Abstract
AIM: To evaluate a different decision tree for safe liver resection and verify its efficiency.
METHODS: A total of 2457 patients underwent hepatic resection between January 2004 and December 2010 at the Chinese PLA General Hospital, and 634 hepatocellular carcinoma (HCC) patients were eligible for the final analyses. Post-hepatectomy liver failure (PHLF) was identified by the association of prothrombin time < 50% and serum bilirubin > 50 μmol/L (the “50-50” criteria), which were assessed at day 5 postoperatively or later. The Swiss-Clavien decision tree, Tokyo University-Makuuchi decision tree, and Chinese consensus decision tree were adopted to divide patients into two groups based on those decision trees in sequence, and the PHLF rates were recorded.
RESULTS: The overall mortality and PHLF rate were 0.16% and 3.0%. A total of 19 patients experienced PHLF. The numbers of patients to whom the Swiss-Clavien, Tokyo University-Makuuchi, and Chinese consensus decision trees were applied were 581, 573, and 622, and the PHLF rates were 2.75%, 2.62%, and 2.73%, respectively. Significantly more cases satisfied the Chinese consensus decision tree than the Swiss-Clavien decision tree and Tokyo University-Makuuchi decision tree (P < 0.01,P < 0.01); nevertheless, the latter two shared no difference (P = 0.147). The PHLF rate exhibited no significant difference with respect to the three decision trees.
CONCLUSION: The Chinese consensus decision tree expands the indications for hepatic resection for HCC patients and does not increase the PHLF rate compared to the Swiss-Clavien and Tokyo University-Makuuchi decision trees. It would be a safe and effective algorithm for hepatectomy in patients with hepatocellular carcinoma.
Keywords: Hepatectomy, Liver failure, Decision tree
Core tip: We have established a decision tree for safe hepatectomy based on four variables: normal or cirrhotic liver, Child-Turcotte-Pugh score, the indocyanine green retention rate at 15 min, and the ratio of reserved functional liver volume to standard liver volume. Post-hepatectomy liver failure (PHLF) has been identified by the “50-50” criteria. The Chinese consensus decision tree expands the indications for hepatic resection for liver tumor and does not increase the PHLF rate compared to the Swiss-Clavien and Tokyo University-Makuuchi decision trees. The Chinese consensus decision tree would be a safe and effective algorithm for hepatectomy in patients with liver tumor.
INTRODUCTION
Hepatectomy and liver transplantation are two curative treatments for liver tumors. Liver transplantation donations are far fewer than the demand in China[1,2]. Hepatectomy is the preferred approach to treating malignant liver cancer. It is a complicated surgery accompanied by high morbidity and mortality, especially for patients with cirrhosis undergoing major hepatectomy. With improvements in technique and peri-operative management, the mortality and complication rates have significantly decreased. Recent studies indicate that the hospital mortality rate is lower than 1%, even approaching zero mortality[3-8].
However, post-hepatectomy complications, especially hepatic failure, are still dreadful complications for the surgeon. The incidence rate of hepatic failure is approximately 0%-32% and accounts for 50%-75% of post-hepatectomy mortality. As the mortality rate after hepatectomy significantly decreases, it becomes an important indicator for evaluating the effect of hepatectomy. Based on the actual situations of patients, certain high-volume centers have created complete and effective management strategies and decision trees for safe hepatectomy. Professor Makuuchi proposed the University of Tokyo standard for hepatectomy[9], and Professor Clavien established the Swiss criteria for safe liver resection.
Advances in liver surgery have reduced blood loss and have led to a decline in morbidity and mortality. However, the mortality rate in extended liver resection, the rate of curative resection for liver malignancies, and postoperative long-term survival remain far from satisfactory. Therefore, we hope the concept of precision will help propel liver surgery into a brand new era. Precise liver surgery has become an important direction of development[10,11].
Due to limitations based on the characteristics of the local population and the available background information, there is no single decision tree for hepatectomy based on the Chinese population. Thus, based on past experience and data, we propose a Chinese decision tree system for hepatectomy.
This study aims to compare the incidence rate of postoperative hepatic failure according to the aforementioned three decision-making systems for hepatectomy with respect to the patients with hepatocellular carcinoma in a single center to identify the best decision tree for safe hepatectomies.
