Abstract
The effect of hyperoxia on activity of the superoxide-sensitive citric acid cycle enzyme aconitase was measured in cultured human epithelial-like A549 cells and in rat lungs. Rapid and progressive loss of > 80% of the aconitase activity in A549 cells was seen during a 24-hr exposure to a PO2 of 600 mmHg (1 mmHg = 133 Pa). Inhibition of mitochondrial respiratory capacity correlated with loss of aconitase activity in A549 cells exposed to hyperoxia, and this effect could be mimicked by fluoroacetate (or fluorocitrate), a metabolic poison of aconitase. Exposure of rats to an atmospheric PO2 of 760 mmHg or 635 mmHg for 24 hr caused respective 73% and 61% decreases in total lung aconitase activity. We propose that early inactivation of aconitase and inhibition of the energy-producing and biosynthetic reactions of the citric acid cycle contribute to the sequelae of lung damage and edema seen during exposure to hyperoxia.
Full text
PDF

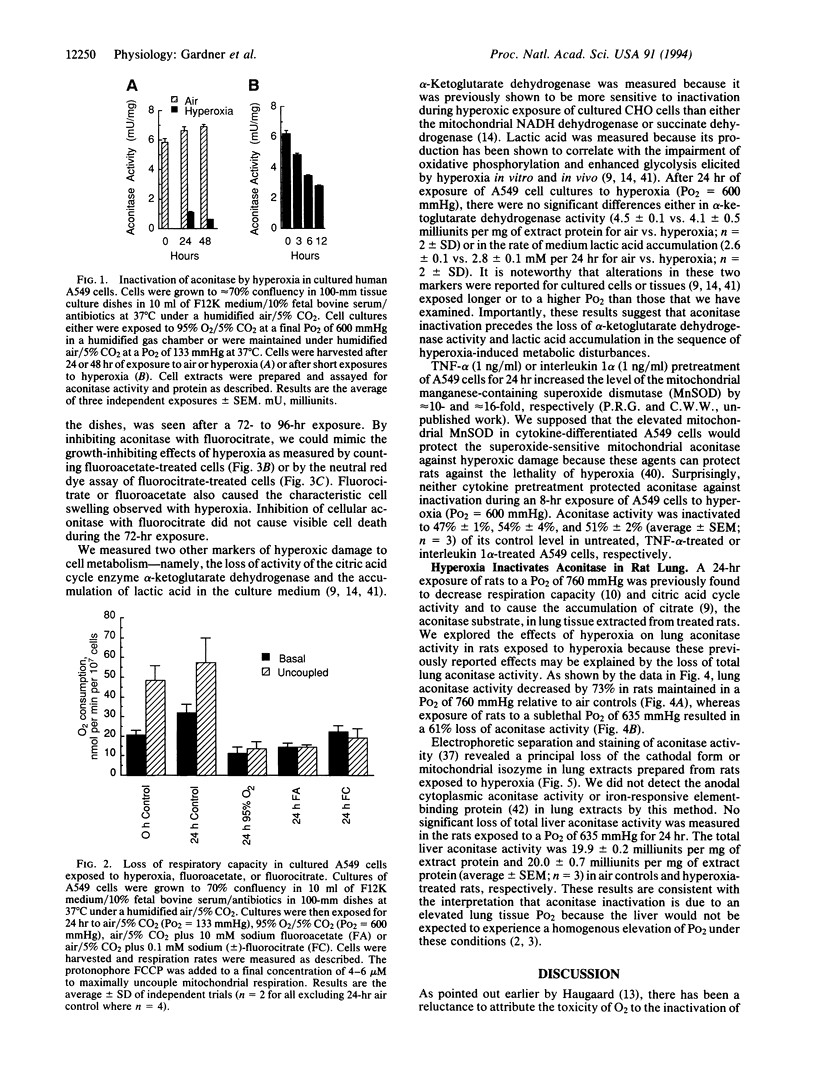
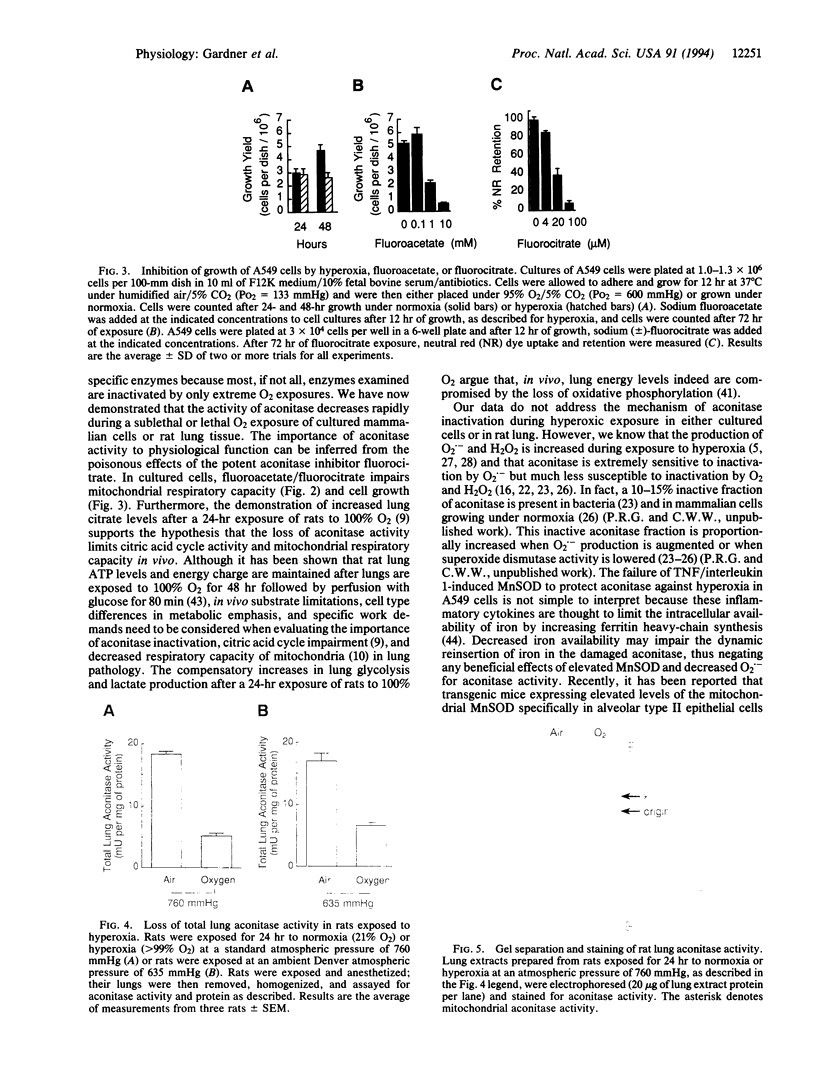

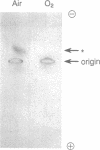
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassett D. J., Bowen-Kelly E., Reichenbaugh S. S. Rat lung glucose metabolism after 24 h of exposure to 100% oxygen. J Appl Physiol (1985) 1989 Feb;66(2):989–996. doi: 10.1152/jappl.1989.66.2.989. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Elbon C. L., Reichenbaugh S. S. Respiratory activity of lung mitochondria isolated from oxygen-exposed rats. Am J Physiol. 1992 Oct;263(4 Pt 1):L439–L445. doi: 10.1152/ajplung.1992.263.4.L439. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Reichenbaugh S. S. Tricarboxylic acid cycle activity in perfused rat lungs after O2 exposure. Am J Physiol. 1992 Apr;262(4 Pt 1):L495–L501. doi: 10.1152/ajplung.1992.262.4.L495. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Evans S. M., Mendes-Mourão J., Chappell J. B. Fluorocitrate inhibition of aconitate hydratase and the tricarboxylate carrier of rat liver mitochondria. Biochem J. 1973 May;134(1):217–224. doi: 10.1042/bj1340217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- Fisher A. B. Energy status of the rat lung after exposure to elevated PO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):56–59. doi: 10.1152/jappl.1978.45.1.56. [DOI] [PubMed] [Google Scholar]
