Abstract
AIM: To assess the impact of Helicobacter pylori (H. pylori) genotypes and patient age and sex on the development of gastric diseases.
METHODS: H. pylori-infected patients (n = 233) referred to the endoscopy unit at Tehran University of Medical Sciences (Tehran, Iran) were diagnosed with chronic gastritis (CG), gastric ulcer (GU), or duodenal ulcer (DU). Brucella blood agar was used for biopsy cultures and H. pylori isolation under microaerobic conditions. H. pylori isolates were confirmed with biochemical tests and through amplification of the 16S rRNA gene. DNA was extracted from fresh cultures of the H. pylori isolates and used for amplification of vacA alleles and the cagA gene. Statistical analysis was performed to determine the association between H. pylori genotypes, age (< 40 years vs > 40 years) and sex of the patient, and gastric diseases.
RESULTS: CG was the most prevalent gastric disease (113/233; 48.5%), compared to GU (64/233; 27.5%) and DU (56/233; 24%). More patients were male, and gastric diseases were more frequent in patients > 40 years (P < 0.05). The percentage of CG and GU patients that were male and female did not show a significant difference; however DU was more common in males (P < 0.05). Interestingly, a diagnosis of CG in patients > 40 years was more common in females (18.5%) than males (11.6%) (P = 0.05), whereas a diagnosis of GU or DU in patients > 40 years was more frequent in males (14.6% vs 10.7% and 12.4% vs 4.3%, respectively). Overall, genotyping of the H. pylori isolates revealed that the vacA s1 (82%), vacA m2 (70%), and cagA+ (72.5%) alleles were more frequent than vacA s2 (18%), vacA m1 (29.2%), and cagA- (all P < 0.05). The vacA s1m2cagA+ genotype was the most prevalent within the three disease groups. vacA s1m2 frequency was 56.2% with a similar occurrence in all diagnoses, while vacA s1m1 appeared more often in DU patients (33.9%). A genotype of vacA s2m2 occurred in 15% of isolates and was more common in CG patients (21.2%); vacA s2m1 was the least common genotype (3%). The vacA s1 allele was found to be a risk factor for DU, vacA s2 for CG, and vacA s1 and vacA s2 for GU (all P < 0.05). The vacA s2m2 genotype was associated with the development of CG and GU compared to DU (P < 0.05). No correlation was found between vacA m or cagA and gastric diseases.
CONCLUSION: The outcome of H. pylori infection is the result of interaction between bacterial genotypes and the age and sex of infected individuals.
Keywords: Age, Gastric disease, Gender, Genotype, Helicobacter pylori
Core tip: Helicobacter pylori (H. pylori) genotype and host and environmental factors have emerged as the risk factors of H. pylori-associated diseases. However, controversies exist regarding the reciprocal interaction between these factors. Results of this study demonstrate that increased age is an important risk factor for gastric ulcers in both males and females, for chronic gastritis in females, and for duodenal ulcers in males. Genotypes vacA s1 and vacA s2m2 emerged as significant risk factors for duodenal ulcers, and chronic gastritis and gastric ulcers, respectively. No correlation was found between vacA m or cagA and gastric diseases.
INTRODUCTION
Analysis of the genetic composition of Helicobacter pylori (H. pylori) has revealed a remarkable heterogeneity in gene content and sequence[1]. This versatile gene reservoir appears to serve as a powerful tool for bacterial adaptation when encountering new conditions in different human hosts[2]. Establishment of H. pylori in gastric epithelium is associated with a persistent induction of inflammatory responses and tissue damage that could lead to development of more critical clinical diagnoses, including chronic gastritis (CG), peptic ulcers (PUs), or gastric cancer[3,4]. An interaction between H. pylori virulence factors, host genetics, and environmental factors is currently thought to determine the extent of tissue damage[5,6]. In this regard, the longevity of H. pylori infection and sex of infected individuals have been investigated as important factors in the development of gastric diseases[7-11]. Many investigators have studied H. pylori virulence factors and proposed several candidate proteins, including vacuolating cytotoxin A (VacA) and cytotoxin-associated gene A (CagA)[12]. In contrast, few studies have focused on host genetics, eating habits, and lifestyle, and the results remain controversial[13-19].
VacA, which occurs in all strains of H. pylori, is regarded as a multifunctional toxin with the potential to insert into the endosomal membranes of epithelial cells, inducing the formation of large vacuoles, and inhibition of antigen presentation[20]. VacA also inserts into the mitochondrial membrane and causes apoptosis[21]. Cellular tight junctions are loosened by VacA, releasing nutrients, such as iron, nickel, sugars, and amino acids, needed for the establishment of H. pylori[22]. Furthermore, VacA inhibits the proliferation of T cells, which helps the bacterium to evade an immune response and establish a chronic infection[23,24]. Although VacA is crucial for colonization of all H. pylori strains, its toxicity is determined by the presence of different allelic types of the signal sequence (s1 and s2) and middle region (m1 and m2). It has been proposed that H. pylori strains carrying vacA s1m1 are highly toxigenic, increasing the risk of PUs or gastric cancer, those with vacA s1m2 less toxigenic, and vacA s2m2 nontoxigenic, while a genotype of vacA s2m1 rarely occurs[25,26]. CagA binds to epithelial cells and causes perturbation of tight junctions, cell polarity, and differentiation[27]. Interaction of CagA with E-cadherin and β-catenin causes interruption of the adhesion of epithelial cells, as well as formation of junctions and growth. Furthermore, CagA induces the production of interleukin-8, which leads to an inflammatory response and tissue damage[28]. These interactions of CagA with epithelial cells lead to destabilization and damage of gastric epithelium, and thus, contribute to H. pylori pathogenesis[22].
Studies have shown that cagA+ strains are often associated with a higher risk of PUs or gastric cancer, compared to cagA- strains[29,30]. It has been suggested that the combination of an active VacA toxin with CagA constitutes an efficient system for generating an appropriate niche for long-term colonization of H. pylori in gastric epithelium. CagA contributes to changes in the gastric epithelium in several ways. It has been demonstrated that CagA protects epithelial cells against apoptotic events induced by VacA, but by inducing proinflammatory and antiapoptotic activities, also causes severe tissue damage, leading to a PU and even gastric cancer[31]. Furthermore, the antiapoptotic activity of CagA has been shown to reduce the rate of turnover of epithelial cells[32], whereas VacA decreases CagA-induced cell scattering and motility[33].