MATERIALS AND METHODS
From January of 2004 to December of 2010, we selected 2457 cases of hepatectomy with indocyanine green (ICG) records at the People’s Liberation Army General Hospital. The inclusion criteria were: (1) complete biochemical data during the perioperative period; (2) availability of preoperative enhanced helical computed tomography (CT) or enhanced magnetic resonance imaging (MRI) data (.dcm format); (3) pathologically confirmed hepatocellular carcinoma; and (4) initial hepatectomy. The exclusion criteria were: (1) INR-negative postoperative blood test; (2) concurrent additional pathological diagnosis; (3) no raw image data (digital format); (4) preoperative bilirubin > 50 μmol/L (2.9 mg/dL); and (5) perioperative period bleeding > 1500 mL. Altogether, 634 cases of hepatectomy were incorporated into this study.
We adopted a standardized procedure for the preoperative patient evaluation and hepatectomy. All patients underwent complete questioning about their medical history and a physical examination. For patients over 65 years old and patients with complications and other surgical risks, we performed a complete evaluation of heart and lung function. Before the surgery, we conducted a complete imaging examination. We reviewed the cases and imaging data and found the necessary content, and we re-inspected cases with missing data. Most cases were treated by the surgical approach of anatomical hepatectomy. For cases that required hepatic portal occlusion, we usually adopted the Pringle approach. Since 2008, some cases involved hepatectomy performed under the simple condition of portal vein occlusion. During the hepatectomy, we adopted a low central venous pressure (< 5 mmHg) and the Trendelenburg position, and a conventional abdominal drainage tube was placed.
When reviewing the cases, the examined preoperative factors included gender, age, preoperative comorbidities, esophageal and gastric varices, and routine preoperative examinations of biochemical meridians, blood, and blood clotting. We recorded the 15-min retention rate (ICGR15) of the indocyanine green excretion test, the Child-Turcotte-Pugh score, and the MELD score. The surgical factors included the recorded surgical time, the type of surgery, tumor size, the amount of bleeding, blood transfusion status, and the intra-operative occlusion method and time.
We recorded the postoperative incidence of hepatic failure, postoperative complication, and mortality. Postoperative hepatic failure was defined based on the 50-50 criteria; that is, four days after the surgery, the total bilirubin was > 50 μmol/L (2.9 mg/dL), and the prothrombin ratio was < 50% (INR > 1.7)[12]. Postoperative mortality was defined as mortality during surgery and the hospital stay. Postoperative ascites was diagnosed for patients who, after surgery, experienced more than 2 L of drainage for three consecutive days, needed a puncture drain again after the peritoneal drainage tube was removed, or had a postoperative hospital duration of more than 30 d because of persistent ascites. Intra-abdominal infection was defined as a positive bacteria culture from the drainage. Postoperative bile leakage was defined as a bilirubin level over 85.5 μmol/L in the postoperative drainage for seven consecutive days (5.0 mg/dL)[13].
Calculation of the relevant liver volume with three-dimensional reconstruction
The liver volume was measured using three-dimensional reconstruction software (EDDA Company, United States) to reconstruct three-dimensional liver images based on enhanced thin CT or MRI scanning. Then, the volume of each liver segment was calculated based on the voxel principle, and the segmentation and surgical planning for the scope of surgery was completed. The error was 5%-8%[14-16].
The standardized liver volume is relatively stable in adults in a physiological state, and its size depends on the human’s body surface area (BSA), which depends on the height and weight of the human body. The ideal liver volume for the normal human body has sufficient reserve function and compensatory potential in the healthy state.
BSA is calculated according to the DuBois formula: BSA (m2) = weight (kg) 0.425 × height (cm) 0.725 × 0.007184.
We adopted the adult standard liver volume formula established by Urata et al[17] from the University of Tokyo in Japan: standard liver volume (SLV) (m2) = 706.2 × BSA (m2) + 2.4.
Whole liver volume = reserved liver volume + pre-hepatic resection liver volume:
Standardized residual liver volume ratio = reserved liver volume/standard liver volume × 100%.