- Flint D. H., Smyk-Randall E., Tuminello J. F., Draczynska-Lusiak B., Brown O. R. The inactivation of dihydroxy-acid dehydratase in Escherichia coli treated with hyperbaric oxygen occurs because of the destruction of its Fe-S cluster, but the enzyme remains in the cell in a form that can be reactivated. J Biol Chem. 1993 Dec 5;268(34):25547–25552. [PubMed] [Google Scholar]
- Flint D. H., Tuminello J. F., Emptage M. H. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993 Oct 25;268(30):22369–22376. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Fridovich I., Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Effect of glutathione on aconitase in Escherichia coli. Arch Biochem Biophys. 1993 Feb 15;301(1):98–102. doi: 10.1006/abbi.1993.1120. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J Biol Chem. 1992 May 5;267(13):8757–8763. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968 Apr;48(2):311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VAN DEN BRENK H. A. Pulmonary damage due to high pressure oxygen breathing in rats. 2. Changes in dehydrogenase activity of rat lung. Aust J Exp Biol Med Sci. 1962 Feb;40:51–56. doi: 10.1038/icb.1962.7. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. MEASUREMENT OF OXYGEN TENSIONS IN CEREBRAL TISSUES OF RATS EXPOSED TO HIGH PRESSURES OF OXYGEN. J Appl Physiol. 1963 Sep;18:869–876. doi: 10.1152/jappl.1963.18.5.869. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Jamieson D., van den Brenk H. A. Electrode size and tissue pO2 measurement in rats exposed to air or high pressure oxygen. J Appl Physiol. 1965 May;20(3):514–518. doi: 10.1152/jappl.1965.20.3.514. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HOLZACH O. The conversion of citrate into cis-aconitate and isocitrate in the presence of aconitase. Biochem J. 1952 Nov;52(3):527–528. doi: 10.1042/bj0520527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Koen A. L., Goodman M. Aconitate hydratase isozymes: subcellular location, tissue distribution and possible subunit structure. Biochim Biophys Acta. 1969;191(3):698–701. doi: 10.1016/0005-2744(69)90363-5. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., Mashino T., Fridovich I. alpha, beta-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987 Apr 5;262(10):4724–4727. [PubMed] [Google Scholar]
