Abstract
Immunoglobulin G4 (IgG4)-related disease is a rare systemic diseases. A 67-year-old male presented at our institution with mild upper abdominal pain and jaundice for 20 d. Laboratory results revealed high levels of IgG4 (15.4 g/L, range: 0.08-1.4 g/L). Computed tomography (CT) showed significant enlargement of the entire pancreas and a capsule-like low-density rim surrounding the whole pancreas. Positron emission tomography/CT revealed increased uneven metabolism of the entire pancreas. Both magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography showed stenosis of the distal common bile duct and proximal main pancreatic duct, and dilation of the proximal common bile duct and extra- and intra-hepatic bile ducts. He was diagnosed with IgG4-related autoimmune pancreatitis. The patient was treated with prednisone for 14 mo. The patient responded well to prednisone but upon cessation of the corticosteroid developed enlargement of the submandibular gland. The patient’s serum IgG4 was elevated at 23.9 g/L. It is important to maintain treatment, so the patient was again treated with prednisone and had a good response. Follow-up of IgG4-related disease is thus necessary.
Keywords: Immunoglobulin G4, Immunoglobulin G4-related disease, Autoimmune pancreatitis, Sialadenitis
Core tip: Immunoglobulin G4 (IgG4)-related disease is a rare systemic disease which affects many organs. Here we report a case of a patient with IgG4-related disease involving the pancreas and metachronous sialadenitis.
INTRODUCTION
Immunoglobulin G4 (IgG4)-related disease (RD) is a rare and often misdiagnosed systemic disease with multiple clinical manifestations and multiorgan involvement[1], though metachronous manifestations are rare. We present a unique case of metachronous IgG4-related autoimmune pancreatitis (AIP) and sialadenitis.
CASE REPORT
A 67-year-old male presented at our institution in March 2012 with mild upper abdominal pain and jaundice for 20 d. The physical examination was unremarkable except for moderate yellowing of the patient’s skin and sclera. Laboratory results revealed high levels of alanine aminotransferase (357 U/L, range: 0-40 U/L), aspartate aminotransferase (362 U/L, range: 0-40 U/L), γ-glutamyltransferase (595.0 U/L, range: 8-50 U/L), alkaline phosphatase (750 U/L, range: 20-110 U/L), total bilirubin (52.70 μmol/L, range: 4.3-22.5 μmol/L), and direct serum bilirubin (29.50 μmol/L, range: 0-8.84 μmol/L). Serum amylase and glucose were within normal ranges, as were carbohydrate antigen, carcinoembryonic antigen, and alpha-fetoprotein. The erythrocyte sedimentation rate was elevated at 32 mm/h (range: 0-15 mm/h). Total IgG was elevated (21.5 g/L, range: 6.0-16.0 g/L), IgG4 was about 11 times the normal limit (15.4 g/L, range: 0.08-1.4 g/L), and γ-globulin was 33.03% (range: 10.7%-20.0%). Antinuclear antibody, anti-mitochondrial antibody, and smooth muscle antibody were all negative.
Radiographs of the chest were unremarkable. Computed tomography (CT) showed significant enlargement of the entire pancreas and a capsule-like low-density rim surrounding the whole pancreas. The proximal portion of the main pancreatic duct was stenotic and no significant dilation of the distal duct was observed. CT also revealed stenosis of the distal common bile duct, dilation of the proximal common bile duct, and dilation of the extra- and intra-hepatic bile ducts. Close examination of the CT scan showed heterogeneous enhancement of the pancreas where the density of the pancreatic body and tail was lower than that of the pancreatic head (Figure 1).
Figure 1.
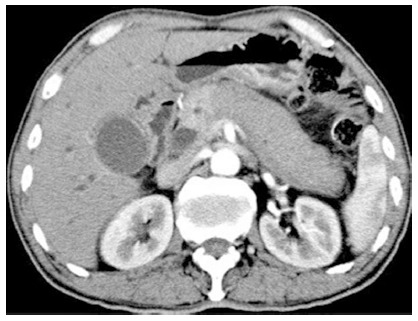
Computed tomography of the enlarged sausage-like pancreas and heterogeneous enhancement of the pancreas in March 2012 prior to steroid treatment.
Endoscopic retrograde cholangiopancreatography (ERCP) found stenosis of the distal common bile duct (about 5 cm in length), dilation of the proximal common bile duct (approximately 2 cm maximum diameter), and dilation of the extra- and intra-hepatic bile ducts. The proximal main pancreatic duct was stenotic (about 5 cm in length) and associated with slight dilation of the distal main pancreatic duct.