Decision trees for hepatectomy
In this study, we compared the Makuuchi decision tree (Figure 1), the Clavien decision tree (Figures 2 and 3), and the Chinese consensus decision tree (Figure 4), which are grouped. For each group, we compared the indication for hepatectomy and the incidence rate of hepatic failure. Professor Makuuchi from Japan replaced the Child-Pugh score with two parameters: whether ascites can be controlled and the total plasma bilirubin level, which is used as an index for assessing the hepatic functional reserve[9]. We adopted the ICGR15 to determine the specific surgical approach for the hepatectomy.
Figure 1.
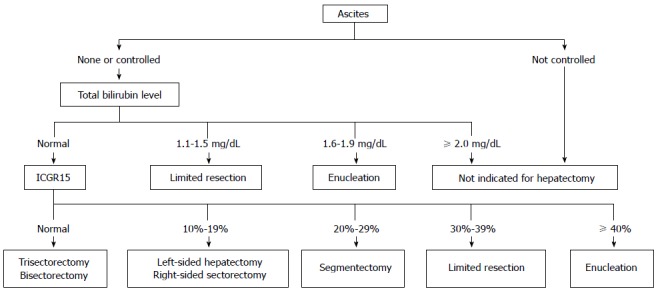
Makuuchi decision tree for a safe hepatectomy.
Figure 2.
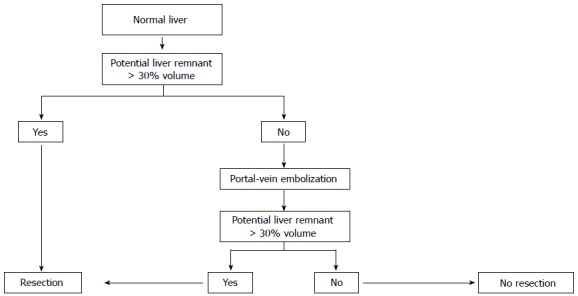
Clavien decision tree for safe hepatectomy in a normal liver.
Figure 3.
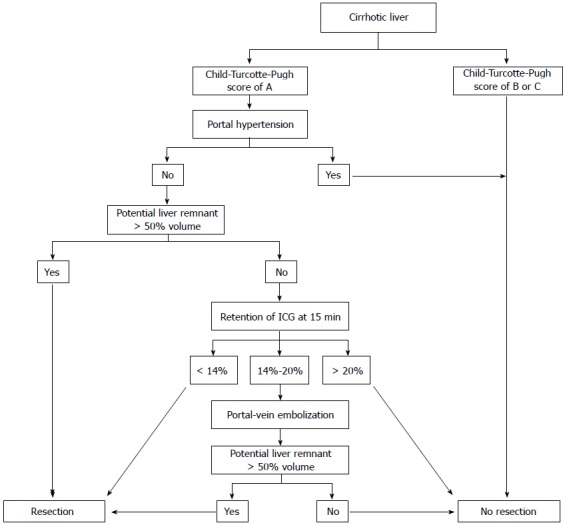
Decision tree from the University of Zurich for the individual evaluation of hepatectomy for a cirrhotic liver.
Figure 4.
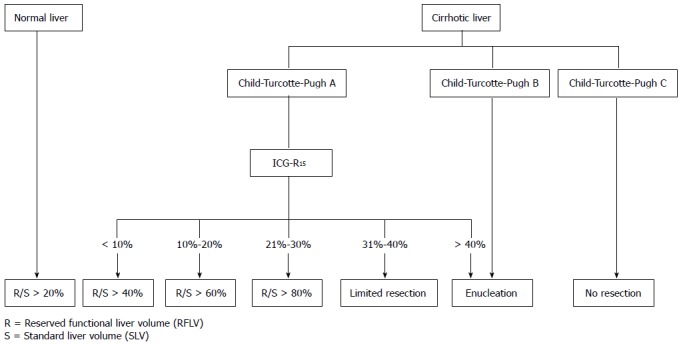
Chinese consensus decision tree for the individual evaluation of the safe extent of hepatectomy.
By combining the parameters of cirrhosis incidence, Child-Pugh score, portal hypertension, and ICG-R15, Professor Clavien of Switzerland determined the corresponding safe extent of hepatectomy[18].
Based on four indexes, namely, cirrhosis, the Child-Pugh score, the 15-min retention rate of indocyanine green (ICGR15), and the standardized residual liver volume rate, we established a decision tree for hepatectomy, termed the Chinese consensus system[10].