Cure of CG[34], gastric ulcer (GU), and duodenal ulcer (DU)[35] with antimicrobial therapy against H. pylori demonstrates that the bacterium is an important risk factor for dyspeptic diseases. However, several studies have observed a correlation between H. pylori-associated gastric atrophy and smoking[13], intake of salt[14], alcohol[15], low levels of dietary beta-carotene, and consumption of soybean products[16]. Furthermore, acid-suppression due to GU[17] and consumption of acid-suppressing drugs[36] have been found to be associated with corpus atrophy in H. pylori-infected patients. In contrast, no correlation between H. pylori-associated gastritis and sex, age, smoking, and coffee intake or between atrophy or intestinal metaplasia and smoking or drinking alcohol was observed in other reports[19]. Furthermore, it is well known that the incidence of H. pylori infection increases with age[7,8], and that aging is an important risk factor for dyspeptic diseases[9,10]. However, there are discrepancies about the role of the sex of the patient in H. pylori-associated dyspeptic diseases[11]. Although reports indicate a reduced incidence of H. pylori infection in some regions of the world due to antimicrobial therapies against H. pylori or other infections[10,37-40], a considerable number of patients in Iran are still referred for endoscopy, seeking relief from dyspeptic diseases. The reported frequency of H. pylori in the general population of Iran is approximately 69%[41], but reaches up to 89% in the northwestern province of Ardabil[42], where 90% of individuals over 40 years-old suffer from H. pylori-associated CG[43]. In this study, H. pylori isolates from 233 patients with CG, GU, or DU were genotyped for vacA alleles and cagA gene. The reciprocal impact of H. pylori genotypes and host age and sex on the development of dyspeptic diseases was assessed.
MATERIALS AND METHODS
Patients
The recruited patients (n = 233; 129 men and 104 women) were randomly selected H. pylori-positive referrals to the endoscopy unit of Shariati Hospital (Tehran University of Medical Sciences, Tehran, Iran) due to complaint of dyspepsia. Patients were stratified based on diagnosis and age: CG, GU, and DU, and < 40 years and > 40 years, respectively.
H. pylori isolation and cultivation
Two antral biopsies were taken from each patient for a rapid urease test and H. pylori cultivation. Biopsies were cultured on selective Brucella agar (Pronadisa, Madrid, Spain) containing 5% defibrinated sheep blood and 10 mg/L vancomycin, 5 mg/L trimethoprim, and 2.5 IU/L polymyxin B (all from MP Biomedical, Santa Ana, CA, United States). Cultures were incubated at 37 °C under microaerobic conditions for 3-5 d. Bacterial isolates were identified as H. pylori on the basis of Gram-stained morphology, positive urease, catalase, and oxidase tests, and amplification of the H. pylori-specific 16S rRNA gene. The purified bacterial isolates were harvested in phosphate-buffered saline (PBS) and stored at -20 °C until further use.
Genotyping of H. pylori isolates
DNA was extracted from fresh cultures of H. pylori isolates with phenol/chloroform as previously described[44]. Genotyping of H. pylori isolates was performed by polymerase chain reaction (PCR) amplification of the cagA gene, vacA signal sequences (s1 or s2), and middle regions (m1 or m2). The primers for amplification are listed in Table 1. Escherichia coli (DSM 0498) and previously PCR-confirmed H. pylori isolates were used as negative and positive controls, respectively. Amplification was carried out in a total volume of 25 μL containing 2.5 μL of 10 × PCR buffer (Sinaclon, Karaj, Iran), 1.5 mmol/L MgCl2, 125 μmol/L of each dNTP (Sinaclon), 1U of Taq DNA polymerase (Sinaclon), 0.5 μmol/L of each primer, and 25 ng of bacterial DNA. Cycling parameters were 94 °C (1 min), optimized annealing temperature for each genes/alleles (1 min), and 72 °C (1 min) for 33 cycles with a final extension at 72 °C (7 min). PCR products were electrophoresed and visualized with a UV transilluminator (UVP, Upland, CA, United States). Amplified fragments of all genes/alleles from the five isolates were purified and sequenced with both forward and reverse primers using BigDye technology, and sequencing reactions were run on an AB13700XL DNA sequencer (Life Technologies of Thermo Fisher Scientific, Waltham, MA, United States). The BLAST program (http://www.ncbi.nlm.nih.gov) was used to match the nucleotide sequences with published sequences in Genbank (data not shown). The size of the PCR products of all genes was similar to those generated from the control H. pylori strains, and sequences showed 99%-100% similarity with the corresponding sequences of the reference H. pylori strains in Genbank (Table 1).
Table 1.
Oligonucleotide primers used for polymerase chain reaction
| Gene | Primer | Sequence (5'→3') | PCR products (bp) | Annealing temperature (°C) | Ref. |
| 16S rRNA | HP1 | GCAATCAGCGTCAGTAATGTT C | 519 | 58.5 | [85] |
| HP2 | GCTAAGAGATCAGCCTATGTCC | ||||
| vacA (s1, s2) | VA1F | ATGGAAATACAAGAAACACACC | s1: 259 | 56.0 | [25] |
| VA1R | CTGCTTGAATGCGCCAAACTTTAATC | s2: 286 | |||
| vacA (m1, m2) | VAG-F | CAATCTGTCCAATCAAGCGAG | m1: 570 | 58.5 | [25] |
| VAG-R | GCGTCAAAATAATTCCAAGG | m2: 645 | |||
| cagA | D008 | ATAATGCTAAATTAGACAACTTGAGCGA | 298 | 58.5 | [86] |
| R008 | TTAGAATAATCAACAAACATCACGCCAT |
PCR: Polymerase chain reaction.
Statistical analysis
Statistical analysis was performed using Pearson’s χ2 and Fisher’s exact probability tests. Kendell’s Tau b correlation coefficient was used to measure the strength of dependence between H. pylori genotypes, and age or sex and gastric disease. Logistic regression analysis was used to predict the outcome of gastric diseases based on age, sex, or H. pylori genotype (SPSS version 20, IBM Corp., Armonk, NY, United States). Statistical significance was defined as P ≤ 0.05.
RESULTS
Classification of patients according to age, sex, and gastric disease
All patients (n = 233) were H. pylori positive, but were diagnosed with one of three diseases, CG, DU, or GU. CG was the most prevalent gastric disease (113/233; 48.5%), compared to GU (64/233; 27.5%) and DU (56/233; 24%). The distribution of patients according to sex, age, and H. pylori genotype are presented in Table 2. A greater percentage of patients were > 40 years of age (168/233; 72.1%) and male (129/233; 55.4%). More patients were male in both age groups: < 40 years, 16.7% (39/233) vs 11.2% (26/233) females, and > 40 years, 38.6% (90/233) were male vs 33.5% (78/233) female. For all diagnoses, more patients were > 40 years (Figure 1, Table 2).