Positron emission tomography (PET)/CT revealed increased uneven metabolism of the entire pancreas, and especially of the pancreatic head (Figure 2). Thickening of the walls of the gall bladder and dilation of the intrahepatic bile duct was also observed. Other organs were normal.
Figure 2.
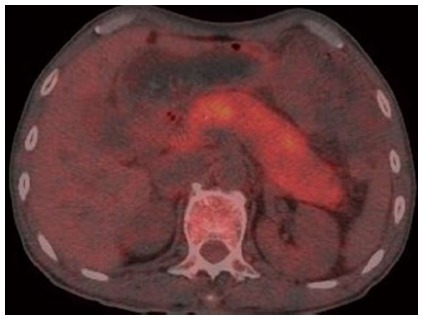
Positron emission tomography/computed tomography showing increased uneven metabolism of the entire pancreas in March 2012.
The patient was diagnosed with AIP. Stents were placed in the bile and pancreatic ducts to manage the patient’s jaundice. The patient was then treated with prednisone at 40 mg/d for 3 wk, tapered by 2.5-5 mg/d every 1-2 wk with a maintenance dose of 7.5 mg/d over a period of 6 mo. Total steroid administration lasted for 14 mo.
Follow-up
During steroid administration, the patient was seen in our hospital in June 2012, November 2012, and August 2014. At the initial follow-up, the patient was asymptomatic and his liver enzyme and IgG4 levels were within the normal ranges. Abdominal CTs in June and November 2012 showed a normal pancreatic form (Figure 3A, B). Follow-up magnetic resonance cholangiopancreatography (MRCP) and ERCP showed reduced dilation of the common bile duct (approximately 1.5 cm maximum diameter) and the extra- and intra-hepatic bile ducts. Slight stenosis of the distal common bile duct and mild dilation of the distal main pancreatic duct were also observed. At the second follow-up period in November 2012, the stents in the bile and pancreatic ducts were removed by ERCP.
Figure 3.
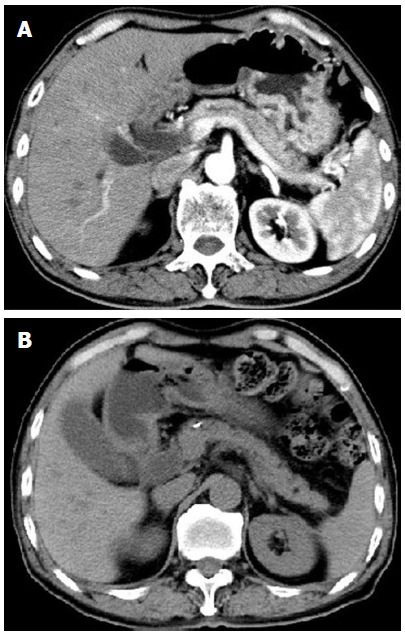
Computed tomography in June and November 2012 revealing the normalized pancreas after steroid treatment.
In 2013, the patient was diagnosed with Mikulicz syndrome and was treated with oral prednisone for 50 d in a local hospital. The patient responded well to the prednisone but upon cessation of the corticosteroid developed enlargement of the submandibular gland. In August 2014, the patient was admitted to our hospital after 8 mo of bilateral submandibular gland enlargement. The patient reported that he did not feel any abdominal discomfort. Physical examination revealed swollen but non-tender bilateral submandibular glands and no jaundice was noted.
Blood values were unremarkable except for serum IgG4 which was elevated at 23.9 g/L. Anti-Sjögren syndrome-related antigen A and Sjögren syndrome type B antigen were both negative. Repeat X-rays of the chest were unremarkable. Abdominal CT and MRCP showed similar findings to those in June and November 2012 (Figure 4, Figure 5). Salivary gland ultrasonography revealed symmetrical bilateral swelling of the submandibular and parotid glands. Magnetic resonance imaging of the salivary glands showed symmetrical bilateral enlargement of the submandibular (4.0 × 2.6 × 5.2 cm) (Figure 6) and parotid (4.7 × 4.5 × 5.8 cm) glands.
Figure 4.
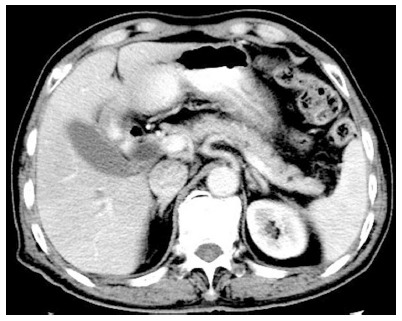
Computed tomography in August 2014 also showing the normal pancreas.