Statistical analysis
We retrospectively collected and recorded the preoperative, intra-operative, and postoperative data. The classification variables were evaluated using a χ2 or Fisher’s exact test, and the continuous variables were compared using a t-test or Mann-Whitney test. We performed logistic regression analysis to evaluate hepatic failure, mortality, or the risk factors of complications after hepatectomy. We defined P < 0.05 as indicating a significant difference. We used SPSS 20.0 software for the statistical analysis.
RESULTS
General information
From January 2004 to December 2010, 634 cases of hepatectomy due to hepatocellular carcinoma at the Department of Hepatobiliary Surgery of People’s Liberation Army General Hospital were included in the study. There were 502 male patients and 132 female patents; the average age was 51.8 years (12.4) (Table 1). There were 452 cases of hepatitis B, 34 cases of hepatitis C, and 25 cases of concurrent hepatitis B and hepatitis C. Additionally, there were 123 cases without infection, 78 cases with portal hypertension, and 34 cases with preoperative TACE. The cases with ICGR15 < 10%, 10%-20%, 20%-30%, 30%-40%, and > 40% were 545 (86.8%), 69 (10.9%), 8 (1.3%), 6 (0.9%), and 6 (0.9%), respectively. The postoperative pathology confirmed that 419 patients had cirrhosis. There were 629 cases (99.2%) with Child-Pugh grade A and five cases (0.8%) with Child-Pugh grade B, and the mean MELD score was 8.4 ± 1.5 (Table 2).
Table 1.
Basic information on the study population
| Factors | n (%) |
| Age (yr), mean (SD) | 51.8 (12.4) |
| Age > 65 yr | 102 (15.1) |
| Male | 502 (79.2) |
| ICG R15 | |
| < 10% | 545 (86.8) |
| 10%-20% | 69 (10.9) |
| 20%-30% | 8 (1.3) |
| 30%-40% | 6 (0.9) |
| > 40% | 6 (0.9) |
| Hepatitis serology | |
| Hepatitis B | 452 (71.3) |
| Hepatitis C | 34 (5.4) |
| Hepatitis B and C | 25 (3.9) |
| Negative or not determined | 123 (19.4) |
| Cirrhosis | 479 (75.5) |
| Portal vein hypertention | 78 (12.3) |
ICG R15: Indocyanine green retention rate at 15 min.
Table 2.
Preoperative biochemical indices and ratings n (%)
| Serum albumin (g/L) | 41.1 (4.0) |
| Platelet count (103/mm3) | 178.6 (75.1) |
| Serum creatinine (μmol/L) | 67.7 (14.0) |
| Serum total bilirubin (μmol/L) | |
| Serum total bilirubin (μmol/L) | 6.3 (14.2) |
| Serum sodium (mmol/L) | 142.0 (2.8) |
| INR, mean (SD) | 1.04 (0.10) |
| CTP score | |
| Class A | 629 (98.2) |
| Class B | 5 (0.8) |
| MELD score, mean (SD) | 8.4 (1.5) |
| Tumor size (cm) | 7.4 (4.9) |
Tumor size: The largest diameter of tumor, even for multiple lesions; CTP score: Child-Turcotte-Pugh score; MELD score: Model for End-stage Liver Disease score.
Among the cases of hepatectomy, there were 119 (18.8%) cases of major hepatectomy (not less than three segments), 90 (14.2%) cases with resection of two segments, 163 cases (25.7%) with resection of one segment, and 262 cases (41.3%) with limited hepatectomy and sub-segmental hepatectomy. The surgery time was 251 ± 52.1 min, and the surgical blood loss was 438 ± 559.7 mL. There were 142 cases (22.4%) with intra-operative blood transfusion. The adopted occlusion method was the Pringle approach, simple portal vein occlusion, and no occlusion in 392 (61.8%), 30 (4.7%), and 202 (33.4%) cases, respectively. The average block time was 20 ± 11.2 min (Table 3).
Table 3.