Table 2.
Distribution of genotypes in Helicobacter pylori isolates n (%)
| Characteristic | CG | GU | DU | Total | |
| No. of cases | 113 (48.5) | 64 (27.5) | 56 (24.0) | 233 (100) | |
| Sex | Female | 62 (54.9) | 27 (42.2) | 15 (26.8) | 104 (44.6) |
| Male | 51 (45.1) | 37 (57.8) | 41 (73.2) | 129 (55.4) | |
| Age | < 40 yr | 43 (38.1) | 5 (7.8) | 17 (30.4) | 65 (27.9) |
| > 40 yr | 70 (61.9) | 59 (92.2) | 39 (69.6) | 168 (72.1) | |
| vacA | s1 | 83 (73.5) | 53 (82.8) | 55 (98.2) | 191 (82.0) |
| s2 | 30 (26.5) | 11 (17.2) | 1 (1.8) | 42 (18.0) | |
| m1 | 32 (28.3) | 16 (25) | 20 (35.7) | 68 (29.2) | |
| m2 | 81 (71.7) | 48 (75) | 34 (60.7) | 163 (70.0) | |
| s1m1 | 24 (21.2) | 15 (23.4) | 19 (33.9) | 58 (24.9) | |
| s1m2 | 59 (52.2) | 38 (59.4) | 34 (60.7) | 131 (56.2) | |
| s2m1 | 6 (5.3) | 1 (1.6) | 0 | 7 (3.0) | |
| s2m2 | 24 (21.2) | 10 (15.6) | 1 (1.8) | 35 (15.0) | |
| cagA | + | 82 (72.6) | 50 (78.1) | 37 (66.1) | 169 (72.5) |
| - | 31 (27.4) | 14 (21.9) | 19 (33.9) | 64 (27.5) | |
| s1m1cagA | + | 17 (15.0) | 10 (15.6) | 10 (17.9) | 37 (15.9) |
| - | 7 (6.2) | 5 (7.8) | 9 (16.1) | 21 (9.0) | |
| s1m2cagA | + | 41 (36.3) | 30 (46.9) | 24 (42.9) | 95 (40.8) |
| - | 18 (15.9) | 8 (12.5) | 10 (17.9) | 36 (15.5) | |
| s2m1cagA | + | 5 (4.4) | 1 (1.6) | 0 | 6 (2.6) |
| - | 1 (0.9) | 0 | 0 | 1 (0.4) | |
| s2m2cagA | + | 19 (16.8) | 9 (14.1) | 1 (1.8) | 29 (12.4) |
| - | 5 (4.4) | 1 (1.6) | 0 | 6 (2.6) | |
| s1m1m2cagA | + | 0 | 0 | 2 (3.6) | 2 (0.8) |
| - | 0 | 0 | 0 | 0 |
CG: Chronic gastritis; DU: Duodenal ulcer; GU: Gastric ulcer.
Figure 1.
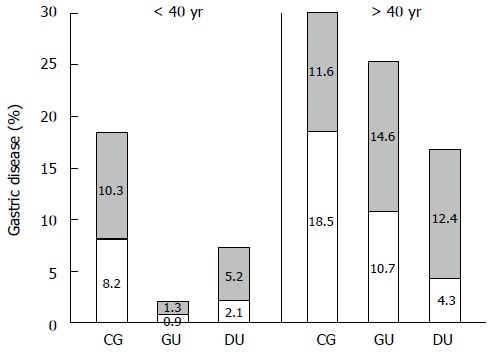
Gastric disease cases are more prevalent in patients > 40 years of age. The percentages of the 233 Helicobacter pylori-positive patients are plotted according to disease diagnosis (CG: Chronic gastritis: GU: Gastric ulcer; and DU: Duodenal ulcer), age (< 40 years and > 40 years), and sex (male: White bars; female: Shaded bars).
Genotype frequencies within H. pylori isolates from CG, GU, and DU patients
In order to determine whether H. pylori isolates genetically differed among patients and/or disease, vacA and cagA genes were amplified from 233 H. pylori DNAs and sequenced. The most frequently detected alleles were vacA s1, vacA m2, and cagA+, at 82.0% (191/233), 70.0% (163/233), and 72.5% (169/233), respectively (Table 2). All vacA s/m genotypes were detected, with vacA s1m2 (131/233; 56.2%) as the most common and vacA s2m1 (7/233; 3.0%) the least common. Moreover, vacA s1m2 was equally prevalent among the three disease groups. For the remaining vacA s/m genotypes, vacA s1m1 was observed in 24.9% (58/233) of cases and most often associated with a diagnosis of DU (33.9%), whereas vacA s2m2 was detected in 15% (35/233) of cases overall, but most often in CG patients (24/233; 21.2%). For all the alleles detected, vacA s1m2 cagA+ (95/233; 40.8%) was the most common genotype observed in the cohort.
Sex, age, and H. pylori genotypes of CG, GU, and DU patients
Statistical analysis was first performed on clinical characteristics of the patients, such as sex of the patient and age. In this part of statistical analysis, each individual disease group was considered separately. Whereas CG and GU were not associated with sex of the patient, In contrast, the increased proportion of male relative to female patients in the DU group was statistically significant (73.2% vs 26.8%, P = 0.001). Increased age (> 40 years) was clearly associated with all diseases (P = 0.011, 0.000, and 0.003 for CG, GU, and DU, respectively).
The frequencies of specific virulence alleles were examined based on gastric disease diagnosis. In all diagnoses, the frequencies of the vacA s1 allele compared to vacA s2 and vacA m2 relative to vacA m1 were higher. The frequency of the vacA s1 allele was higher than vacA s2 (73.5% vs 26.5% and 82.8% vs 17.2%, P = 0.000), and vacA m2 was higher than vacA m1 (71.7% vs 28.3% and 75.0% vs 25.0%, P = 0.000) in CG and GU patients, respectively. In addition, in DU patients, the frequency of the vacA s1 allele was significantly higher than vacA s2 (98.2% vs 1.8%, P = 0.000), and the vacA m2 allele was at a higher frequency than vacA m1 (60.7% vs 35.7%, P = 0.000). Finally, the vacA s1m1m2 genotype was only detected in 2/56 (3.6%) female DU patients. cagA was detected in significantly more H. pylori strains derived from CG (72.6%; P = 0.000) and DU (66.1%; P = 0.016) patients. H. pylori isolates with vacA s1m2cagA± genotypes exhibited the highest frequency (56.3%) overall with similar prevalence among CG, GU, and DU patients (Table 2, Figure 2).
