upstream of transcription start site.
bDownstream: variant overlaps 1-kb region downstream of transcription end site.
Table 2.
Summary of indels in fly
| INDELs | Chr2L | Chr2R | Chr3L | Chr3R | ChrX | Chr4 |
|---|---|---|---|---|---|---|
| No. sites overall | 236 300 | 195 139 | 243 431 | 225 411 | 177 697 | 3927 |
| Variant density (per kb) | 10.2785 | 9.2518 | 8.7421 | 8.0950 | 7.9859 | 3.0391 |
| No. in splicing | 155 | 153 | 140 | 172 | 82 | 6 |
| No. in UTR3 | 6711 | 7318 | 7371 | 8063 | 7135 | 134 |
| No. in UTR5 | 4293 | 4432 | 4712 | 4613 | 3596 | 79 |
| No. in upstreama | 16 877 | 16 368 | 16 841 | 16 417 | 10 128 | 241 |
| No. in downstreamb | 14 759 | 13 530 | 14 953 | 14 398 | 10 528 | 274 |
| No. in intergetic | 81 545 | 57 305 | 85 089 | 68 854 | 61 799 | 1137 |
| No. in intron | 108 098 | 92432 | 110 465 | 108 684 | 81 361 | 1991 |
| No. in exon | 3862 | 3601 | 3860 | 4210 | 3068 | 65 |
| Synonymous | 1353 | 1219 | 1103 | 1352 | 666 | 35 |
| Nonsynonymous | 2485 | 2351 | 2721 | 2832 | 2414 | 25 |
| Stop loss | 2 | 3 | 6 | 10 | 3 | 0 |
| Stop gain | 57 | 85 | 75 | 78 | 22 | 8 |
aUpstream: Variant overlaps 1-kb region upstream of transcription start site.
bDownstream: Variant overlaps 1-kb region downstream of transcription end site.
Both Tables 1 and 2 show that, the variant density, which is defined as the total number of variants per kb, is similar for the four arms of chromosome 2 and 3 at about 1 polymorphism per 20 base pairs. Variant density for the X chromosome is markedly lower. This is true even when we take the fact that variants on the X chromosome from the X chromosome lethal project are excluded. This is consistent with the idea that the X chromosome is under higher purify selection as males only have a single copy (19, 20).
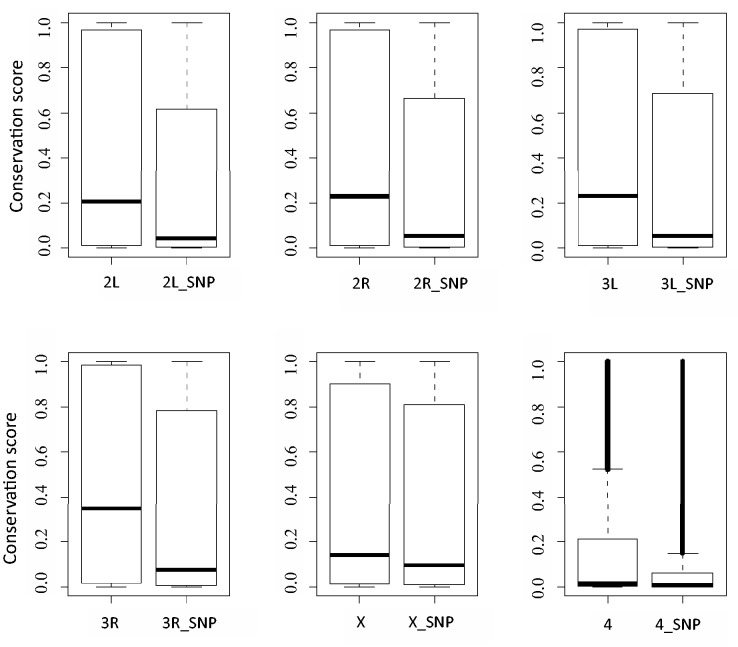
As expected, the vast majority of the selected variants are likely to be benign polymorphisms for the following reasons. First, variants are enriched in noncoding region as about 70% of them are located in intergenic or intronic regions. Second, for variants that map to exons, 65% are synonymous, not affecting the amino acid sequence of the final protein product. Third, these variants tend to be less conserved bases. As shown in Figure 1, the average level of evolutionary conservation for the polymorphism bases is lower than that across the genome. It is worth noting that though many polymorphism sites are highly conserved, evolutionary conservation score alone is not sufficient to predict the functional importance of a given base.