Relevant surgical indices n (%)
| Operating time (min) | 251 (52) |
| Porta hepatis clamping time (min) | 20 (11.2) |
| Estimated blood loss (mL) | 438 (559.7) |
| Extent of hepatectomy | |
| Subsegmentectomy or limited resection | 262 (41.3) |
| Segmentectomy | 163 (5.7) |
| Bisegmentectomy | 90 (14.2) |
| Major hepatectomy (3 or more segments) | 119 (18.8) |
| Intraoperative RBC transfusion | |
| Yes | 142 (22.4) |
| No | 492 (77.6) |
| Blocking methods | |
| Portal vein blocking | 30 (4.7) |
| Pringle maneuver | 392 (61.8) |
| No | 212 (33.4) |
RBC: Red blood cells.
The postoperative mortality for these patents was 0.16% (1/634). In one case, the patient died of hepatic failure after surgery. The overall complication rate was 44.9% (Table 4). In 19 cases, the patients suffered hepatic failure after surgery. In 40 cases, there was postoperative bile leakage. In seven cases, the patients suffered postoperative bleeding. In four cases, the patients suffered reoperation-related complications. In 62 cases, the patients suffered pleural effusion or thoracic puncture and drainage.
Table 4.
Postoperative complications of hepatectomy
| Overall morbidity | 44.90% |
| Surgical morbidity | |
| Posthepatectomy liver failure | 19% |
| Biliary leakage | 40% |
| Posthepatectomy hemorrhage | 7% |
| Perihepatic abscess | 22% |
| Wound infection | 42% |
| Cholangitis | 5% |
| Pleural effusion | 62% |
| Portal vein thrombosis | 3% |
| Medical morbidity | |
| Respiratory insufficiency/failure | 12% |
| Renal failure | 6% |
| Pneumonia | 28% |
| Cardiac arrhythmia | 20% |
| Pulmonary embolism | 2% |
| Cardiac failure | 8% |
| Myocardial infarction | 5% |
| Relaparotomy | 4% |
The numbers of cases satisfying the Makuuchi decision tree of the University of Tokyo, the Clavien decision tree of the University of Zurich, and the Chinese consensus decision tree were 573, 581, and 622, respectively. The incidence of hepatic failure in these groups was 2.62%, 2.75%, and 2.73%, respectively. Meanwhile, the incidences of hepatic failure in cases outside the decision tree were 5.66%, 6.56%, and 16.67%, respectively (Table 5).
Table 5.
Postoperative liver failure rate according to criteria
| Criteria | Yes | No |
| Swiss | 2.75% | 5.66% |
| Japan | 2.62% | 6.56% |
| Chinese | 2.73% | 16.67% |
The multivariate analysis indicates that liver cirrhosis, abdominal infection, major hepatectomy, and total hospital days are related to hepatic failure after hepatectomy (Table 6). Therefore, this analysis demonstrates that liver cirrhosis, abdominal infection, and major hepatectomy are the main factors that affect hepatic failure. The total hospital duration of the patients with hepatic failure is significantly prolonged.
Table 6.
Multivariate logistic analysis of hepatic failure
| Variables in the equation | Sig. |
| Liver cirrhosis | 0.009 |
| Bile leakage | 0.199 |
| Ascites | 0.184 |
| Abdominal infection | 0.014 |
| Major hepatectomy | 0.006 |
| Bleeding | 0.878 |
| Height (cm) | 0.710 |
| Weight (kg) | 0.361 |
| HB (g/dL) | 0.819 |
| BSA (m2) | 0.384 |
| BMI (kg/m2) | 0.733 |
| Kmin | 0.356 |
| ICGR15 | 0.510 |
| T12 (min) | 0.617 |
| EHBF (L/min) | 0.472 |
| Total hospital duration | 0.017 |
DISCUSSION
Hepatitis B is an epidemic disease in China. Approximately 85% (or more) of patients with hepatocellular carcinoma also have cirrhosis; therefore, reducing the risk of hepatectomy with cirrhosis has always been a focus of hepatectomy. Hepatectomy has always been affected by the range of surgical options due to its complexity, high surgery morbidity, and mortality. In the past several decades, due to increasing improvements in liver surgery techniques and careful treatment during the perioperative period, the hospital mortality rate for hepatectomy has decreased, the safety of the surgery has increased, and the indications for hepatectomy have expanded.