Figure 2.
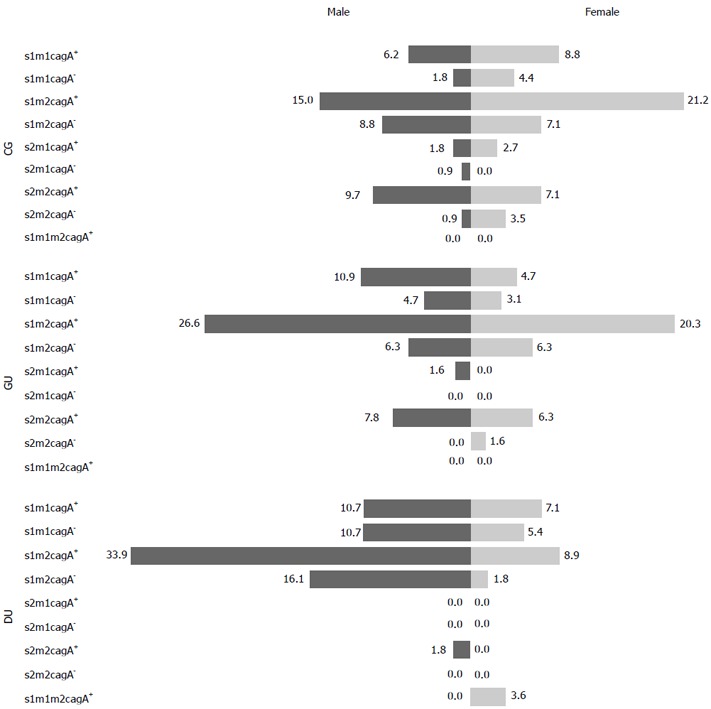
Distribution of Helicobacter pylori genotypes according to sex. vacA s1m2 cagA+ was the most common genotype in both males (dark bars) and females (light bars) of all three gastric diseases. CG: Chronic gastritis; GU: Gastric ulcer; DU: Duodenal ulcer.
Reciprocal impact of host age and sex and H. pylori genotypes on the development of gastric diseases
Clinical characteristics of patients were analyzed for associations with disease development. Increased age clearly emerged as a risk factor for all dyspeptic disease diagnoses. As indicated above, the number of patients in the three disease groups was significantly greater in patients > 40 years than those < 40 years. Increased age appeared as an important risk factor for GU in both males and females, compared to CG and DU (P = 0.000). Furthermore, being male was correlated with DU and female with CG (P = 0.000), whereas no significant correlation was found between the development of GU and being male or female.
A strong correlation was found between specific vacA s genotypes and gastric disease diagnosis. The vacA s1 genotype was a risk factor for development of DU and vacA s2 for CG (P = 0.000); however, both of these genotypes were equally associated with the development of GU. There was no correlation between vacA m or cagA+ alleles and gastric disease. For the combination of vacA s, vacA m, and cagA genotypes in gastric disease, only vacA s2m2 was found to have an association with the development of CG and GU compared to DU (P = 0.004). The frequency of vacA s1m2cagA+ and vacA s1m2cagA- strains was higher in male patients with DU compared to female patients (P = 0.004 and 0.011, respectively). Statistically significant differences were not observed in CG and GU patients (Figures 1 and 2).
DISCUSSION
Understanding the etiology of the development of gastric diseases will help to develop strategies for prevention and treatment. This study addressed age and sex of the patient and H. pylori bacterial genotype as major factors contributing to disease development in a cohort of Iranian patients. Among 233 patients, 48.5% were diagnosed with CG, 27.5% GU, and 24.0% DU. All three diseases were more common in patients > 40 years (72.1%). The most significant difference between patients < 40 and > 40 years was observed in the GU group. In addition, CG was found to be more frequent in females and DU in males; however, GU was similarly prevalent in males and females. Finally, genotyping of the H. pylori isolates indicated that the vacA s1 allele in combination with being male was a significant risk factor for DU, and that vacA s2m2 and being female were risk factors for CG and GU. However, no correlation was found between alleles of vacA m or cagA and dyspeptic diseases.
In developing countries, the prevalence of H. pylori infection reaches up to 80% before the age of 50 years and in developed countries, 50% of individuals older than 60 years are infected[45]. The reported H. pylori-infection rate in the adult population of Brazil ranged from 35.3%[46] to 97.9%[47]. In another study performed in Brazil, the incidence of H. pylori-related gastritis increased with age in women in their 50s and men in their 70s. Furthermore, the frequency of dyspepsia in patients over 70 years was twofold greater than in young adults, and two thirds of dyspeptic patients were women[10]. In Japan, the prevalence of H. pylori was considerably high (85%) and increased with age, from 26% in subjects 16-20 years up to 61% in those 50-64 years[48]. In Africa, an increased prevalence of H. pylori infection was detected in older patients[49]. In a study on 1391 Albanian subjects, H. pylori seropositivity was more prevalent in females > 40 years[50]. A cross-sectional study in the United Kingdom demonstrated a significant association between H. pylori seropositivity and males, shorter height, tobacco consumption, and lower socioeconomic status[45]. In a study from Brazil, the most prevalent gastric disease was CG (72.3%), with GU at 5.1% and DU at 6%. Gastroesophageal alterations were detected in 16.7% of these cases. Sex and age played no role in the development of CG; however, being male and older age were associated with GU, whereas being male alone was linked to the development of DU[51]. A similar prevalence of GU and DU and an association with being male was also observed in another study performed in Southern Brazil[11]. However, in a third study in Brazil, GU and DU were significantly more frequent in women[52].
Reports indicate that males and females become similarly infected with H. pylori[53]. However, the clinical outcome depends on the longevity and severity of the inflammatory response to H. pylori infection in each individual[54]. An increasing body of evidence indicates that the consequences of H. pylori infection are more severe in males; however, the contributing factors are currently unknown. Although the prevalence of H. pylori in males and females was found to be similar, as determined by the rapid urease test and stained biopsy smear examination, higher levels of IgG were observed in males[55]. Furthermore, being male, having polymorphism at the interleukin-1β promoter, and overexpression of interleukin-1β have all been associated with increasing the risk of atrophic gastritis and gastric adenocarcinoma in H. pylori-infected patients[56,57]. It has been demonstrated that gastrin, a hormone which stimulates the proliferation of epithelial cells[58], can lead to gastric cancer if overexpressed, especially in the context of H. pylori infection[59]. In Sweden, higher levels of antibodies against VacA and CagA in H. pylori-infected patients were associated with increased risk of the development of gastric cancer by twofold when compared with control patients without H. pylori infection[60]. H. pylori and aging have also been found to be strongly associated with an increased risk of atrophy and the development of intestinal metaplasia in gastric mucosa[19,61]. In Japan, intestinal metaplasia was evident in a considerable number of males (90%) over the age of 50 years compared to females in the same age group or younger individuals overall[62]. In the United States, the incidence of gastric cancer in males has been reported to be five times higher than in females[63].
