Abstract
Conventional endoscopic resection techniques such as endoscopic mucosal resection or endoscopic submucosal dissection are powerful tools for treatment of gastrointestinal neoplasms. However, those techniques are restricted to superficial layers of the gastrointestinal wall. Endoscopic full-thickness resection (EFTR) is an evolving technique, which is just about to enter clinical routine. It is not only a powerful tool for diagnostic tissue acquisition but also has the potential to spare surgical therapy in selected patients. This review will give an overview about current EFTR techniques and devices.
Keywords: Endoscopic full-thickness resection, Over-the-scope-clip, Colorectal adenoma, Colorectal carcinoma, Endoscopic gastrointestinal surgery
Core tip: Endoscopic full-thickness resection is an evolving technique, which is just about to enter clinical routine. It is not only a powerful tool for diagnostic tissue acquisition but also has the potential to spare surgical therapy in selected patients. This review gives an overview about the current status of endoscopic full-thickness resection. General principles, indications and resection techniques and -devices will be discussed in detail on the basis of currently available literature.
INTRODUCTION
Flexible endoscopy was initially established as pure diagnostic procedure but has evolved to an important therapeutic modality over the last years. As a therapeutic instrument, Conway et al[1] recently called endoscopy “truly the queen of minimally invasive interventions, being less morbid than surgery and without the radiation exposure of interventional radiologic interventions”. Advanced techniques like endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) are well investigated methods for endoscopic resection of gastrointestinal neoplasms[2,3]. However, those techniques are limited to the superficial layers of the gastrointestinal (GI) wall, namely mucosa and submucosa. Although “superficial” resection may be sufficient for the majority of indications (e.g., colorectal adenomas), full-thickness resection of the GI wall may be necessary in a subset of cases. For example, non-lifting lesions or neoplasms arising from deeper layers than the submucosa are difficult if not impossible to treat with conventional techniques due to the increased risk of perforation. Endoscopic full-thickness resection (EFTR) with secure defect closure may offer a safe and -compared to surgical resection- minimally invasive approach for those lesions. Moreover, diagnostic yield of full thickness resection specimen may be higher compared to endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), e.g., in case of T1-carcinomas. Hence, there is a clinical need for EFTR. However, for many years, it has not entered routine endoscopic practice due to the lack of safe techniques and devices. But what qualifies an EFTR device for broad clinical use? Basic requirements of such a device are: (1) safe and reliable defect closure; and (2) good endoluminal maneuverability. The ideal device would also be relatively easy use not only in the hands of experts. The very first device for flexible endoluminal full-thickness resection was introduced back in 2001 by Schurr and colleagues[4]. This prototype over-the-scope device had a flexible shaft and a multifunctional front-end incorporating tissue retractors and a stapling/cutting mechanism. Although it has been used successfully in the left-sided colon in animal experiments, the device has never entered clinical practice, probably due to its large dimensions and the limited endoluminal maneuverability. In the following couple of years, no major innovations had been published concerning EFTR devices suitable for routine clinical use. However, extensive experimental research on Natural Orifice Transluminal Endoscopic Surgery (NOTES) has led to substantial progress in the field of conservative and endoscopic management of GI wall defects[5]. This resulted not only in improvements in the therapy of accidental perforations, but also has opened the door for clinical use of EFTR. One major innovation for example was the introduction of the over-the-scope-clips (OTSC). Based on this closure technique, an over-the-scope full-thickness resection device (FTRD, Ovesco Endoscopy, Tuebingen, Germany, see below) was developed and recently CE-marked. Compared to the very first FTRD, the device is much smaller, easier to use and suitable for resections in the entire colon. With those and other recent developments, EFTR is now just about to enter clinical practice and may be the next logical step towards more extended endoscopic resections[6]. This review will give an overview over the current status of experimental and clinical EFTR techniques and devices.
LITERATURE SEARCH
The MEDLINE database was searched for articles describing endoscopic full-thickness resection. Keywords included “EFTR”, “Endoscopic full-thickness resection” and “gastrointestinal AND endoscopic full-thickness resection”. Additionally, we created ontology-based search queries on EFTR and used the “Ontovigilance” search engine (more information at http://www.ontovigilance.org) to search for reports not listed in MEDLINE.
The “Ontovigilance” search engine is an innovative prototype system for semantic search based on ontologies. Here, a so called Search Ontology, specifically designed for the identification of EFRT-related content, was applied. The specific ontology allows the expert the formal specification of domain concepts (e.g., EFTR; Over-the-scope clip etc.) search terms associated to the domain, and rules describing domain concepts in order to generate complex search queries connected with Boolean operators[7]. The following search queries were used: (“endoscopic full thickness resection” OR eFTR) (“endoscopic full thickness resection” OR eFTR) AND (“over the scope” OR Ovesco OR OTSC OR FTRD); (“endoscopic full thickness resection” OR eFTR) AND (suture OR T-Tag OR Plicator OR Overstitch OR GERDX); (“endoscopic full thickness resection” OR eFTR) AND (Stapler). To avoid duplicate search results, the following databases were excluded: Synmed, Slideshare, Mdlinx, Eventscribe, Bioportfolio, EM-consulte, and Researchgate). Articles dealing with non-gastrointestinal endoscopy and articles in other than English or German language were also excluded. We further excluded articles on laparoscopic or combined endoscopic/laparoscopic procedures. Animal studies as well as human case reports and studies were included.
CURRENT INDICATIONS FOR EFTR
Indications for EFTR substantially differ in the upper and lower GI tract. Although not yet clinical “routine”, all mentioned indications are already applicable in clinical practice with existing EFTR techniques and devices. The indications for EFTR in the colorectum are mainly suitable for resections with the FTRD System.
Upper GI tract
Subepithelial tumors (SET) arising from (or infiltrating) the muscularis propria are the most frequent indication for EFTR. Those tumors are difficult, if not impossible to resect with other endoscopic techniques due to the high risk of perforation.
Non-lifting recurrent or previously untreated non-ampullary duodenal adenomas may also be effectively resected by EFTR. However, this can not yet considered to be a routine indication because data on duodenal EFTR is very limited.
Lower GI tract
Recurrent adenomas with negative lifting sign. Although recurrency rate after EMR has recently described to be low[3], endoscopic re-treatment of those lesions is difficult. ESD has been reported to be effective for those lesions[2] but is technically challenging and harbours a substantial risk of perforation due to scarring even in the hands of experienced endoscopists. EFTR, especially with one-step closure/resection devices, may be technically easier, more time effective and safer. However, comparative studies are not yet available.
Incomplete resected non-lifting adenomas. Similar to recurrent adenomas, scarring represents a major problem for endoscopic re-treatment. EFTR may be a good indication for resection of those residual lesions.
Non-lifting adenomas without previous treatment. Those lesions are suspicious for invasive carcinoma. In those cases, EFTR may be done as primarily diagnostic resection. Compared to ESD, a full-thickness resection may increase the diagnostic yield and help to stratify in low-risk or high-risk situation. In particular, submucosal infiltration depth may be determined more accurately by the pathologist.
Re-resection of T1-carcinomas. When a T1 carcinoma is incidentally diagnosed in a lesion, which has been resected with conventional endoscopic methods and R-status and/or submucosal infiltration depth can not be determined accurately by the pathologist, diagnostic EFTR may be the method of choice to obtain a full-thickness resection. In case of low-risk lesions (submucosal infiltration depth < 1000 μm, G1 or G2, no lymphatic vessel invasion, R0-resection), it is even therapeutic. Compared to ESD, EFTR may also be the more radical approach resulting in lower recurrency rates. However, comparative studies are lacking.