Figure 1.
Comparison of evolutionary conservation scores between a whole chromosome and its polymorphism sites. In each plot, the left boxplot depicts conservation scores of a whole chromosome, such as ‘2L’ for chromosome 2L; the right boxplot displays conservation scores of which polymorphisms exists, for example ‘2L_SNP’ representing polymorphism sites of chromosome 2L.
Interestingly, we identified a total of 150 000 variants that corresponds to non-sense mutations, frame shifts and splice site mutations. These represent potential loss of function (LOF) mutations. These variants may affect genes that do not play an essential role in viability or cause an obvious phenotype (21). Second, a detrimental variant may be compensated for by a different variant, present elsewhere in the genome. Third, it is possible that a variant only affects a subset of transcript, isoforms or functional domains of a gene. This is particularly likely when a variant is located within alternatively spliced exons. Finally, nonsense mutations towards the end of a protein coding region may lead to a minor truncation of the protein with subtle functional consequences (22). To exclude the last two possibilities, we applied stringent criteria by which only LOF mutations that fall within constitutive exons and not within the last coding exons are considered. As a result, a total of 1094 genes that have clear LOF mutations when in a homozygous state in at least one strain is identified and these genes are considered dispensable for obvious developmental defects. When applied, this dispensable gene list allows exclusion of variants from these genes during the filtering process, further improving the selectivity in classifying deleterious mutations. We noticed that some dispensable genes are known lethal genes. Therefore, we provided an option for users whether to filter out variants within dispensable gene regions.
PLATFORM FUNCTIONS
To facilitate the usage of the database by individual researcher, we have implemented several web based functionalities to the database, allowing querying, annotating and filtering.
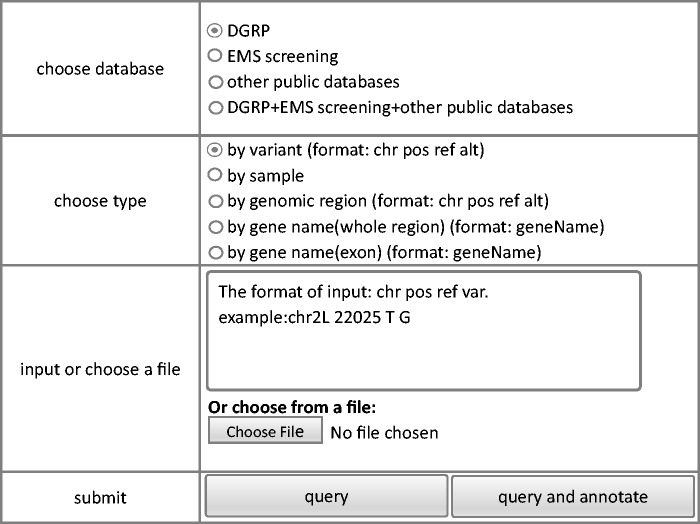
The criteria based query module allows extraction of variance information from the database. The interface of the query module is shown in Figure 2. To make it easy for all users, we keep the interface of FlyVar very simple. Data can be queried based on fly strain ID, genomic coordinate, a given genomic interval, and gene ID. When users have chosen way of querying, corresponding input format and an example could be shown in the right input position of webpage. For example, as shown in Figure 2, the querying way was chosen as ‘by variant’, corresponding input format and an example ‘The format of input:chr pos ref var. example:chr2L 22025 T G’ is presented immediately in the box of‘input or choose a file’part. The query output includes chromosome, genomic coordinate, reference sequence, and variant sequence. In addition, the ID of the fly strain(s) in which the variant was identified is included. We also designed a query by sample function, in case the specific strain ID in a dataset is meaningful. Furthermore, to facilitate user access to the DGRP collection, all variants from individual DGRP strains can be obtained by querying the corresponding strain ID. Finally, variant frequency in the DGRP collection is also provided with links to the corresponding strains at the FlyBase website (23).
Figure 2.
Steps of query model of FlyVar database.