The frequencies of vacA s1 (82%), vacA s2 (18%), vacA m1 (29.2%), vacA m2 (70.8%), and cagA (72.5%) were within ranges reported by other studies performed on patients in Iran: vacA s1, 68%-80%; vacA s2, 20%-32%; vacA m1, 30%-70%; vacA m2, 27%-70%[64,65]; and cagA, 44%[66] to 91%[67]. vacA s1 was detected in 73.5%, 82.8%, and 98.2% of CG, GU, and DU patients, respectively, whereas vacA s2 was detected in 26.5%, 17.2%, and 1.8%. vacA m1 was found in 28.3% of CG, 25% of GU, and 35.7% of DU patients, and vacA m2 in 71.7% CG, 75% GU, and 60.7% of DU patients. The cagA gene was detected in most H. pylori isolates (66.1%-78.1%). The most frequent genotype among the 233 isolates was vacA s1m2cagA+ (40.8%) followed by vacA s1m1cagA+ (15.9%), and vacA s1m2cagA- (15.5%), with a similar distribution among gastric disease diagnoses. The frequency of vacA s2m2cagA+ genotype was lower, at 12.4%. Reports indicate that the s1 genotype is very common in East Asian countries, but with no relationship to the clinical outcomes of infection, whereas vacA m1 is more frequent in North East Asia and vacA m2 in South Asia[30,68]. Several studies in Western countries have shown that individuals infected with H. pylori strains carrying vacA s1 or m1 alleles are at a higher risk of PU or gastric cancer when compared to those infected with vacA s2 or vacA m2-carrying strains[69,70]. In this cohort, genotypes vacA s1m1 and vacA s2m2 were detected at a high frequency. The H. pylori vacA s1m1 genotype is in fact common worldwide, ranging from 42% to 84%[71] around the globe, whereas vacA s2m2 varies from 0% to 57%[71,72]. The frequencies of the vacA s1m1 genotype within the isolates of this study exhibited no significant difference among gastric disease diagnoses (21.2%, 23.4%, and 33.9% for CG, GU, and DU patients, respectively). However, the frequencies of the vacAs2m2 genotype were significantly higher in CG and GU patients compared to DU patients (21.2%, 15.6%, and 1.8%, respectively). In a study from Japan, H. pylori strains with the vacA s1m1 genotype were isolated from 59.2%, 79.2%, and 87.5% of CG, GU, and gastric cancer patients, respectively[73]. Furthermore, the vacA s1m2 genotype was found in 17.3%, 7.9%, and 27.2% of isolates from CG, GU, and DU patients, respectively. The vacA s2m2 genotype was more common in H. pylori isolates from CG (22.4%) than GU (11.9%), DU (10.5%), and gastric cancer (4.2%) patients.
Although the frequency of cagA was high (72.5%) in our cohort, an association of cagA+ genotypes with the development of CG, GU, and DU was not observed. The frequency of cagA has been reported to range from 50% in some Middle Eastern countries[74] to 88% in Europe and North America[75,76] and 99% in many East Asian countries[77,78]. Studies in Western countries have revealed a significant association of cagA+ H. pylori strains with severe gastritis, PU, and gastric cancer[29,30,79]. However, such a relationship was not found between cagA+ strains and PU, gastric cancer, and non-ulcer dyspepsia in Far Eastern countries[80]. In a study from Italy, 72% (132/193) of H. pylori isolates were cagA+, and cagA positivity was associated with PU and gastric cancer but not gastritis[81]. It has been proposed that the vacA s1m1 genotype is often linked to the presence of cagA and the vacA s2m2 genotype with its absence[25,82]. In Alaska, cagA was detected in 85% of H. pylori isolates; however, no correlation was found between the cagA+ or cagA- genotype and development of gastric diseases. In the same study, 66% of vacA s2m2-carrying H. pylori strains contained the cagA gene[12].
Results of this study demonstrate that gastric diseases are significantly more frequent in patients > 40 years. Being male and the vacA s1 genotype played an important role in the development of DU. Aging and the vacA s2m2 genotype were associated with a diagnosis of GU, and being female and the vacA s2m2 genotype with CG. However, no correlation was found between vacA m or cagA and gastric diseases. A large body of evidence indicates that the heterogeneity of H. pylori underlies the diversity of gastric diseases observed. This bacterial genetic diversity appears to be the result of recombination processes that evolved for the purpose of long-term colonization in humans, despite eliciting chronic inflammatory responses[83]. In this regard, investigators believe that VacA and CagA act together to stimulate signals in epithelial cells, affecting cell structure, differentiation and behavior[27], and are balanced with the damage needed for long-term colonization[31,84]. Results of this study indicate that VacA and CagA are mainly involved in the colonization of H. pylori in the human stomach. However, the interplay between H. pylori genotypes and age and sex of the human hosts is likely to determine the severity of the gastric disease diagnosis.
COMMENTS
Background
Helicobacter pylori (H. pylori) infection has been regarded as a risk factor for gastric diseases, ranging from chronic gastritis to more severe outcomes, such as peptic ulcers, gastric cancer, and mucosa-associated lymphoid tissue lymphoma. H. pylori has a remarkable heterogeneous genetic reservoir which may enable efficient bacterial adaptation to the gastric niche in different patients. Disease development is potentially the result of the interaction between H. pylori virulence factors, VacA, and CagA and the host, which leads to inflammation and tissue damage. Thus, underlying the clinical outcome of H. pylori infection may be the interplay between virulence factors, host genetics, and environmental factors. However, despite extensive research on H. pylori-related diseases, the impact of risk factors alone or in concert has not been thoroughly evaluated. Therefore, it remains possible that the age and sex of infected individuals play important roles in determining the outcome of H. pylori infection.