Adenomas at difficult anatomic locations not suitable for “conventional” endoscopic resection. Adenomas involving a diverticulum can be effectively resected by EFTR[8]. Moreover, EFTR has been proposed for adenomas involving the appendical orifice[9]. In those cases EFTR may be a minimally invasive alternative for surgical resection.
Subepithelial tumors (SET). Although a rare indication, EFTR has been reported to be feasible for resection of small subepithelial lesions such as neuroendocrine tumors[10,11]. Conventional resection techniques like ESD harbour a significant risk of perforation, especially when the tumor infiltrates or (arises from) the muscularis propria.
Diagnostic resections in patients with suspected motility disorders. Compared to standard full-thickness biopsies, EFTR may increase the diagnostic yield in patients with suspected aganglionosis such as Hirschsprung’s disease[12-14].
GENERAL PRINCIPLES OF EFTR
Full thickness resection naturally results in GI wall defect. Hence, the mainstay of EFTR is secure defect closure. Generally, there are two different approaches combining EFTR and defect closure: (1) Full thickness resection followed by closure of the wall defect; and (2) Securing GI wall patency by creation of GI wall duplication (with serosa-to-serosa apposition) before resection (Figure 1).
Figure 1.
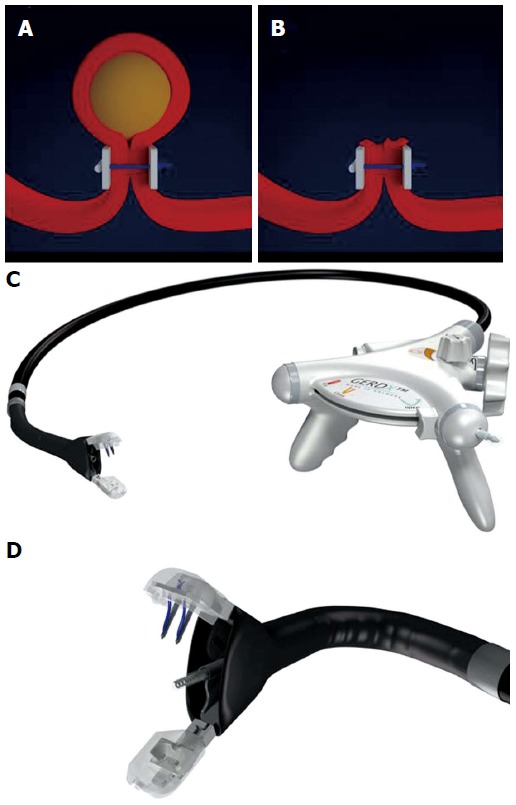
Endoscopic full-thickness resection with prior transmural suturing. A: Transmural sutures are placed underneath a subepithelial tumor (schematic illustration); B: The sutures are securing gastric wall patency after full-thickness resection; C: The GERDXTM device (G-Surg, Seeon, Germany); D: The tip of GERDXTM device with opened branches and the central tissue retractor.
EFTR followed by subsequent perforation closure has been described to be feasible and effective[15-17]. However, reliable sealing of large (> 2 cm) GI wall defects may be difficult even with modern closure devices like Over-the-scope Clips[18]. Another potential problem is that with creation of a large wall defect, loss of air/CO2 may lead to collapse of the GI lumen and failure to maintain a reasonable operative field. Several studies investigating non-insufflation techniques and countertraction devices/platforms have addressed this problem, but all devices are still at the stage of prototypes and far away from clinical use[19-21]. Therefore, securing GI wall patency before resection may be a potentially safer and easier approach[22]. In analogy to surgical standards, this resection technique is based on creating a GI wall duplication with serosa-to-serosa apposition and therefore requires not only a mucosal/submucosal but rather a transmural closure mechanism. Depending on the anatomic resection site (stomach vs colonic) and thickness of the GI wall, transmural suturing techniques or closure with over-the-scope clips prior to resection have been described to be clinically feasible[9,23-26], whereas endoluminal stapler-assisted methods are still in the experimental stage of development[27]. Another potential advantage of prior closure is that in case of tumor fragmentation during resection [e.g., in case or large gastric gastrointestinal stromal tumors (GIST)], tumor seeding into the abdominal cavity may be avoided.
EFTR followed by perforation closure may lead to spillage of gastrointestinal content into the abdominal cavity, which may result in intraperitoneal infection. However, extensive research on NOTES has clearly demonstrated that abdominal infection during transgastric interventions is uncommon. Moreover, experimental studies have shown that the degree of peritoneal bacterial contamination can be reduced by antibiotics and decontamination of endoscopic entry routes. Also, bacterial contamination of the peritoneum does not necessarily correlate with relevant infection[28,29]. However, data on transcolonic interventions is limited and risk of bacterial seeding is likely to be higher for this access route[30,31]. Therefore, at least for colonic resections, reliable single-step closure/resection devices may be preferable compared to step-by-step closure modalities in order to minimize exposure of the peritoneal cavity to the bowel lumen[32].
EFTR TECHNIQUES
Submucosal endoscopic tumor resection
The concept of “submucosal tunnelling” in the esophagus was initially introduced by Inoue et al[33] for peroral endoscopic myotomy (POEM). Only a few years later, this technique was applied for resection of esophageal subepithelial tumors (SET) arising from the muscularis propria[34,35]. In analogy to the POEM procedure, a mucosal incision at least 5 cm proximal to the tumor is created and the endoscope is introduced into the submucosal space. The tumor is subsequently enucleated in ESD technique. After extracting the tumor from the tunnel, the mucosal incision is finally closed with through-the-scope (TTS) clips. The beauty of this technique is that even if a full thickness resection has been performed, the intact mucosal layer over the resection site covers the perforation and protects from mediastinitis or peritonitis. Hence, in contrast to other EFTR techniques, endoscopic closure of the resection site is not required. The largest study published to date included 85 SET (60 esophageal and 9 gastric). The tumors were mainly arising from the superficial muscularis propria (MP, 88.2%) and had a mean size of 19.2 mm (range 10-30 mm). Complete resection was achieved in 100% of cases with a mean procedure time of 57.2 min. Pneumothorax occurred in 7.1%, subcutaneous emphysema in 9.4% and pneumoperitoneum im 4.7%, respectively[36]. Other smaller studies reported success rates between 78% and 100% and complication rates between 13% and 33%[35,37,38]. The most common complications reported are pneumothorax, subcutaneous and mediastinal emphysema and pneumoperitoneum. While occurrence of pneumothorax generally requires a chest drain, air leakage into the mediastinum, the abdominal cavity and the subcutaneous tissue may not be considered as a “complication” rather than a natural consequence when the MP is perforated/resected. As long as the covering mucosa over the perforation is preserved, leakage of esophageal or gastric content is prevented. In the clinical studies published to date, no severe intraabdominal or mediastinal infections have been reported. Hence, submucosal endoscopic tumor resection using a tunnelling technique is feasible and relatively safe for tumors originating from the MP in the esophagus and cardia.
EFTR with subsequent clip closure
Gastric resections: Multiple asian studies reported on pure endoscopic full-thickness resection of gastric SET with subsequent defect closure. A study by Zhou et al[15] reported full thickness resection of 26 gastric SETs with a mean tumor sizes of 2.8 cm (1.2-4.5 cm) arising from the muscularis propria. The tumors were resected using ESD technique and the gastric wall defect was closed with standard through-the-scope clips. Complete resection rate was 100% with a mean procedure time of 105 min; no major complications were reported. Two other groups recently confirmed these results in a similar studies on 35 and 48 patients[16,17]. Another more recent retrospective study reported on a similar resection technique in 20 patients with gastric SETs. In this study, the wall defects were closed with clips and endoloops, severe complications where not reported, en bloc resection rate was as high as 100%[39]. Ye et al[40] recently also showed excellent results with a similar closure technique in 51 patients. There are two studies comparing laparoscopic resection vs EFTR with secondary clip closure. Both studies did not show significant differences in complete resection rate, operation time and length of hospital stay, indicating that the endoscopic approach may be an alternative strategy for such lesions[17,41].