In China, hepatectomy is the primary method for treating hepatocellular carcinoma. With improvements in both technology and the treatment capabilities during the perioperative period, the mortality and complication rates of hepatectomy have significantly declined, and the hospital mortality of this study is 0.16%, which is similar to that of our previous studies[19]. The indications for surgery have gradually expanded. However, hepatic failure after hepatectomy still has a relatively high incidence rate and accounts for 18%-75% of postoperative deaths[20].
The decision-making system for hepatectomy consists of decision trees for hepatectomy proposed by individual centers based on scientific theory and practical experience[3,4,9,10,18,21,22]. At present, the primary decision-making systems are based on the following three decision-making systems: the Makuuchi decision-making system of Japan, the Clavien system, and the Chinese consensus decision-making system for hepatectomies.
The Makuuchi decision-making system was generated in the late 1990s, when the mortality rate of hepatectomy was approximately 10%, which is much higher than the current level. At present, the mortality rate is lower than 1%[20,23,24]. Therefore, the safety of hepatectomy was the main problem confronted by hepatobiliary surgery at that time. From the perspective of safety, Professor Makuuchi proposed the decision tree for hepatectomy according to the existing data and clinical experience. In the subsequent 1245 hepatectomies, there were no reported deaths, which confirmed that the safety of this decision tree is very good[4,8]. However, it appears to be too general to assume that patients with ICG-R15 > 40% can still undergo liver surgery. It is not sufficiently accurate to use the type of hepatectomy surgery and the amount of resectable liver segment to characterize the safe limit of hepatectomy. For example, for an individual patient with cirrhosis, right hepatic atrophy, and left liver enlargement as well as good hepatic function reserve, it is safe to perform regular right hemihepatectomy, whereas it is dangerous to perform regular left hemihepatectomy. Moreover, in this standard, whether ascites can be controlled and the Child-Pugh score be replaced with the bilirubin level seem to be crude determinants.
In contrast with Asian experiences, European experts believe that a clinical situation with a Child-Pugh score above grade B, portal hypertension, and ICG-R15 > 20% is a contraindication for hepatectomy. The Zurich decision tree is based on the assumption that for a normal liver, a safe hepatectomy can only be performed if the residual liver volume (potential liver volume) exceeds 30% of the functional liver volume (Total liver volume-Tumor Volume), and it is also safe to achieve this index through preoperative portal vein embolization. However, many cases in which the hepatectomy can be performed safely miss this cut-off[25]. Previous study confirmed that cirrhosis patients have the same preoperative index, evolution process during the perioperative period, incidence rate of postoperative hepatic failure, complication rate, hospital time, and survival rate regardless of whether they also have portal hypertension[26-30]. Italian scholars also suggested that the clinical situation in which the cirrhosis patients have portal hypertension should be viewed as an absolute contraindication for hepatectomy; for patients with Child-Pugh grade A, the short-term and long-term postoperative effects are similar to the effects in patients with normal portal vein pressure.
Based on the characteristics of hepatectomy for the Chinese population, hepatocellular carcinoma, and the fact that the theory and practice of other decision trees are not suitable for China and its surgical conditions, we established the Chinese consensus decision tree. The safe resection extent for hepatectomy is established according to the proportion of reserved liver volume relative to the liver volume of the patient. Among patients with liver disease, the liver volume differs considerably, and the function of different parts of the liver also differs. Therefore, it is inaccurate to set the safe amount of reserved liver volume according to the proportion of diseased liver volume. Moreover, it is also very difficult to generally set the safe limit of resection with pen and paper using the same percentage of reserved liver volume without considering the degree to which the liver function is impaired. There is no specification regarding the extent of hepatectomy when less than 50% of the liver will remain.
Applying the minimum residual liver volume or the necessary functional liver volume and SLV is a relatively reliable method for setting the safe limit of hepatectomy. We selected liver parenchymal disease, Child-Pugh score, and ICGR-15 as the assessment standards to classify the reserved hepatic function. The ratio (F/S) between EFLV and SLV is used to set the safe extent of hepatectomy and to establish a decision tree for the individual evaluation of the safe limit of hepatectomy.