Research frontiers
Reports on the risk factors involved in development of H. pylori-associated gastric diseases are controversial. H. pylori-associated gastric atrophy has been correlated to smoking, intake of salt, alcohol, or low beta-carotene, consumption of soybean products, and even acid-suppressing drugs. No correlation with age, sex, smoking, or coffee intake, however, has been observed in other studies. Currently, the relationship between bacterial, host, and environmental factors has only been examined in a few studies with larger numbers of patients. The incidence of H. pylori infection is considerably high in Iran (69%-80%); correspondingly, the frequency of referrals to endoscopy rooms due to complaint of dyspepsia is also high. Therefore, knowledge of the risk factors may contribute to the management and/or prevention of the more severe consequences of H. pylori infection in high-risk patients.
Innovations and breakthroughs
The focus of this study was to assess the potential impact of individual factors, including host age and sex and H. pylori genotypes, on the development of H. pylori-associated chronic gastritis (CG), gastric ulcer (GU), and duodenal ulcer (DU). Results indicated that age and sex were associated with the development of gastric disease in the context of H. pylori infection, and specific H. pylori genotypes were differentially associated with the diagnosis of CG, GU, and DU.
Applications
Increased age, being female, and the vacA s2m2 genotype were risk factors for CG, increased age in males and females and vacA s2m2 for GU, and increased age and vacA s1 for DU. Accordingly, for prevention and control of H. pylori-associated gastric diseases, results of this study might help to identify high-risk patients, particularly in the Iranian population.
Terminology
GU is a defect in gastric mucosa that penetrates deep into the muscularis mucosa. The sensation of indigestion is described as burning and can be relieved by antacid. DU, the duodenal deformity caused by acid and pepsin from the duodenal mucosa, is often associated with pain in the upper stomach, vomiting, bleeding, perforation, and obstruction, and is also relieved by taking antacids. CG is the inflammation of gastric mucosa, mainly caused by H. pylori infection. CG usually has no definite symptoms, but the patient is susceptible to the development of GU.
Peer-review
The relationship between H. pylori genotypes and host age and sex on the development of H. pylori-associated gastric diseases was investigated. Increased age (> 40 years) was found to be a risk factor for CG, GU, and DU. Furthermore, being female and vacA s2m2 were risk factors for CG, vacA s2m2 for GU, and vacA s1 for DU in males. No correlation between H. pylori alleles vacA m or cagA and gastric diseases was observed. Therefore, the disease outcome of H. pylori infection may be a direct result of the interaction of specific bacterial genotypes with the age and sex of infected individuals.
Footnotes
Supported by Research Council of the University of Tehran.
Institutional review board statement: The study was approved by the research Ethics Committee of Tehran University of Medical Sciences. All patients signed written informed consent.
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: Parts of the study have been presented at the XXVIIth International Workshop on Helicobacter and Microbiota in Chronic Digestive Inflammation and Gastric Cancer.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 4, 2015
First decision: February 10, 2015
Article in press: May 7, 2015
P- Reviewer: Chmiela M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Baltrus DA, Amieva MR, Covacci A, Lowe TM, Merrell DS, Ottemann KM, Stein M, Salama NR, Guillemin K. The complete genome sequence of Helicobacter pylori strain G27. J Bacteriol. 2009;191:447–448. doi: 10.1128/JB.01416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19:597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akopyanz N, Bukanov NO, Westblom TU, Kresovich S, Berg DE. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall DG, Coleman DC, Sullivan DJ, Xia H, O’Moráin CA, Smyth CJ. Genomic DNA fingerprinting of clinical isolates of Helicobacter pylori using short oligonucleotide probes containing repetitive sequences. J Appl Bacteriol. 1996;81:509–517. doi: 10.1111/j.1365-2672.1996.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 7.Iijima K, Ohara S, Koike T, Sekine H, Shimosegawa T. Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scand J Gastroenterol. 2004;39:709–716. doi: 10.1080/00365520410005911. [DOI] [PubMed] [Google Scholar]
- 8.Sasidharan S, Lachumy SJ, Ravichandran M, Latha LY, Gegu SR. Epidemiology of Helicobacter pylori among multiracial community in Northern Peninsular, Malaysia: effect of age across race and gender. Asian Pac J Trop Med. 2011;4:72–75. doi: 10.1016/S1995-7645(11)60037-0. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643–654. doi: 10.1111/j.1365-2036.2004.01897.x. [DOI] [PubMed] [Google Scholar]
- 10.Mapel D, Roberts M, Overhiser A, Mason A. The epidemiology, diagnosis, and cost of dyspepsia and Helicobacter pylori gastritis: a case-control analysis in the Southwestern United States. Helicobacter. 2013;18:54–65. doi: 10.1111/j.1523-5378.2012.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saul C, Teixeira CR, Pereira-Lima JC, Torresini RJ. [Prevalence reduction of duodenal ulcer: a Brazilian study. (retrospective analysis in the last decade: 1996-2005)] Arq Gastroenterol. 2007;44:320–324. doi: 10.1590/s0004-28032007000400008. [DOI] [PubMed] [Google Scholar]
- 12.Miernyk K, Morris J, Bruden D, McMahon B, Hurlburt D, Sacco F, Parkinson A, Hennessy T, Bruce M. Characterization of Helicobacter pylori cagA and vacA genotypes among Alaskans and their correlation with clinical disease. J Clin Microbiol. 2011;49:3114–3121. doi: 10.1128/JCM.00469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kneller RW, You WC, Chang YS, Liu WD, Zhang L, Zhao L, Xu GW, Fraumeni JF, Blot WJ. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst. 1992;84:1261–1266. doi: 10.1093/jnci/84.16.1261. [DOI] [PubMed] [Google Scholar]
- 14.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504–509. [PubMed] [Google Scholar]
- 15.Dixon MF. Campylobacter pylori and chronic gastritis. In: Rathbone BJ, Heatlly RV, editors. Campylobacter pylori and Gastroduodenal Disease. Oxford: Blackwell Scientific; 1989. pp. 106–116. [Google Scholar]
- 16.Tsugane S, Kabuto M, Imai H, Gey F, Tei Y, Hanaoka T, Sugano K, Watanabe S. Helicobacter pylori, dietary factors, and atrophic gastritis in five Japanese populations with different gastric cancer mortality. Cancer Causes Control. 1993;4:297–305. doi: 10.1007/BF00051331. [DOI] [PubMed] [Google Scholar]