Although the studies mentioned report excellent results with no serious complications, it must be emphasized that defect closure with standard clips is usually only possible for small gastric perforations. Moreover, concerns have been raised whether closure of only the mucosal layer is sufficient after EFTR[42]. A porcine study compared closure of NOTES gastrostomies by either TTS clips or over-the-scope clips (OTSC)[43]. In the TTS-clip group, 3 minor and 1 major leaks were observed and four pigs developed peritonitis. No leaks occurred in the OTSC group, and necropsy with microscopic evaluation of the perforation sites showed that OTSC led to a deeper defect closure within the submucosal or muscular layer, respectively. Another potential advantage of OTSC closure is that OTSC deployment is a single-step procedure compared to step-by-step closure with TTS clips (+/- Endoloops) which may reduce the time of peritoneal exposure to gastric contents. Multiple clinical studies have shown high effectivity of OTSC for treatment of GI wall perforations, fistulas and leaks[10,44-47]. EFTR followed by defect closure with OTSC was clinically evaluated in the EndoResect study[48]. 20 patients with gastric SET ≤ 3 cm were enrolled; 14 of them were resected using a double channel endoscope, a tissue retractor and a monofilament snare. Perforation occurred in six patients, all of which could be closed by OTSC application; mean procedure time was 44 min. Although this approach is very interesting because of its technical simplicity, most of the procedures in the study were done under laparoscopic control. Guo et al[49] most recently reported on EFTR of gastric SETs with subsequent OTSC defect closure. All interventions were done without laparoscopic assistance and successful defect closure was achieved in 100% of cases. Mean time for OTSC closure was as low as 4.9 min, reflecting the simplicity of the procedure. However, all tumors in this study had a size of ≤ 2 cm, so the resulting wall defects should have been relatively small. Although OTSCs are preferable over TTS-Clips for gastric defects > 1 cm[5], secure closure requires apposition of the borders of the defect which may be difficult if impossible in case of larger perforations.
Colonic resections: Endoscopic resection of the colonic wall can generally be achieved in 3 ways: (1) Traction of the colonic with a forceps or an anchoring device and snare resection using a 2-channel endoscope; (2) suction of the colonic wall into a cap followed by snare resection; and (3) cutting of the wall with a knife in ESD-technique. Defect closure can then be performed with TTS-Clips or OTSCs.
Ahmed et al[50] experimentally compared traction- vs suction to retract the colonic wall for EFTR. The suction-resection technique resulted in larger resection specimen but was associated with more injury to the adjacent viscera compared to the traction technique; closure of the defect was not attempted in this study. Raju et al[51] published a porcine experimental study where EFTR was performed with a band-ligation-resection device. Transverse closure of the circular perforations with TTS clips was unsuccessful in 3/11 cases, whereas longitudinal closure resulted in a leak proof seal in 6 of 7 cases. EFTR using a ESD-like technique with subsequent TTS-clip closures has been reported to be clinically feasible for resection of colonic SET[52]. However, in 2 of 16 patients required laparoscopic closure of the colonic wall defect and 2 patients developed signs of peritonitis. Apart from this report, there are no other clinical studies available following this approach[53].
von Renteln et al[54] evaluated a grasp-and-snare technique using a double channel endoscope and tissue anchor (Ovesco endoscopy, Tuebingen) in a porcine study. Resection yielded specimen up to 5.5 cm. Secure OTSC closure in the pigs with large defects) 2.4-5-5 cm in only 9 of 20 cases. In contrast, when an endoloop was used to secure the resection base before EFTR, resection specimen were smaller (1.2-2.2 cm) and OTSC closure led to efficient sealing of the defects in all cases. This indeed does reflect the clinical experience that colonic perforations up to approximately 2.5-3 cm can be closed sufficiently with OTSC whereas bigger defects can often not be sealed reliably.
EFTR with subsequent suturing
While transmural suturing is a hallmark procedure in open and laparoscopic GI surgery, endoluminal suturing with flexible instruments is technically much more difficult and still an area of intensive research. Roughly, there are 3 categories of endoscopic suturing methods: dedicated suturing devices, through-the-scope catheter based devices and multitasking platforms[1]. The Over- Stitch suturing device (Apollo Endosurgery Inc, Austin, Tex) is a commercially available device which is mounted on the tip of a endoscope and which was designed to create single-knot sutures. There are reports on successful closures of post-ESD mucosal defects[55] and a gastric fistula[56]. Chiu et al[57] demonstrated feasibility of EFTR using a master and slave transluminal endoscopic robot and closed the gastric perforations successfully with the Apollo Overstitch in two live porcine models. There are alos publications on the EagleClaw suturing device which uses a similar principle and has been used for closure of gastrostomies and other various indications[1,58,59]. However, to our knowledge there are no studies which further investigated EFTR with defect closure using these over-the-scope suturing devices.
Our group reported on defect closure after resection of gastric GIST by means of full-thickness suturing with the PlicatorTM, Suturing device (NDO Surgical, Inc, Mansfield, Mass)[23]. This device was originally designed for endoscopic antireflux therapy and deploys transmural PTFE (Polytetraflourethylene)-pledgeted sutures. Although resection and closure of the gastric wall defect were successful in both cases we followed a “suture first, cut later” approach for the future cases (see below)[24].
Ikeda et al[60] were the first to investigate gastric EFTR with perforation closure using T-Tags. The tissue apposition system (TAS, Ethicon, Blue Ash, Ohio, United States) is a through-the-scope instrument and consists of a needle, which is used to transmurally place T-tags at the edges of the perforation and a knot-tying device. T-Tags have been used to to close wide colon perforations, esophageal, gastric and duodenal defects[61-65]. Raju et al[66] reported on colonic EFTR with subsequent closure using T-Tags. Suture closure of 2 cm defects was successful in 19/20 pigs with a median duration of 41 min for 4 sutures. Eighteen animals survived without signs of clinical distress and well-healed scars without peritonitis on necropsy at two weeks. One animal failed to thrive and necropsy revealed mild peritonitis, small abscesses, distant adhesions and 2 mm insufficiency at the suture site, respectively. Although T-Tag closure seemed to be promising in numerous studies, the TAS has been withdrawn form the market and is no longer commercially available.
Mori et al[21] demonstrated the experimental use of a Double-arm-bar Suturing System (DBSS) to close gastric defects after EFTR. Similar to the OverStich system, the device is mounted on an endoscope and allow serial single-stitch sutures. Closures were compared with hand-sewn sutures and OTSCs. No significant difference was found in the leak tests between the hand-sewn group and the DBSS, while burst pressures were significantly higher in the DBSS and hand-sewn group vs the OTSC group. The utility of the device was also demonstrated in two porcine video case reports recently[20,67]. It is noteworthy, that all interventions with the DBSS were done without air/CO2-insufflation, a mechanical countertraction device was used to maintain an operative field. Both the countertraction device and the DBSS are still in the stage of early prototypes and are not clinically approved.