Liver volume analysis is the most important determinant factor for whether hepatectomy or liver transplantation is selected to treat liver cancer. The maximum resectable volume of liver depends on the liver function. In the People’s Liberation Army General Hospital, conventionally, the preoperative liver volume is calculated using enhanced CT, and liver function is estimated through the indocyanine green retention test before every hepatectomy. The equilibrium between the range of hepatectomy and liver function is closely related to postoperative complications and mortality[25,31]. The range of hepatectomy can usually be evaluated through the CT volume analysis of the relevant liver segment. This detailed information is helpful for making the most reasonable decision about the hepatectomy. The CT volume analysis can be implemented in 2D or 3D. Our 3D analysis adopts the principle of basin analysis[32]. According to each portal vein or hepatic venous system, we accurately analyze the range of each segment and then calculate the liver volume.
Because the 50-50 criteria are indices for evaluating hepatic failure after hepatectomy for patients with a normal bilirubin range, this study excluded patients with hepatocellular carcinoma and preoperative jaundice (with total bilirubin > 50 mmol/L). Blood transfusions during the perioperative period will certainly affect the bilirubin level. In the original experiment, we excluded patients with an intra-operative blood transfusion when evaluating the 50-50 criteria[33]. However, according to previous studies and our experience, we believe that the likelihood that a blood transfusion in the perioperative period affects postoperative bilirubin and coagulation is very small; therefore, in this study, we did not exclude the relevant cases with blood transfusion.
Our studies indicate that the numbers of cases that satisfy the Makuuchi decision tree of the University of Tokyo, the Clavien decision tree of the University of Zurich, and the Chinese consensus decision tree were 573, 581, and 622, respectively, and the corresponding incidence of hepatic failure was 2.62%, 2.75%, and 2.73%, respectively. The incidence of hepatic failure in cases that did not satisfy the decision tree was 5.66%, 6.56%, and 16.67%, respectively. In comparison with the Makuuchi decision system of Japan and the Clavien system, the Chinese consensus decision tree expands the indications for hepatectomy without significantly increasing the incidence of hepatic failure after hepatectomy and is a safe and effective decision tree.
The multivariate analysis indicates that liver cirrhosis, abdominal infection, major hepatectomy, and total hospital duration are related to hepatic failure after hepatectomy and thus indicates that liver cirrhosis, abdominal infection, and major hepatectomy are the main factors that affect hepatic failure. The total hospital duration for patients with hepatic failure is significantly prolonged, which is consistent with previous research findings.
In summary, this study indicates that, in comparison with the Makuuchi decision tree of the University of Tokyo and the Clavien decision tree of the University of Zurich, the Chinese consensus decision tree can expand the scope of hepatectomy without significantly increasing the incidence of postoperative hepatic failure and that it is a safe and effective decision tree. Liver cirrhosis, abdominal infection, and major hepatectomy are the main factors that affect hepatic failure after hepatectomy.
COMMENTS
Background
Although many criteria have been established for safe hepatic resection, the evaluation of post-hepatectomy liver failure (PHLF) associated with several competing decision trees remains unknown.
Research frontiers
This article aims to compare a new embracing algorithm with two widely accepted criteria for safe liver resection and verify its efficiency in a large Chinese cohort.
Innovations and breakthroughs
In this study, the authors established a decision tree for safe hepatectomy based on four variables: normal or cirrhotic liver, the Child-Turcotte-Pugh score, the indocyanine green retention rate at 15 min, and the ratio of reserved functional liver volume to standard liver volume. They proved that the Chinese consensus decision tree expands the indications for hepatic resection for HCC patients and does not increase the PHLF rate compared to the Swiss-Clavien and Tokyo University-Makuuchi decision trees.
Applications
The Chinese consensus decision tree seems to be a safe and effective guideline for hepatectomy in patients with hepatocellular carcinoma.
Peer-review
PHLF is the most dreadful complication and it has many different definitions. In future, it may need to test those in a prospective study. In this large population, retrospective study, they proved the efficiency of the new criteria in elective patients. It is beneficial for elective hepatocellular carcinoma patients.
Footnotes
Supported by Grants from the Project of the National Science and Technology Major Project, No. 2012BAI06B01; and Postdoctoral Science Foundation funded project, No. 201003781.