- 17.Maaroos HI, Salupere V, Uibo R, Kekki M, Sipponen P. Seven-year follow-up study of chronic gastritis in gastric ulcer patients. Scand J Gastroenterol. 1985;20:198–204. doi: 10.3109/00365528509089657. [DOI] [PubMed] [Google Scholar]
- 18.Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CM, Appelmelk BJ, Schenk BE, Meuwissen SG. Role of Helicobacter pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol Suppl. 1997;223:28–34. [PubMed] [Google Scholar]
- 19.Ohkuma K, Okada M, Murayama H, Seo M, Maeda K, Kanda M, Okabe N. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol. 2000;15:1105–1112. doi: 10.1046/j.1440-1746.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 20.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Iidependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Papini E, Satin B, Norais N, de Bernard M, Telford JL, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Invest. 1998;102:813–820. doi: 10.1172/JCI2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji X, Fernandez T, Burroni D, Pagliaccia C, Atherton JC, Reyrat JM, Rappuoli R, Telford JL. Cell specificity of Helicobacter pylori cytotoxin is determined by a short region in the polymorphic midregion. Infect Immun. 2000;68:3754–3757. doi: 10.1128/iai.68.6.3754-3757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 25.Atherton JC, Cao P, Peek RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 26.Letley DP, Lastovica A, Louw JA, Hawkey CJ, Atherton JC. Allelic diversity of the Helicobacter pylori vacuolating cytotoxin gene in South Africa: rarity of the vacA s1a genotype and natural occurrence of an s2/m1 allele. J Clin Microbiol. 1999;37:1203–1205. doi: 10.1128/jcm.37.4.1203-1205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 31.Oldani A, Cormont M, Hofman V, Chiozzi V, Oregioni O, Canonici A, Sciullo A, Sommi P, Fabbri A, Ricci V, et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009;5:e1000603. doi: 10.1371/journal.ppat.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimuro H, Suzuki T, Nagai S, Rieder G, Suzuki M, Nagai T, Fujita Y, Nagamatsu K, Ishijima N, Koyasu S, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Tegtmeyer N, Zabler D, Schmidt D, Hartig R, Brandt S, Backert S. Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: antagonistic effects of the vacuolating cytotoxin VacA. Cell Microbiol. 2009;11:488–505. doi: 10.1111/j.1462-5822.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoleni LE, Sander GB, Francesconi CF, Mazzoleni F, Uchoa DM, De Bona LR, Milbradt TC, Von Reisswitz PS, Berwanger O, Bressel M, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–1936. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 35.Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949–1958. doi: 10.1046/j.1365-2036.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018–1022. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- 37.Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Bu XL, Wang QY, Hu PJ, Chen MH. Decreasing seroprevalence of Helicobacter pylori infection during 1993-2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 39.Fialho AM, Braga AB, Braga Neto MB, Carneiro JG, Rocha AM, Rodrigues MN, Queiroz DM, Braga LL. Younger siblings play a major role in Helicobacter pylori transmission among children from a low-income community in the Northeast of Brazil. Helicobacter. 2010;15:491–496. doi: 10.1111/j.1523-5378.2010.00791.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima S, Nishiyama Y, Yamaoka M, Yasuoka T, Cho E. Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J Gastroenterol Hepatol. 2010;25 Suppl 1:S99–S110. doi: 10.1111/j.1440-1746.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- 41.Nouraie M, Latifi-Navid S, Rezvan H, Radmard AR, Maghsudlu M, Zaer-Rezaii H, Amini S, Siavoshi F, Malekzadeh R. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter. 2009;14:40–46. doi: 10.1111/j.1523-5378.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 42.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, Yazdanbod A, Shokoohi B, Mashayekhi A, Arshi S, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107:113–118. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Russell DW. Molecular cloning: A Laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 45.Jackson L, Britton J, Lewis SA, McKeever TM, Atherton J, Fullerton D, Fogarty AW. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter. 2009;14:108–113. doi: 10.1111/j.1523-5378.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 46.Ito LS, Oba SM, Hamajima N, Marie SK, Uno M, Shinjo SK, Kino A, Lavilla F, Inoue M, Tajima K, et al. Helicobacter pylori seropositivity among 963 Japanese Brazilians according to sex, age, generation, and lifestyle factors. Jpn J Cancer Res. 2001;92:1150–1156. doi: 10.1111/j.1349-7006.2001.tb02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida Cunha RP, Alves FP, Rocha AM, Rocha GA, Camargo LM, Nogueira PO, Camargo EP, Queiroz DM. Prevalence and risk factors associated with Helicobacter pylori infection in native populations from Brazilian Western Amazon. Trans R Soc Trop Med Hyg. 2003;97:382–386. doi: 10.1016/s0035-9203(03)90063-0. [DOI] [PubMed] [Google Scholar]
- 48.Kimura K. Gastritis and gastric cancer. Asia. Gastroenterol Clin North Am. 2000;29:609–621. doi: 10.1016/s0889-8553(05)70133-3. [DOI] [PubMed] [Google Scholar]
- 49.Dube C, Nkosi TC, Clarke AM, Mkwetshana N, Green E, Ndip RN. Helicobacter pylori antigenemia in an asymptomatic population of Eastern Cape Province, South Africa: public health implications. Rev Environ Health. 2009;24:249–255. doi: 10.1515/reveh.2009.24.3.249. [DOI] [PubMed] [Google Scholar]
- 50.Monno R, Volpe A, Basho M, Fumarola L, Trerotoli P, Kondili LA, Bino S, Schinaia N, Dentico P. Helicobacter pylori seroprevalence in selected groups of Albanian volunteers. Infection. 2008;36:345–350. doi: 10.1007/s15010-008-6338-6. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki RB, Cola RF, Cola LT, Ferrari CG, Ellinger F, Therezo AL, Silva LC, Eterovic A, Sperança MA. Different risk factors influence peptic ulcer disease development in a Brazilian population. World J Gastroenterol. 2012;18:5404–5411. doi: 10.3748/wjg.v18.i38.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattar R, dos Santos AF, Eisig JN, Rodrigues TN, Silva FM, Lupinacci RM, Iriya K, Carrilho FJ. No correlation of babA2 with vacA and cagA genotypes of Helicobacter pylori and grading of gastritis from peptic ulcer disease patients in Brazil. Helicobacter. 2005;10:601–608. doi: 10.1111/j.1523-5378.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 53.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 54.McColl KE. What remaining questions regarding Helicobacter pylori and associated diseases should be addressed by future research? View from Europe. Gastroenterology. 1997;113:S158–S162. doi: 10.1016/s0016-5085(97)80031-5. [DOI] [PubMed] [Google Scholar]