EFTR with prior transmural suturing
In 2008, our group reported on the concept of applying transmural sutures underneath gastric SETs prior to EFTR[23]. We used a device originally designed for endoscopic anti-refllux therapy (PlicatorTM,NDO Surgical, Inc, Mansfield, Mass) to place two non-resorbable transmural PTFE-pledgeted sutures underneath the tumor. Thereby, a full-thickness duplication with serosa-to-serosa apposition was created and the “Pseudopolyp” was then resected with a snare above the suture (Figures 1 and 2). In 2011, a second series with four patients undergoing successful EFTR after deploying resorbable sutures was published[24]. Recently, our group reported on EFTR of gastric SET in a series of 31 patients using this “suture-first-cut-later” technique[25]. Mean tumor size was 20.5 mm (range 8-48 mm). Macroscopically complete en bloc resection could be achieved in 100 %, R0-resection rate was 90.3% with a median procedure time of 60 min. Perforation occurred in three patients; in all cases, the perforation was successfully closed with additional transmural sutures. When compared to OTSC application before resection (see below), this method is applicable for tumors up to a size of about 4 cm. The suturing device was originally designed to work in retroflex position, so technique is especially suitable for tumors in the proximal corpus, cardia and even in the fundus. In comparison to the clip closure techniques described above, patency of the gastric wall is secured not only by mucosal closure but rather by full- thickness suturing with serosa-to-serosa apposition. This technique meets surgical standards for defect closure and may result in a more secure and durable gastric wall repair especially for resection of large lesions. A major limitation of this method the need of special endoscopic equipment. Moreover, the devices are relatively large and can exclusively be used in the stomach. Handling of the devices also require a certain experience, e.g., in endoscopic anti-reflux therapy. The PlicatorTM device from NDO is not any more commercially available. However, a new CE-marked single-use device is available in Europe now (GERDXTM, G-Surg, Seeon, Germany) (Figure 1). It uses the same suturing technique as the PlicatorTM but works with a hydraulic closure mechanism. This device was used for the last two cases in our series and seems to be as effective as the PlicatorTM. The device is currently being evaluated in a prospective study initiated by our group.
Figure 2.
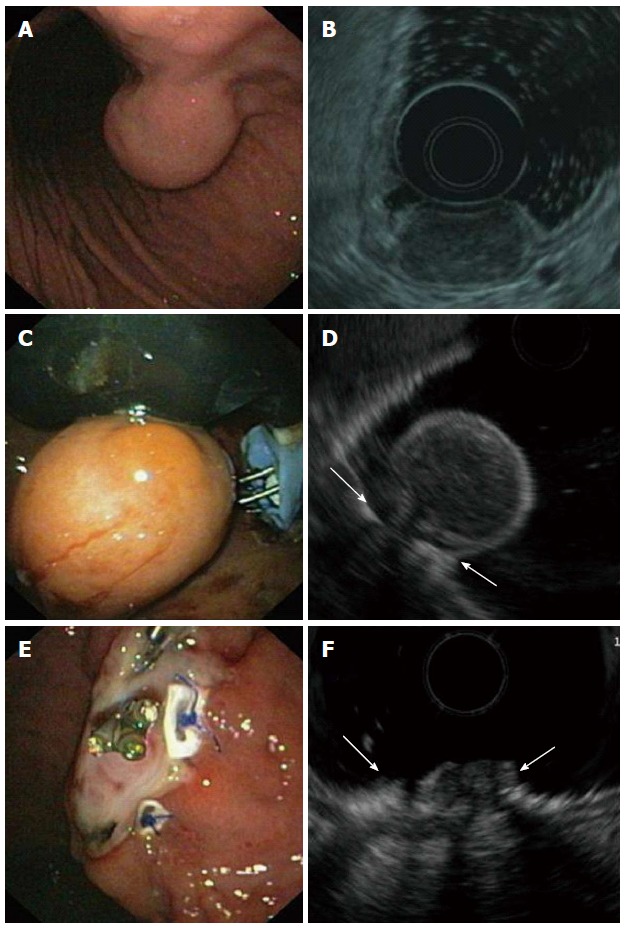
Endoscopic full-thickness resection of a gastric gastrointestinal stromal tumors after prior transmural suturing. A: Endoscopic image of the subepithelial tumor in the gastric corpus; B: EUS-image showing an inhomogeneous tumor arising from the muscularis propria; C: Two transmural sutures are deployed underneath the tumor using the PlicatorTM suturing device; D: EUS image after suturing. The PTFE pledges are indicated with arrows; E: Resection site. The transmural sutures are securing gastric wall patency; F: EUS image of the resection site. The PTFE pledges are indicated with arrows. EUS: Endoscopic ultrasonography.
EFTR with flexible stapler devices
The very first report on a one-step endoluminal full thickness resection device was published as early as 2001 by Schurr et al[4]. The device was a combination of a semicircular stapler and scalpel; it was also equipped with flexible tissue graspers. The device had a central lumen to accommodate a conventional flexible endoscope. For resection, the tissue graspers were used to pull the colonic wall into a resection chamber at the head of the device, thereby creating a full-thickness duplication. After application of 11 staples, the tissue above the staples was cut with the integrated scalpel and the resection specimen was retrieved within the resection chamber. This was the very first study to show that full thickness bowel wall resection was feasible with a flexible one-step instrument. The results of this study were confirmed by Rajan et al[68] in animal experiments, yielding resection specimen with a mean diameter of 3.6 cm. This novel device technique represented a major advantage in EFTR as the concept of a one-step closure-and-cut technique without exposition of the peritoneal cavity to the bowel lumen and content. However, due to the technical requirements of a stapling mechanism, the device was quite big with limited maneuverability and could not be advanced beyond the left-sided colon. Therefore, the stapling closure approach was left and replaced by a more simple OTSC-based technique which resulted in the recently CE marked FTRD (Full Thickness Resection Device, see below)[69].
In 2006, Kaehler et al[27] demonstrated EFTR with the SurgAssist System (Power Medical Interventions Deutschland GmbH). The SurgAssist combines a 20 cm long flexible shaft with a linear stapling device and allows electronically controlled remote release of conventional stapler magazines. An endoscope is introduced simultaneously to allow endoscopic vision and to retract the gastric wall. In the study, the technique was shown to be feasible for EFTR in the gastric corpus in 3 human exenterates. The technique has also been applied in 2 clinical cases for successful full thickness resection of a T1 carcinoma and a carcinoid tumor in the gastric corpus[70]. There is also a porcine study reporting on successful closure of gastric defects after NOTES[71]. The SurgAssist device was also investigated in a non-survival porcine study by Evans et al[72]. EFTR was successful in only 2 cases. In the other 2, resection was limited to the submucosa. Furthermore, parallel introduction of the endoscope and the stapler caused one severe tear in the esophagus. In two animals, the endoscope was introduced through a gastrostomy port, which allowed better visualisation and also better countertraction of the tissue. All studies claimed limited intragastric maneuverability of the device; moreover, parallel introduction of scope and stapler device through the esophagus may be difficult and risky. There is a more recent study reporting on successful ex vivo closures of NOTES colostomies using a novel flexible stapler (Covidien North Haven, CT, United States)[73]. Although all studies mentioned show that endoscopic full-thickness stapling is feasible, this technique has not been followed consequently and is still far away from routine use for full thickness resection. The main reason may be that due the technical requirements, currently available stapling devices are too large in diameter, not flexible enough and show limited intraluminal maneuverability. Further technical improvements including miniaturisation seem to be necessary before use in clinical routine.
EFTR after OTSC application/FTRD
The concept of OTSC application followed by snare resection above the clip was reported in several clinical retrospective studies. An American group reported about resection of small subepithelial tumors in different locations with a mean tumor size of 13.4 mm[74]. Lesions were located in the duodenum, in the esophagus, in the stomach and in the rectum. After OTSC-deployment, all lesions were resected successfully. R0-resection was achieved in all but one cases, respectively. A recent retrospective German series included 17 patients with a variety of indications including SET and relapsed or R1-resected colonic adenomas/carcinomas; 17/17 resections were done in the lower GI tract[11]. Technical success was 94% with a R0 resection rate of 100%. A drawback of this technique is that the size of the cap limits the maximum size of the lesion.