The annotation module provides a user-friendly interface to annotate variants input by the users. Currently, Drosophila melanogaster Release 5.1 reference (23, 24) and an annotation tool ANNOVAR (25) are used to perform functional annotation. Webpage of annotation module is shown in Figure 3. Input with standard vcf format or user defined tab-separated plain text are both accepted. For the custom plain text file, only essential information to define a variant, including chromosome, coordinate, reference base, and variant base are required. This module will generate two output files: the first file contains annotation for all the variants while the second file contains annotation for only variants that affect protein coding.
Figure 3.

Steps of annotation model of FlyVar database.
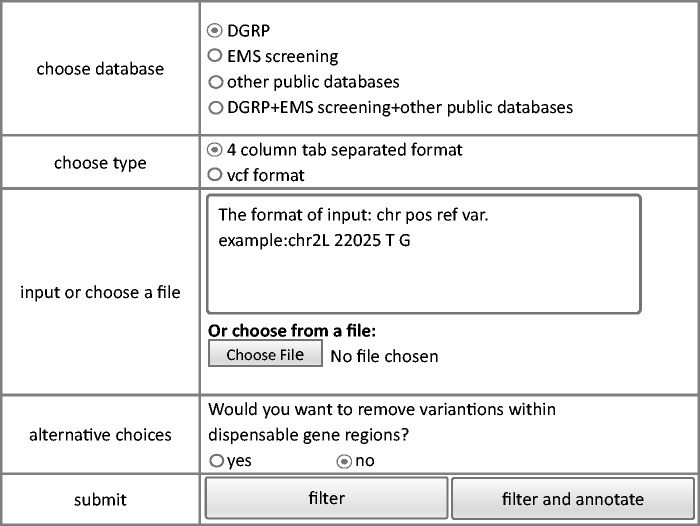
We also developed a module that permits filtering of raw sequence data to eliminate potentially benign variants. The module removes homozygous polymorphisms stored in the database resulting in a narrower list of candidate mutations. To avoid potential deleterious mutations, only homozygous variants were considered benign and filtered out. Same style of filtering interface as query module and annotation module is shown in Figure 4. Similar to the annotation module, both vcf and user defined tab delimited files can be used as input. The output file has the same format as the input file and only contains variants that are not observed in the database.
Figure 4.
Steps of filtering model of FlyVar database.
Since some dispensable genes are known lethal genes, an option, whether variants in dispensable genes are filtered out or not, is provided. Based on the annotation file of collected LOF mutations which have been stored in our database and could be downloaded from ‘dispensable gene’ webpage, users could make their own decision about how to utilize dispensable genes. If dispensable gene regions were included into filtering process, the rest of variants after filtering would be separated into two parts, variants not in dispensable gene regions and variants within dispensable gene regions. Input variants within dispensable genes could also be annotated to ease decision making.
Considering that the number of variants that need to be processed can be large, we provide support for batch file input processing as well as a standard interactive interface. This interactive input feature allows processing of up to 200 000 variants at once. For a larger number of variants, a batch file containing the variants can be uploaded and results will be sent back to users through email.
Besides querying or annotating or filtering online, users also could download the whole database into their local disk.
Data downloading module organizes data according to its project source to ease data selection.
By now, FlyVar is a publicly and freely available for all potential users including profit and non-profit organizations. After logging in, users could give comments or suggestion.
DISCUSSION
Here, we present a set of integrative web-based tools that allow individual research labs to process NGS data directly. This is the first of its kind that specifically addresses an increasing need for processing NGS data within the Drosophila research community. The FlyVar database contains data derived from the latest public WGS data. As a result, the number of variants collected in our database is several orders of magnitude larger than existing databases, greatly enhancing its utility. Upon filtering, the number of remaining variants will be dramatically reduced, resulting in a smaller, more accurate list of candidate mutations. The chrX mutation sequencing project delivered proof-of-principle of the usefulness of this database (9). Finally, to allow easy usage and access of the database, a set of interactive web based tools have been implemented that enable straightforward data processing without the requirement for programming or special computational resources.
There are several aspects that we are currently improving. First, copy number variations will be included in the near future. Second, we will further improve our annotation of variants. For example, annotation from the modENCODE (26) data can be included to annotate the potential effects a variant might have on a gene regulatory element. Third, as it is important to keep the database growing, we are developing tools that allow the community to easily contribute their private data. Finally, we plan to adopt cloud computing to the database and tools to make big data sets more accessible for the researchers.