Figure 5.
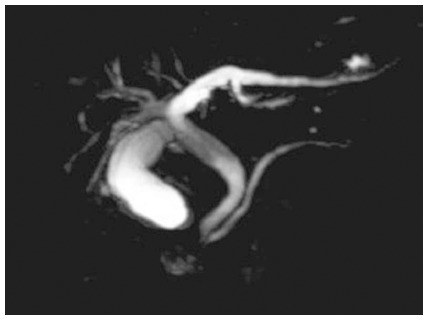
Magnetic resonance cholangiopancreatography showing stenosis of the distal common bile duct and proximal main pancreatic duct, and dilation of the proximal common bile duct and extra- and intra-hepatic bile ducts.
Figure 6.
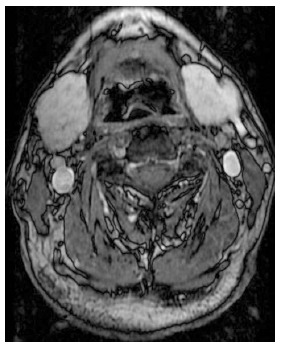
Magnetic resonance imaging of the salivary glands showing symmetrical bilateral enlargement of the submandibular gland (4.0 × 2.6 × 5.2 cm).
The patient was again treated with 40 mg/d prednisone for 4 wk, following which, ultrasonography of the submandibular and parotid glands was normal.
DISCUSSION
Diagnosis of autoimmune pancreatitis
The concept of AIP was first proposed by Yoshida et al[2] in 1995. The incidence of AIP was reported as 0.82 per 100000 cases, or roughly 4%-13% of chronic pancreatitis cases. The male to female ratio has been reported as 2.85:1.0 with 95% of patients over 45 years of age[3]. AIP is currently classified into 2 subgroups, type 1 and type 2. Type 1 AIP, the most common, frequently involves extra-pancreatic organs and is accompanied by IgG4-positive plasmacytes and/or elevated serum IgG4 levels. IgG4-RD was proposed to describe this condition[4,5], and AIP type 1 is a pancreatic manifestation of IgG4-RD.
As this case presented with elevated serum IgG4, was responsive to corticosteroid treatment, and had pancreatic imaging consistent with the diagnosis of AIP, it met the Asia and International Consensus Diagnostic Criteria for AIP[6,7]. It is important to discriminate AIP from pancreatic and bile duct carcinoma. Misdiagnosis of carcinoma can possibly lead to unnecessary surgery. In past years, 2.5% of AIP cases in North America underwent pancreaticoduodenectomy due to misdiagnosis of pancreatic cancer[8]. In this case, on CT the pancreas appeared consistent in shape (sausage-like) with a diagnosis of AIP. ERCP showed stenosis of the distal common bile and proximal main pancreatic duct without significant dilation of the main distal pancreatic duct. Systemic PET/CT examination showed inflammatory characteristics in the entire pancreas. The tumor markers CA19-9, carcinoembryonic antigen, and alpha-fetoprotein were normal. Furthermore, the patient was very responsive to corticosteroids and at follow-up there was no sign of a tumor, so cancer of the pancreas and bile duct was ruled out.
Treatment of AIP
As per the treatment guidelines, this case was treated with oral corticosteroids and stents placed in the proximal main pancreatic duct and the distal common bile duct[9]. Follow-up imaging showed restoration of normal pancreatic morphology, but stenosis of the distal common bile duct and proximal main pancreatic duct remained, indicating that the pancreas recovered more quickly than the bile and pancreatic ducts. This founding was similar to another study reported in the literature[10]. Because fibrotic lesions have a poor response to steroids[11], IgG4-related fibrosis of the pancreatic and bile ducts was not completely ruled out. In accordance with published guidelines[9], steroids should be maintained for at least 3 years for proper management of AIP. This case did not meet this as steroids were only administered for 14 mo, so it was possible that the steroid maintenance therapy was not sufficient for this patient.
IgG4-related sialadenitis
After withdrawal of the steroid for 7 mo, the patient developed swelling of the bilateral submandibular glands. Subsequent steroid treatment for 50 d was effective, but upon cessation, the patient developed bilateral parotid edema. Examination showed normal pancreatic morphology but bilateral symmetrical swelling of the submandibular and parotid glands. Serum IgG4 was again elevated. Together with a history of AIP, this condition met the diagnostic criteria of IgG4-RD[12]. IgG4-RD often simultaneously involves multiple organs, but sialadenitis here was asynchronous with AIP. The involvement of autoimmune mechanisms in IgG4-RD is an underlying possibility[13], but it is not clear if different organs have different pathogenesis.