The novel “Full thickness resection device” (FTRD, Ovesco Endoscopy, Tübingen, Germany) was designed for one-step colonic EFTR after OTSC application. Similar to the OTSC system, it can be mounted over a standard colonoscope and consists of a long transparent applicator cap carrying a modified 14 mm OTSC. Compared to the conventional OTSC system, the cap is much longer (23 mm vs 6 mm) and can therefore incorporate more tissue. A 13 mm monofilament high frequency (HF) snare is a preloaded in the tip of the cap. The handle of the snare runs on the outer surface of the scope underneath a plastic sheath (Figure 3). For resection, a grasping forceps (or a tissue anchor) is advanced through the working channel of the scope, the lesion is pulled into the cap thereby creating a full thickness duplication of the colonic wall (Figures 4 and 5). Immediately after clip deployment, the tissue above the clip is resected with the snare above the clip. The device was firstly introduced in 2011 and evaluated in several porcine studies[69,75-77]. In the most recent study, EFTR was done in 11 pigs at one or two sites, divided into three study sessions/groups, respectively[69]. Animals were euthanized after 7 or 28 d. The colonic resections were carried out without complications yielding specimen with an average diameter between 3.1 and 5.4 cm. No immediate or delayed perforations or leakages were observed, the serosa had primarily healed after 28 d in all cases. To date, there are three published reports on clinical use of the device. Our group was the first to report on successful EFTR of 3 recurrent non-lifting colonic adenomas[26]. At the same time, a Swiss group published a video case demonstrating successful EFTR of an adenoma arising from a diverticulum[8]. Furthermore, we recently reported on 25 patients who underwent EFTR in the colorectum at two centers[9]. The majority of indications were non-lifting adenomas, resection sites were spread throughout the colorectum with 40% being in the right-sided colon. Technical success was 83.3% and R0-resection rate 75%, respectively. In this study, we did not observe any immediate or delayed perforation or major bleeding. However, two patients developed a post-polypectomy syndrome after coecal resections which may reflect local serositis after the transmural intervention. This data suggests that EFTR with the FTRD is feasible, effective and safe. The major limitation of the system is the maximum size of the lesion to resect. This strongly depends of the mobility of the colonic wall; whereas resection specimen up to 5.4 cm have been reported in experiments with healthy porcine colon[69], the median diameter in the mentioned clinical study was 24 mm (range 12-40 mm)[9]. Moreover, the long cap limits endoscopic view and flexibility of the endoscope tip so that advancement of the scope thorough the sigmoid or beyond colonic flexures can be difficult. The device was just recently CE marked for colonic EFTR and is commercially available in Europe. Although it has also been used for duodenal resections (Schmidt et al, manuscript accepted) we would like to stress that it is currently not approved for use in the upper GI tract. Full thickness resection in the stomach may not be possible due to the thickness of the gastric wall. Furthermore, the outer diameter (21 mm) of the device and its sharp edges limit peroral introducability, so that modifications of the device seem to be necessary before routine use in the upper GI tract.
Figure 3.
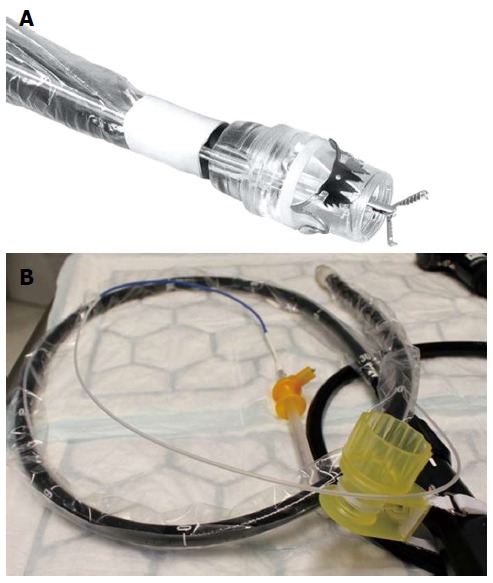
Full thickness resection device (Ovesco Endoscopy, Tuebingen, Germany). A: Tip of a colonoscope with the mounted FTRD. A grasping forceps is advanced through the working channel of the scope; B: The assembled FTRD on a colonoscopy. FTRD: Full thickness resection device.
Figure 4.
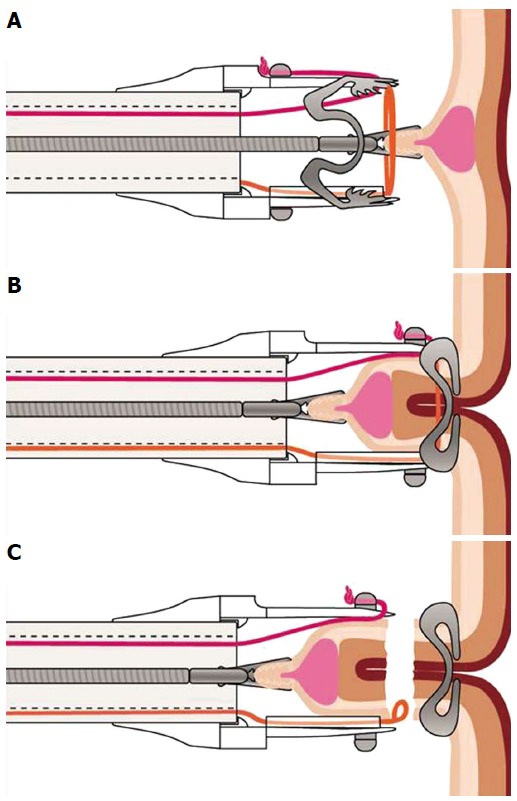
Schematic image of the resection procedure with the full-thickness resection device. A: The lesion is grasped with a forceps and pulled into the cap thereby creating a full-thickness duplication of the colonic wall; B: The over-the-scope clip is deployed; C: The tissue above the clip is resected with the integrated snare.
Figure 5.
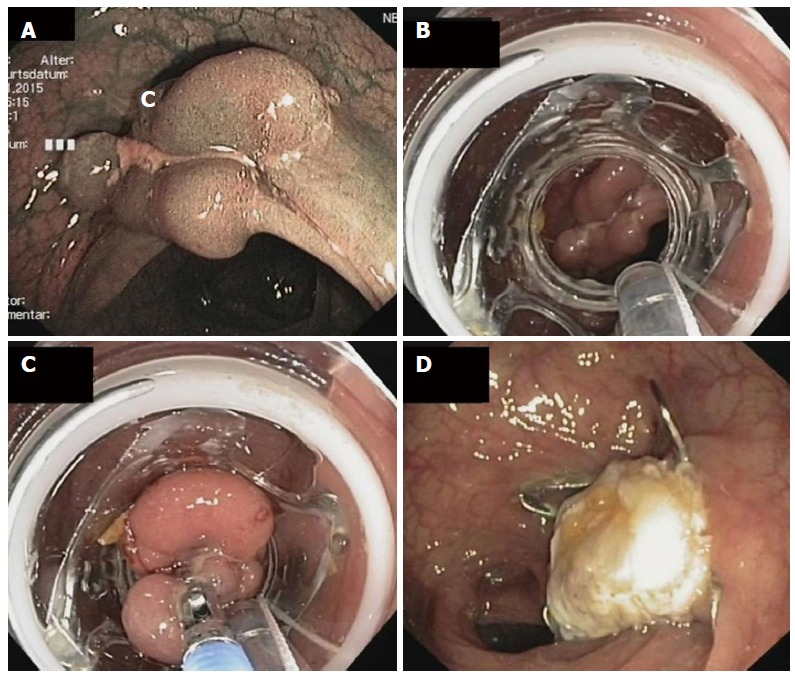
Endoscopic full thickness resection of a non-lifting recurrent adenoma in a patient with a polyposis syndrome. A: Endoscopic image showing a polypoid and centrally depressed non-lifting adenoma (2.5 cm) in the sigmoid (Narrow band imaging mode); B: View with the mounted full thickness resection device (FTRD, Ovesco Endoscopy, Tübingen, Germany); C: The lesion is pulled into the cap with a forceps; D: Resection site. The over-the-scope clip is securing gastric wall patency.