ACKNOWLEDGEMENTS
The authors thank Tinghe Ding, Dr Hua Zhong and Dr Stephen Richards for helpful discussion. We also thank Jason Scott Salvo for editing the manuscript.
Funding
NIH RC4-grant (1RC4GM096355-01 to H.J.B. and R.C.); NSFC grant (61472086) to F.W.
Conflict of interest. None declared.
REFERENCES
- 1.Harris H. (1971) Polymorphism and protein evolution. The neutral mutation-random drift hypothesis. J. Med. Genet., 8, 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Muzny D.M., Reid J.G., et al. (2013) Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med., 369, 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams D.R., Sincan M., Fuentes Fajardo K., et al. (2012) Analysis of DNA sequence variants detected by high-throughput sequencing. Hum. Mutat., 33, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.1000 Genomes Project Consortium. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature, 491, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenstiel J.P., Noll A.C., Griffiths J.A., et al. (2009) Identification of EMS-induced mutations in Drosophila melanogaster by whole-genome sequencing. Genetics, 182, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay T.F.C., Richards S., Stone E.A., et al. (2012) The Drosophila melanogaster Genetic Reference Panel . Nature, 482, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley C.H., Stevens K., Cardeno C., et al. (2012) Genomic variation in natural populations of Drosophila melanogaster. Genetics, 192, 533–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger J., Suzuki T., Senti K.A., et al. (2001) Genetic mapping with SNP markers in Drosophila. Nat. Genet., 29, 475–481. [DOI] [PubMed] [Google Scholar]
- 9.Nele A., Haelterman L.J., Li Y., et al. (2014) Large-scale identification of chemically induced mutations in Drosophila melanogaster. Genome Res., 24, 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D., Ahlford A., Schnorrer F., et al. (2008) High-resolution, high-throughput SNP mapping in Drosophila melanogaster. Nat. Methods, 5, 323–329. [DOI] [PubMed] [Google Scholar]
- 11.Chen D., Berger J., Fellner M., et al. (2009) FLYSNPdb: a high-density SNP database of Drosophila melanogaster. Nucleic Acids Res, 37(Database issue), D567–D570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoskins R.A., Phan A.C., Naeemuddin M., et al. (2001) Single nucleotide polymorphism markers for genetic mapping in Drosophila melanogaster. Genome Res., 11, 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casillas S., Petit N., Barbadilla A. (2005) DPDB: a database for the storage, representation and analysis of polymorphism in the Drosophila genus. Bioinformatics, 21 (Suppl.2), ii26–ii30. [DOI] [PubMed] [Google Scholar]
- 14.Huang W., Massouras A., Inoue Y., et al. (2014) Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res., 24, 1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W., Massouras A., Inoue Y., et al. (2014) Genome Res., ftp://ftp.hgsc.bcm.edu/DGRP/freeze2_Feb_2013/vcf_files/. [Google Scholar]
- 16.Fabian D.K., Kapun M., Nolte V., et al. (2012) Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol. Ecol., 21, 4748–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remolina S.C., Chang P.L., Leips J., et al. (2012) Genomic basis of aging and life-history evolution in Drosophila melanogaster. Evolution, 66, 3390–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolaczkowski B., Kern A.D., Holloway A.K., et al. (2011) Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics, 187, 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begun D.J., Aquadro C.F. (1992) Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature, 356, 519–520. [DOI] [PubMed] [Google Scholar]
- 20.Singh N.D., Petrov D.A. (2007) Evolution of gene function on the X chromosome versus the autosomes. Genome Dyn., 3, 101–118. [DOI] [PubMed] [Google Scholar]
- 21.Young M.W., Judd B.H. (1978) Nonessential sequences, genes, and the polytene chromosome bands of Drosophila melanogaster. Genetics, 88, 723–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacArthur D.G., Balasubramanian S., Frankish A., et al. (2012) A systematic survey of loss-of-function variants in human protein-coding genes. Science, 335, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.dos Santos G., Schroeder A.J., Goodman J.L.et al. (2015) FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res., doi: 10.1093/nar/gku1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith C.D., Shu S.Q., Mungall C.J., et al. (2007) The Release 5.1 annotation of Drosophila melanogaster heterochromatin. Science, 316, 1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Li M., Hakonarson H. (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res., 38, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstein M.B., Lu Z.J., Van Nostrand E.L., et al. (2010) Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science, 330, 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]