- LOTSPEICH W. D., PETERS R. A., WILSON T. H. The inhibition of aconitase by 'Inhibitor fractions' isolated from tissues poisoned with fluoroacetate. Biochem J. 1952 Apr;51(1):20–25. doi: 10.1042/bj0510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Modulation of the fumarases of Escherichia coli in response to oxidative stress. Arch Biochem Biophys. 1993 Mar;301(2):379–384. doi: 10.1006/abbi.1993.1159. [DOI] [PubMed] [Google Scholar]
- MORRISON J. F., PETERS R. A. Biochemistry of fluoroacetate poisoning: the effect of fluorocitrate on purified aconitase. Biochem J. 1954 Nov;58(3):473–479. doi: 10.1042/bj0580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Pulmonary granular pneumocytes. Loss of mitochondrial granules during hyperoxia. J Cell Biol. 1973 Oct;59(1):246–250. doi: 10.1083/jcb.59.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS R. A. Further experiments on the inhibition of aconitase by enzymically prepared fluorocitric acid. Biochem J. 1961 May;79:261–268. doi: 10.1042/bj0790261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum R. M., Wittner M., Lenger M. Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab Invest. 1969 Jun;20(6):516–528. [PubMed] [Google Scholar]
- Smyk-Randall E., Brown O. R., Wilke A., Eisenstark A., Flint D. H. Near ultraviolet light inactivation of dihydroxyacid dehydratase in Escherichia coli. Free Radic Biol Med. 1993 Jun;14(6):609–613. doi: 10.1016/0891-5849(93)90142-h. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Miller L. L., Miller S. C., Torti S. V., Torti F. M. Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts. Relationship to the induction of ferritin heavy chain. J Biol Chem. 1991 Apr 15;266(11):7257–7261. [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- White C. W., Ghezzi P., Dinarello C. A., Caldwell S. A., McMurtry I. F., Repine J. E. Recombinant tumor necrosis factor/cachectin and interleukin 1 pretreatment decreases lung oxidized glutathione accumulation, lung injury, and mortality in rats exposed to hyperoxia. J Clin Invest. 1987 Jun;79(6):1868–1873. doi: 10.1172/JCI113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]