LIMITATIONS OF EFTR AND FUTURE DIRECTIONS
With the development of secure endoscopic closure techniques, major progress has recently been made in (at least partly) transferring an experimental technique into clinical routine. The best example is surely the OTSC-based FTRD which will truly change the clinical management of colorectal non-lifting lesions and will obviate the need for surgical therapy in selected patients. This over-the-scope system shows nicely how existing endoscopic components can be put together to create a safe, highly efficient and easy to use one-step resection device. Another example of applying existent devices for new indications is the suture-first-cut-later technique with the Plicator/GERDX device, which was originally designed for endoscopic anti-reflux therapy. Both techniques also demonstrate that the concept of securing GI wall patency before resection may be a safer and -with the current endoscopic techniques- easier approach compared to secondary defect closure. However, for more extended and complex resections, neither OTSC-assisted nor rather cumbersome suturing devices will suffice to reach the precision of a laparoscopic or open surgical operation. To achieve this, several developments seem to be necessary. More sophisticated and miniaturized stapler devices may facilitate secure and precise wall resections in the future. In our view, one-step stapler devices may be even more important than single-step endoluminal suturing instruments. More extended wall resections as well as suturing require countertraction. In the instruments available, this is achieved by rather primitive tissue retractors running through the working channel of the scope or through the suturing instrument itself. The ideal endoluminal EFTR device suitable for extended resections would be equipped two arms which can be moved independently of each other enabling traction and countertraction like in laparoscopic surgery. Although such prototype platforms have been investigated[57,78], those devices currently still seem quite far away from clinical use.
Looking at recent advances in the field of resection and closure techniques, it is tempting to state that EFTR is “the next logical step towards more extended oncological resections”[6]. However, it is noteworthy to say that all those innovative techniques still need to be investigated systematically. The majority of studies cited in this review are preclinical studies with a very limited amount of animal models or retrospective non-controlled clinical series. Although most resection and closure techniques seem to be feasible and safe, there is still a significant lack of prospective clinical trials. At least for the FTRD System, two prospective German trials have been initiated. The “WALL RESECT” study (NCT02362126) is a single-arm multicentre study investigating efficacy and safety of the device for resection of (mainly non-lifting) lesions in the colorectum. The “FIRE” study (NCT02353533) is a randomized monocentric trial investigating EFTR vs EMR for “difficult-to-resect” colorectal adenomas. There is also a prospective uncontrolled study investigating efficacy and safety of GERDX-mediated resection of gastric SETs (“FROST” study, NCT Nr pending), which has just started to recruit patients. The next step to would certainly be to directly compare those techniques with the surgical standard.
Furthermore, at this early stage of development it is not yet clear to which extend endoscopic interventions will be able to replace oncologic surgical resections. A minimal-invasive endoluminal approach may be ideal for lesions with low risk of tumor seeding like advanced adenomas, “small” mesenchymal tumors or even a subset of early carcinomas. For more advanced lesions, more extended resection including lymph node dissection is generally necessary, and at least at this point of development, this is not possible with the endoscopic-endoluminal approach.
In summary, recent developments have finally brought EFTR into clinical routine for selected indications. This progress has again pushed the frontiers of endoluminal resections towards transmural interventions. However, prospective clinical trials as well as technical improvements regarding resection/closure devices and - platforms are necessary for further development of this evolving technique.
ACKNOWLEDGMENTS
We thank Timo Weiland from Novineon CRO (Tübingen, Germany) for creating the search queries for the Ontovigilance Software. We thank Ovesco Endoscopy (Tübingen, Germany) and G-Surg (Seeon, Germany) for providing the images.
Footnotes
Supported by the Bundesministerium für Bildung und Forschung (BMBF, KMU-innovativ: OntoVigilance SWS365-065, FKZ 01|S12038A) within a subcontract with novineon GmbH (partly).
Conflict-of-interest statement: Arthur Schmidt and Karel Caca have received lectures fees from Ovesco Endoscopy for full-thickness resection device training courses. The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 7, 2015
First decision: April 23, 2015
Article in press: July 3, 2015
P- Reviewer: Arezzo A, Imaeda H, Mann O S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Conway NE, Swanström LL. Endoluminal flexible endoscopic suturing for minimally invasive therapies. Gastrointest Endosc. 2015;81:262–269.e19. doi: 10.1016/j.gie.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Kuroki Y, Hoteya S, Mitani T, Yamashita S, Kikuchi D, Fujimoto A, Matsui A, Nakamura M, Nishida N, Iizuka T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25:1747–1753. doi: 10.1111/j.1440-1746.2010.06331.x. [DOI] [PubMed] [Google Scholar]
- 3.Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57–65. doi: 10.1136/gutjnl-2013-305516. [DOI] [PubMed] [Google Scholar]
- 4.Full thickness resection device (FTRD) for endoluminal removal of large bowel tumours: development of the instrument and related experimental studies. Minim Invasive Ther Allied Technol. 2001;10:301–309. doi: 10.1080/136457001753337357. [DOI] [PubMed] [Google Scholar]
- 5.Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693–711. doi: 10.1055/s-0034-1377531. [DOI] [PubMed] [Google Scholar]
- 6.Meining A. Endoscopic full-thickness resection: the logical step toward more extended endoscopic oncologic resections? Endoscopy. 2015;47:101–102. doi: 10.1055/s-0034-1391372. [DOI] [PubMed] [Google Scholar]
- 7.Uciteli A, Goller C, Burek P, Siemoleit S, Faria B, Galanzina H, Weiland T, Drechsler-Hake D, Bartussek W, Herre H. Search Ontology, a new approach towards Semantic Search. GI Ed Proc LNI. 2014;232:667–672. [Google Scholar]
- 8.Valli PV, Kaufmann M, Vrugt B, Bauerfeind P. Endoscopic resection of a diverticulum-arisen colonic adenoma using a full-thickness resection device. Gastroenterology. 2014;147:969–971. doi: 10.1053/j.gastro.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015:Epub ahead of print. doi: 10.1055/s-0034-1391781. [DOI] [PubMed] [Google Scholar]
- 10.Mönkemüller K, Peter S, Toshniwal J, Popa D, Zabielski M, Stahl RD, Ramesh J, Wilcox CM. Multipurpose use of the ‘bear claw’ (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc. 2014;26:350–357. doi: 10.1111/den.12145. [DOI] [PubMed] [Google Scholar]