- 55.Harris P, Perez-Perez G, Zylberberg A, Rollán A, Serrano C, Riera F, Einisman H, García D, Viviani P. Relevance of adjusted cut-off values in commercial serological immunoassays for Helicobacter pylori infection in children. Dig Dis Sci. 2005;50:2103–2109. doi: 10.1007/s10620-005-3015-9. [DOI] [PubMed] [Google Scholar]
- 56.Sipponen P, Marshall BJ. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am. 2000;29:579–592, v-vi. doi: 10.1016/s0889-8553(05)70131-x. [DOI] [PubMed] [Google Scholar]
- 57.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 58.Iwase K, Evers BM, Hellmich MR, Guo YS, Higashide S, Kim HJ, Townsend CM. Regulation of growth of human gastric cancer by gastrin and glycine-extended progastrin. Gastroenterology. 1997;113:782–790. doi: 10.1016/s0016-5085(97)70172-0. [DOI] [PubMed] [Google Scholar]
- 59.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 60.Enroth H, Kraaz W, Engstrand L, Nyrén O, Rohan T. Helicobacter pylori strain types and risk of gastric cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:981–985. [PubMed] [Google Scholar]
- 61.Fontham ET, Ruiz B, Perez A, Hunter F, Correa P. Determinants of Helicobacter pylori infection and chronic gastritis. Am J Gastroenterol. 1995;90:1094–1101. [PubMed] [Google Scholar]
- 62.Imai T, Murayama H. Time trend in the prevalence of intestinal metaplasia in Japan. Cancer. 1983;52:353–361. doi: 10.1002/1097-0142(19830715)52:2<353::aid-cncr2820520229>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 63.El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368–372. doi: 10.1136/gut.50.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabiri H, Maleknejad P, Yamaoka Y, Feizabadi MM, Jafari F, Rezadehbashi M, Nakhjavani FA, Mirsalehian A, Zali MR. Distribution of Helicobacter pylori cagA, cagE, oipA and vacA in different major ethnic groups in Tehran, Iran. J Gastroenterol Hepatol. 2009;24:1380–1386. doi: 10.1111/j.1440-1746.2009.05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doosti A, Ghasemi-Dehkordi P. Helicobacter pylori vacA genotypes in Shahrekordian (Iran) H. pylori-positive patients. Res J Biol Sci. 2009;4:11–15. [Google Scholar]
- 66.Siavoshi F, Malekzadeh R, Daneshmand M, Ashktorab H. Helicobacter pylori endemic and gastric disease. Dig Dis Sci. 2005;50:2075–2080. doi: 10.1007/s10620-005-3010-1. [DOI] [PubMed] [Google Scholar]
- 67.Talebkhan Y, Mohammadi M, Mohagheghi MA, Vaziri HR, Eshagh Hosseini M, Mohajerani N, Oghalaei A, Esmaeili M, Zamaninia L. cagA gene and protein status among Iranian Helicobacter pylori strains. Dig Dis Sci. 2008;53:925–932. doi: 10.1007/s10620-007-9978-y. [DOI] [PubMed] [Google Scholar]
- 68.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cover TL, Halter SA, Blaser MJ. Characterization of HeLa cell vacuoles induced by Helicobacter pylori broth culture supernatant. Hum Pathol. 1992;23:1004–1010. doi: 10.1016/0046-8177(92)90261-z. [DOI] [PubMed] [Google Scholar]
- 70.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect. 2009;15:835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Doorn LJ, Figueiredo C, Mégraud F, Pena S, Midolo P, Queiroz DM, Carneiro F, Vanderborght B, Pegado MD, Sanna R, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–830. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 72.Kim SY, Woo CW, Lee YM, Son BR, Kim JW, Chae HB, Youn SJ, Park SM. Genotyping CagA, VacA subtype, IceA1, and BabA of Helicobacter pylori isolates from Korean patients, and their association with gastroduodenal diseases. J Korean Med Sci. 2001;16:579–584. doi: 10.3346/jkms.2001.16.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsunari O, Shiota S, Suzuki R, Watada M, Kinjo N, Murakami K, Fujioka T, Kinjo F, Yamaoka Y. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012;50:876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al Qabandi A, Mustafa AS, Siddique I, Khajah AK, Madda JP, Junaid TA. Distribution of vacA and cagA genotypes of Helicobacter pylori in Kuwait. Acta Trop. 2005;93:283–288. doi: 10.1016/j.actatropica.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Miehlke S, Kibler K, Kim JG, Figura N, Small SM, Graham DY, Go MF. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 76.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chomvarin C, Namwat W, Chaicumpar K, Mairiang P, Sangchan A, Sripa B, Tor-Udom S, Vilaichone RK. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12:30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 78.Lai CH, Kuo CH, Chen YC, Chao FY, Poon SK, Chang CS, Wang WC. High prevalence of cagA- and babA2-positive Helicobacter pylori clinical isolates in Taiwan. J Clin Microbiol. 2002;40:3860–3862. doi: 10.1128/JCM.40.10.3860-3862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sezikli M, Guliter S, Apan TZ, Aksoy A, Keles H, Ozkurt ZN. Frequencies of serum antibodies to Helicobacter pylori CagA and VacA in a Turkish population with various gastroduodenal diseases. Int J Clin Pract. 2006;60:1239–1243. doi: 10.1111/j.1742-1241.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 80.Palli D, Menegatti M, Masala G, Ricci C, Saieva C, Holton J, Gatta L, Miglioli M, Vaira D. Helicobacter pylori infection, anti-cagA antibodies and peptic ulcer: a case-control study in Italy. Aliment Pharmacol Ther. 2002;16:1015–1020. doi: 10.1046/j.1365-2036.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- 81.Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91–99. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 82.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller A, Falkow S, Amieva MR. Helicobacter pylori and gastric cancer: what can be learned by studying the response of gastric epithelial cells to the infection? Cancer Epidemiol Biomarkers Prev. 2005;14:1859–1864. doi: 10.1158/1055-9965.EPI-04-0820. [DOI] [PubMed] [Google Scholar]
- 84.Gangwer KA, Shaffer CL, Suerbaum S, Lacy DB, Cover TL, Bordenstein SR. Molecular evolution of the Helicobacter pylori vacuolating toxin gene vacA. J Bacteriol. 2010;192:6126–6135. doi: 10.1128/JB.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Y, Redlinger TE, Avitia R, Galindo A, Goodman K. Isolation and genotyping of Helicobacter pylori from untreated municipal wastewater. Appl Environ Microbiol. 2002;68:1436–1439. doi: 10.1128/AEM.68.3.1436-1439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]