Prognosis
IgG4-RD responds well to treatment with corticosteroids, but there is a high likelihood of recurrence after ceasing steroid administration, and approximately 92% of AIP recurs within 3 years[14]. Continued administration of corticosteroids may be effective in preventing recurrence. Currently, it is not clear if IgG4-RD is related to neoplastic processes, but it is worth noting that there are reports in the literature of cancer with AIP and/or IgG4-related sialadenitis[15,16]. This patient will continue to be monitored for future recurrence.
COMMENTS
Case characteristics
A 67-year-old male with IgG4-related disease involving the pancreas and metachronous sialadenitis.
Clinical diagnosis
Autoimmune pancreatitis and sialadenitis.
Differential diagnosis
Pancreatic cancer, primary sclerosing cholangitis, lymphoma.
Laboratory diagnosis
Total bilirubin 52.70 μmol/L; direct bilirubin 29.50 μmol/L; IgG4 15.4 g/L.
Imaging diagnosis
Computed tomography showed significant enlargement of the entire pancreas and a capsule-like low-density rim surrounding the whole pancreas. Magnetic resonance imaging of the salivary glands indicated symmetrical bilateral enlargement of the submandibular and parotid glands.
Treatment
The patient was treated with prednisone.
Related reports
Immunoglobulin G4-related disease (IgG4-RD) is a systemic disease which affects multiple organs and follow up is important.
Term explanation
IgG4-RD is a systemic disease with multiple clinical manifestations and multiorgan involvement.
Experiences and lessons
For the patient with autoimmune pancreatitis, attention should be paid to multiorgan involvement during follow-up.
Peer-review
This article reported a case of multiple manifestations of IgG4-RD, including the pancreas and parotids and it is helpful to obtain more attention for this underdiagnosed disease.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Beijing Military General Hospital Institutional Review Board.
Informed consent statement: Study participant provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 16, 2015
First decision: April 13, 2015
Article in press: June 26, 2015
P- Reviewer: Buijs J, Berger Z, Haruta I S- Editor: Yu J L- Editor: Cant MR E- Editor: Zhang DN
References
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 3.Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Sano H, et al. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1–e5. doi: 10.1097/MPA.0b013e3181bd64a1. [DOI] [PubMed] [Google Scholar]
- 4.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 6.Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403–408. doi: 10.1007/s00535-008-2205-6. [DOI] [PubMed] [Google Scholar]
- 7.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 8.Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH, et al. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853–858; discussion 858-859. doi: 10.1097/01.SLA.0000071516.54864.C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471–477. doi: 10.1007/s00535-010-0221-9. [DOI] [PubMed] [Google Scholar]
- 10.Du SY, Fang L, Zhang MG, Fan YH. Clinical and Imaging analysis of bile duct damage caused by autoimmune pancreatitis. Clin J Gastroenterol Hepatol. 2014;23:686–688. [Google Scholar]
- 11.Nakazawa T, Naitoh I, Ando T, Hayashi K, Okumura F, Miyabe K, Yoshida M, Ohara H, Joh T. A case of advanced-stage sclerosing cholangitis with autoimmune pancreatitis not responsive to steroid therapy. JOP. 2010;11:58–60. [PubMed] [Google Scholar]
- 12.Okazaki K, Uchida K, Ikeura T, Takaoka M. Current concept and diagnosis of IgG4-related disease in the hepato-bilio-pancreatic system. J Gastroenterol. 2013;48:303–314. doi: 10.1007/s00535-012-0744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto M, Naishiro Y, Suzuki C, Kokai Y, Suzuki R, Honda S, Abe T, Takahashi H, Shinomura Y. Proteomics analysis in 28 patients with systemic IgG4-related plasmacytic syndrome. Rheumatol Int. 2010;30:565–568. doi: 10.1007/s00296-009-1030-4. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–1507. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Miyatani H, Sawada Y, Yoshida Y. A case of pancreas cancer with autoimmune pancreatitis. Pancreas. 2006;33:208–209. doi: 10.1097/01.mpa.0000232329.35822.3a. [DOI] [PubMed] [Google Scholar]
- 16.Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Hum Pathol. 2009;40:881–886. doi: 10.1016/j.humpath.2008.10.020. [DOI] [PubMed] [Google Scholar]