- 11.Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76–79. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 12.Fritscher-Ravens A, Milla P, Ellrichmann M, Hellwig I, Böttner M, Hadeler KG, Wedel T. A novel endoscopic prototype device for gastric full-thickness biopsy for the histopathologic diagnosis of GI neuromuscular pathology: in vivo porcine long-term survival study (with videos) Gastrointest Endosc. 2013;77:262–271. doi: 10.1016/j.gie.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Rajan E, Gostout CJ, Aimore Bonin E, Moran EA, Locke RG, Szarka LA, Talley NJ, Deters JL, Miller CA, Knipschield MA, et al. Endoscopic full-thickness biopsy of the gastric wall with defect closure by using an endoscopic suturing device: survival porcine study. Gastrointest Endosc. 2012;76:1014–1019. doi: 10.1016/j.gie.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan E, Gostout CJ, Lurken MS, Talley NJ, Locke GR, Szarka LA, Sumiyama K, Bakken TA, Stoltz GJ, Knipschield MA, et al. Endoscopic “no hole” full-thickness biopsy of the stomach to detect myenteric ganglia. Gastrointest Endosc. 2008;68:301–307. doi: 10.1016/j.gie.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–2931. doi: 10.1007/s00464-011-1644-y. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Yu L, Yang S, Li X, Ding J, Chen L, Xu Y, Shi R. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A. 2014;24:171–176. doi: 10.1089/lap.2013.0370. [DOI] [PubMed] [Google Scholar]
- 17.Huang LY, Cui J, Lin SJ, Zhang B, Wu CR. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol. 2014;20:13981–13986. doi: 10.3748/wjg.v20.i38.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258–2274. doi: 10.1007/s00464-012-2754-x. [DOI] [PubMed] [Google Scholar]
- 19.Mori H, Kobara H, Fujihara S, Nishiyama N, Rafiq K, Oryu M, Fujiwara M, Suzuki Y, Masaki T. Feasibility of pure EFTR using an innovative new endoscopic suturing device: the Double-arm-bar Suturing System (with video) Surg Endosc. 2014;28:683–690. doi: 10.1007/s00464-013-3266-z. [DOI] [PubMed] [Google Scholar]
- 20.Mori H, Rafiq K, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Tani J, et al. Development of pure endoscopic full-thickness resection with mechanical countertraction and double-armed bar suturing systems. Gastrointest Endosc. 2014;79:24–25. doi: 10.1016/j.gie.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Mori H, Rafiq K, Kobara H, Fujihara S, Nishiyama N, Oryuu M, Suzuki Y, Masaki T. Innovative noninsufflation EFTR: sufficient endoscopic operative field by mechanical counter traction device. Surg Endosc. 2013;27:3028–3034. doi: 10.1007/s00464-013-2846-2. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt A, Bauder M, Riecken B, Caca K. Endoscopic resection of subepithelial tumors. World J Gastrointest Endosc. 2014;6:592–599. doi: 10.4253/wjge.v6.i12.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Renteln D, Schmidt A, Riecken B, Caca K. Gastric full-thickness suturing during EMR and for treatment of gastric-wall defects (with video) Gastrointest Endosc. 2008;67:738–744. doi: 10.1016/j.gie.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 24.Walz B, von Renteln D, Schmidt A, Caca K. Endoscopic full-thickness resection of subepithelial tumors with the use of resorbable sutures (with video) Gastrointest Endosc. 2011;73:1288–1291. doi: 10.1016/j.gie.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015;47:154–158. doi: 10.1055/s-0034-1390786. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology. 2014;147:740–742.e2. doi: 10.1053/j.gastro.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 27.Kaehler GF, Langner C, Suchan KL, Freudenberg S, Post S. Endoscopic full-thickness resection of the stomach: an experimental approach. Surg Endosc. 2006;20:519–521. doi: 10.1007/s00464-005-0147-0. [DOI] [PubMed] [Google Scholar]
- 28.Fritscher-Ravens A, Arlt A. Safety notes: how to avoid infections in natural orifice transluminal endoscopic surgery. Endoscopy. 2011;43:58–62. doi: 10.1055/s-0030-1256033. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff A, Vetter S, von Renteln D, Caca K, Kähler G, Eickhoff JC, Jakobs R, Riemann JF. Effectivity of current sterility methods for transgastric NOTES procedures: results of a randomized porcine study. Endoscopy. 2010;42:748–752. doi: 10.1055/s-0030-1255597. [DOI] [PubMed] [Google Scholar]
- 30.Meining A, Feussner H, Swain P, Yang GZ, Lehmann K, Zorron R, Meisner S, Ponsky J, Martiny H, Reddy N, et al. Natural-orifice transluminal endoscopic surgery (NOTES) in Europe: summary of the working group reports of the Euro-NOTES meeting 2010. Endoscopy. 2011;43:140–143. doi: 10.1055/s-0030-1256128. [DOI] [PubMed] [Google Scholar]
- 31.Meining A, Spaun G, Fernández-Esparrach G, Arezzo A, Wilhelm D, Martinek J, Spicak J, Feussner H, Fuchs KH, Hucl T, et al. NOTES in Europe: summary of the working group reports of the 2012 EURO-NOTES meeting. Endoscopy. 2013;45:214–217. doi: 10.1055/s-0032-1326205. [DOI] [PubMed] [Google Scholar]
- 32.Kopelman Y, Siersema PD, Bapaye A, Kopelman D. Endoscopic full-thickness GI wall resection: current status. Gastrointest Endosc. 2012;75:165–173. doi: 10.1016/j.gie.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H, Kudo SE. [Per-oral endoscopic myotomy (POEM) for 43 consecutive cases of esophageal achalasia] Nihon Rinsho. 2010;68:1749–1752. [PubMed] [Google Scholar]
- 34.Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–230. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 35.Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231–235. doi: 10.1055/s-0031-1291720. [DOI] [PubMed] [Google Scholar]
- 36.Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524–530. doi: 10.1007/s00464-013-3197-8. [DOI] [PubMed] [Google Scholar]
- 37.Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos) Gastrointest Endosc. 2012;75:195–199. doi: 10.1016/j.gie.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Kim SJ, Lee TH, Chung IK, Park SH, Kim EO, Lee HJ, Cho HD. Human applications of submucosal endoscopy under conscious sedation for pure natural orifice transluminal endoscopic surgery. Surg Endosc. 2013;27:3016–3020. doi: 10.1007/s00464-013-2844-4. [DOI] [PubMed] [Google Scholar]
- 39.Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329–334. doi: 10.1055/s-0032-1326214. [DOI] [PubMed] [Google Scholar]
- 40.Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978–1983. doi: 10.1007/s00464-014-3421-1. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Huang LY, Wu CR, Cui J, Jiang LX, Zheng HT. Endoscopic full-thickness resection of gastric stromal tumor arising from the muscularis propria. Chin Med J (Engl) 2013;126:2435–2439. [PubMed] [Google Scholar]
- 42.Zhang Y, Fan Z. Is closure of only the mucosal layer really sufficient? Endoscopy. 2014;46:82. doi: 10.1055/s-0033-1358951. [DOI] [PubMed] [Google Scholar]
- 43.von Renteln D, Vassiliou MC, Rothstein RI. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy. 2009;41:1056–1061. doi: 10.1055/s-0029-1215241. [DOI] [PubMed] [Google Scholar]
- 44.Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of gastrointestinal fistulae--a meta-analysis. Minim Invasive Ther Allied Technol. 2012;21:249–258. doi: 10.3109/13645706.2012.694367. [DOI] [PubMed] [Google Scholar]
- 45.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 46.Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos) Gastrointest Endosc. 2012;76:202–208. doi: 10.1016/j.gie.2012.03.250. [DOI] [PubMed] [Google Scholar]