Institutional review board statement: The study was reviewed and approved by the PLA General Hospital Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflicting interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 2, 2014
First decision: December 2, 2014
Article in press: April 17, 2015
P- Reviewer: Ehrenpreis ED, Takeda R S- Editor: Yu J L- Editor: Logan S E- Editor: Zhang DN
References
- 1.Makuuchi M, Tamura S, Sugawara Y. New national policy for deceased organ donation in China. Hepatobiliary Surg Nutr. 2013;2:307–308. doi: 10.3978/j.issn.2304-3881.2013.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Wang H, Fan ST, Zhao B, Zhang Z, Hao L, Huo F, Liu Y. The national program for deceased organ donation in China. Transplantation. 2013;96:5–9. doi: 10.1097/TP.0b013e3182985491. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206; discussion 1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 9.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Yang S, Zeng J, Cai S, Ji W, Duan W, Zhang A, Ren W, Xu Y, Tan J, et al. Precision in liver surgery. Semin Liver Dis. 2013;33:189–203. doi: 10.1055/s-0033-1351781. [DOI] [PubMed] [Google Scholar]
- 11.Qian NS, Liao YH, Cai SW, Raut V, Dong JH. Comprehensive application of modern technologies in precise liver resection. Hepatobiliary Pancreat Dis Int. 2013;12:244–250. doi: 10.1016/s1499-3872(13)60040-5. [DOI] [PubMed] [Google Scholar]
- 12.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828, discussion 828-829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Dello SA, Stoot JH, van Stiphout RS, Bloemen JG, Wigmore SJ, Dejong CH, van Dam RM. Prospective volumetric assessment of the liver on a personal computer by nonradiologists prior to partial hepatectomy. World J Surg. 2011;35:386–392. doi: 10.1007/s00268-010-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuBray BJ, Levy RV, Balachandran P, Conzen KD, Upadhya GA, Anderson CD, Chapman WC. Novel three-dimensional imaging technique improves the accuracy of hepatic volumetric assessment. HPB (Oxford) 2011;13:670–674. doi: 10.1111/j.1477-2574.2011.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takamoto T, Hashimoto T, Ogata S, Inoue K, Maruyama Y, Miyazaki A, Makuuchi M. Planning of anatomical liver segmentectomy and subsegmentectomy with 3-dimensional simulation software. Am J Surg. 2013;206:530–538. doi: 10.1016/j.amjsurg.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 18.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZQ, Xu LN, Yang T, Zhang WZ, Huang XQ, Liu R, Cai SW, Zhang AQ, Feng YQ, Zhou NX, et al. [Liver resection: single center experiences of 2008 consecutive resections in 20 years] Zhonghua Waike Zazhi. 2008;46:1314–1321. [PubMed] [Google Scholar]
- 20.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka N, Okamoto E, Kuwata K, Tanaka N. A multiple regression equation for prediction of posthepatectomy liver failure. Ann Surg. 1984;200:658–663. doi: 10.1097/00000658-198411000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanaka N, Okamoto E, Oriyama T, Fujimoto J, Furukawa K, Kawamura E, Tanaka T, Tomoda F. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994;219:342–346. doi: 10.1097/00000658-199404000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makuuchi M, Sano K. Surgical treatment of HCC: update topics. Updates Surg. 2011;63:67–68. doi: 10.1007/s13304-011-0069-4. [DOI] [PubMed] [Google Scholar]
- 24.Dokmak S, Ftériche FS, Borscheid R, Cauchy F, Farges O, Belghiti J. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013;15:908–915. doi: 10.1111/hpb.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. “State of the art” in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797–803. doi: 10.1007/s00268-008-9878-0. [DOI] [PubMed] [Google Scholar]
- 26.Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 27.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 28.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 29.Ruzzenente A, Valdegamberi A, Campagnaro T, Conci S, Pachera S, Iacono C, Guglielmi A. Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol. 2011;17:5083–5088. doi: 10.3748/wjg.v17.i46.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 2013;15:78–84. doi: 10.1111/j.1477-2574.2012.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clavien PA, Oberkofler CE, Raptis DA, Lehmann K, Rickenbacher A, El-Badry AM. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010;52:715–729. doi: 10.1002/hep.23713. [DOI] [PubMed] [Google Scholar]
- 32.D’Onofrio M, De Robertis R, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014;6:62–71. doi: 10.4329/wjr.v6.i4.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]