- 47.Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603–608. doi: 10.1016/j.cgh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4–11. doi: 10.1055/s-0032-1325760. [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015:Epub ahead of print. doi: 10.1007/s00464-015-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed I, Shibukawa G, Groce R, Poussard A, Brining D, Raju GS. Study of full-thickness endoluminal segmental resection of colon in a porcine colon model (with videos) Gastrointest Endosc. 2007;65:696–702. doi: 10.1016/j.gie.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 51.Raju GS, Ahmed I, Shibukawa G, Poussard A, Brining D. Endoluminal clip closure of a circular full-thickness colon resection in a porcine model (with videos) Gastrointest Endosc. 2007;65:503–509. doi: 10.1016/j.gie.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Wang XY, Zhou PH, Li QL, Zhang Y, Zhong Y, Chen W, Ma L, Ishaq S, Qin W, et al. Endoscopic full-thickness resection of colonic submucosal tumors originating from the muscularis propria: an evolving therapeutic strategy. Endoscopy. 2013;45:770–773. doi: 10.1055/s-0033-1344225. [DOI] [PubMed] [Google Scholar]
- 53.Brigic A, Symons NR, Faiz O, Fraser C, Clark SK, Kennedy RH. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013;27:3520–3529. doi: 10.1007/s00464-013-2946-z. [DOI] [PubMed] [Google Scholar]
- 54.von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc. 2010;71:1267–1273. doi: 10.1016/j.gie.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 55.Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos) Gastrointest Endosc. 2014;79:503–507. doi: 10.1016/j.gie.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 56.Kantsevoy SV, Thuluvath PJ. Successful closure of a chronic refractory gastrocutaneous fistula with a new endoscopic suturing device (with video) Gastrointest Endosc. 2012;75:688–690. doi: 10.1016/j.gie.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Chiu PW, Phee SJ, Wang Z, Sun Z, Poon CC, Yamamoto T, Penny I, Wong JY, Lau JY, Ho KY. Feasibility of full-thickness gastric resection using master and slave transluminal endoscopic robot and closure by Overstitch: a preclinical study. Surg Endosc. 2014;28:319–324. doi: 10.1007/s00464-013-3149-3. [DOI] [PubMed] [Google Scholar]
- 58.Chiu PW, Lau JY, Ng EK, Lam CC, Hui M, To KF, Sung JJ, Chung SS. Closure of a gastrotomy after transgastric tubal ligation by using the Eagle Claw VII: a survival experiment in a porcine model (with video) Gastrointest Endosc. 2008;68:554–559. doi: 10.1016/j.gie.2008.03.1110. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, Chiu PW, Teoh AY, Lam CC, Ng EK, Lau JY. Endoscopic suturing is superior to endoclips for closure of gastrotomy after natural orifices translumenal endoscopic surgery (NOTES): an ex vivo study. Surg Endosc. 2014;28:1342–1347. doi: 10.1007/s00464-013-3280-1. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda K, Fritscher-Ravens A, Mosse CA, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc. 2005;62:122–129. doi: 10.1016/s0016-5107(05)00517-1. [DOI] [PubMed] [Google Scholar]
- 61.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA. Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc. 2007;65:134–139. doi: 10.1016/j.gie.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 62.Raju GS, Shibukawa G, Ahmed I, Brining D, Poussard A, Xiao SY, Coe J, Cropper M, Martin D, Hull J. Endoluminal suturing may overcome the limitations of clip closure of a gaping wide colon perforation (with videos) Gastrointest Endosc. 2007;65:906–911. doi: 10.1016/j.gie.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 63.Fritscher-Ravens A, Hampe J, Grange P, Holland C, Olagbeye F, Milla P, von Herbay A, Jacobsen B, Seehusen F, Hadeler KG, et al. Clip closure versus endoscopic suturing versus thoracoscopic repair of an iatrogenic esophageal perforation: a randomized, comparative, long-term survival study in a porcine model (with videos) Gastrointest Endosc. 2010;72:1020–1026. doi: 10.1016/j.gie.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda K, Mosse CA, Park PO, Fritscher-Ravens A, Bergström M, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection: circumferential cutting method. Gastrointest Endosc. 2006;64:82–89. doi: 10.1016/j.gie.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 65.Park PO, Bergström M, Ikeda K, Fritscher-Ravens A, Mosse S, Kochman M, Swain P. Endoscopic pyloroplasty with full-thickness transgastric and transduodenal myotomy with sutured closure. Gastrointest Endosc. 2007;66:116–120. doi: 10.1016/j.gie.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Raju GS, Malhotra A, Ahmed I. Colonoscopic full-thickness resection of the colon in a porcine model as a prelude to endoscopic surgery of difficult colon polyps: a novel technique (with videos) Gastrointest Endosc. 2009;70:159–165. doi: 10.1016/j.gie.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Mori H, Kobara H, Kazi R, Fujihara S, Nishiyama N, Masaki T. Balloon-armed mechanical counter traction and double-armed bar suturing systems for pure endoscopic full-thickness resection. Gastroenterology. 2014;147:278–280.e1. doi: 10.1053/j.gastro.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 68.Rajan E, Gostout CJ, Burgart LJ, Leontovich ON, Knipschiel MA, Herman LJ, Norton ID. First endoluminal system for transmural resection of colorectal tissue with a prototype full-thickness resection device in a porcine model. Gastrointest Endosc. 2002;55:915–920. doi: 10.1067/mge.2002.124099. [DOI] [PubMed] [Google Scholar]
- 69.Schurr MO, Baur FE, Krautwald M, Fehlker M, Wehrmann M, Gottwald T, Prosst RL. Endoscopic full-thickness resection and clip defect closure in the colon with the new FTRD system: experimental study. Surg Endosc. 2015;29:2434–2441. doi: 10.1007/s00464-014-3923-x. [DOI] [PubMed] [Google Scholar]
- 70.Kaehler G, Grobholz R, Langner C, Suchan K, Post S. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy. 2006;38:86–89. doi: 10.1055/s-2005-921181. [DOI] [PubMed] [Google Scholar]
- 71.Meireles OR, Kantsevoy SV, Assumpcao LR, Magno P, Dray X, Giday SA, Kalloo AN, Hanly EJ, Marohn MR. Reliable gastric closure after natural orifice translumenal endoscopic surgery (NOTES) using a novel automated flexible stapling device. Surg Endosc. 2008;22:1609–1613. doi: 10.1007/s00464-008-9750-1. [DOI] [PubMed] [Google Scholar]
- 72.Evans JA, Rosato FE, Ginsberg GG. Gastrostomy port assisted full-thickness gastric resection by using the peroral SurgASSIST introduced via an oroesophageal overtube in a porcine model. Gastrointest Endosc. 2007;65:684–687. doi: 10.1016/j.gie.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Sodergren M, Clark J, Beardsley J, Bryant T, Horton K, Darzi A, Teare J. A novel flexible endoluminal stapling device for use in NOTES colotomy closure: a feasibility study using an ex vivo porcine model. Surg Endosc. 2011;25:3266–3272. doi: 10.1007/s00464-011-1703-4. [DOI] [PubMed] [Google Scholar]
- 74.Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Mönkemüller K. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014;46:758–761. doi: 10.1055/s-0034-1365513. [DOI] [PubMed] [Google Scholar]
- 75.Schurr MO, Baur F, Ho CN, Anhoeck G, Kratt T, Gottwald T. Endoluminal full-thickness resection of GI lesions: a new device and technique. Minim Invasive Ther Allied Technol. 2011;20:189–192. doi: 10.3109/13645706.2011.582119. [DOI] [PubMed] [Google Scholar]
- 76.von Renteln D, Kratt T, Rösch T, Denzer UW, Schachschal G. Endoscopic full-thickness resection in the colon by using a clip-and-cut technique: an animal study. Gastrointest Endosc. 2011;74:1108–1114. doi: 10.1016/j.gie.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 77.von Renteln D, Rösch T, Kratt T, Denzer UW, El-Masry M, Schachschal G. Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci. 2012;57:1298–1303. doi: 10.1007/s10620-012-2039-1. [DOI] [PubMed] [Google Scholar]
- 78.Thompson CC, Ryou M, Soper NJ, Hungess ES, Rothstein RI, Swanstrom LL. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery applications (with video) Gastrointest Endosc. 2009;70:121–125. doi: 10.1016/j.gie.2008.11.007. [DOI] [PubMed] [Google Scholar]


