Abstract
Objective
To increase the use of evidence-informed approaches to diagnosis, investigation, and treatment of headache for patients in primary care.
Quality of evidence
A comprehensive search was conducted for relevant guidelines and systematic reviews published between January 2000 and May 2011. The guidelines were critically appraised using the AGREE (Appraisal of Guidelines for Research and Evaluation) tool, and the 6 highest-quality guidelines were used as seed guidelines for the guideline adaptation process.
Main message
A multidisciplinary guideline development group of primary care providers and other specialists crafted 91 specific recommendations using a consensus process. The recommendations cover diagnosis, investigation, and management of migraine, tension-type, medication-overuse, and cluster headache.
Conclusion
A clinical practice guideline for the Canadian health care context was created using a guideline adaptation process to assist multidisciplinary primary care practitioners in providing evidence-informed care for patients with headache.
Headache is one of the most common reasons patients seek help from family physicians. The estimated lifetime prevalence of headache is 66%: 14% to 16% for migraine, 46% to 78% for tension-type headache, and 0.1% to 0.3% for cluster headache.1–3 In Canada, at least 2.6 million adult women and nearly 1 million men experience migraine.4 About 90% of migraine sufferers report moderate to severe pain, with 75% reporting impaired function and 33% requiring bed rest during an attack.5 The economic effects of headache are also substantial. It is estimated that headache accounts for 20% of work absences.6
Vast quantities of over-the-counter medications are taken for headache disorders, and treatment is often suboptimal.1,7 Although most migraine sufferers use acute treatment to relieve their headaches, a substantial number of people who might benefit from prophylactic therapy do not receive it—more than 1 in 4 migraineurs are candidates for preventive therapy.5,8
Better information and education for patients and health professionals is essential to improving management of headache in primary care, which should lead to prompt diagnosis and more effective treatment.9 To help address this, a consortium of organizations and clinicians from Alberta developed the Guideline for Primary Care Management of Headache in Adults.10
Scope
The Alberta guideline is intended to assist any primary care practitioner responsible for the assessment and management of headaches in adults. The guideline’s main focus is primary headache disorders (eg, migraine, tension-type, and cluster headache) and medication-overuse headache. Some advice is also provided for the diagnosis and investigation of secondary headache disorders and the management of cervicogenic headache and temporomandibular joint disorder. The guideline will be helpful to a range of primary health care professionals, including family physicians, physical therapists, occupational therapists, nurses, nurse practitioners, pharmacists, psychologists, and chiropractors.
Development
Leadership
The lead organizations involved in developing the guideline were Toward Optimized Practice (TOP), which develops and disseminates primary care guidelines in Alberta, and the Institute of Health Economics (IHE). Three multidisciplinary committees were formed to coordinate guideline production.
The Steering Committee provided operational oversight.
The Guideline Development Group (GDG) formulated the recommendations and comprised 9 family physicians, 2 neurologists, an osteopathic physician, a chiropractor, 2 physical therapists, an occupational therapist, a nurse, a pharmacist, 2 psychologists, and a health technology assessment specialist.
The Advisory Committee advised the Steering Committee on strategic matters and included representatives from the Alberta College of Family Physicians, the Alberta College of Physicians and Surgeons, Alberta Health Services, Alberta Health, the Pain Society of Alberta, and a chronic pain patient advocacy group, as well as experts in guideline development and dissemination.
A research team of health technology assessment researchers with methodologic expertise from the IHE assisted the Steering Committee and GDG.11
Literature review
The Alberta guideline was developed using a guideline adaptation process, which takes advantage of existing high-quality guidelines and allows guideline developers to modify the recommendations from these seed guidelines to meet the needs of the local health care setting.12 Guideline adaptation is a popular alternative to de novo guideline development owing to the need to reduce duplication and constrain costs in the creation of evidence-informed guidelines.13–15
The research team collaborated with experienced medical librarians to systematically search for existing clinical practice guidelines (CPGs) published between January 2000 and May 2011. The search identified 64 guidelines, 18 of which were deemed relevant after application of specific selection criteria developed by the research team and content experts from the GDG.11 The quality of the guidelines was appraised independently by 2 reviewers (C.M. and N.A.S.) using the AGREE (Appraisal of Guidelines for Research and Evaluation) instrument,16,17 which was modified to reduce the subjectivity of the item scoring and to enable the differentiation of good- from poor-quality guidelines.18 Although an updated AGREE tool was published in May 2009,19,20 the research team elected to use the original instrument in order to maintain consistency with previous guidelines produced by TOP and the IHE. Of the 18 potentially eligible guidelines, 6 were scored as good quality and were chosen as seed guidelines.
Two reviewers (C.M. and N.A.S.) extracted the following information into standardized evidence tables: the source of the guideline, the recommendations, the number and types of studies used to create the recommendations (eg, 5 randomized controlled trials), and the strength of the recommendations. A total of 187 recommendations were tabulated. Discordant recommendations were highlighted in the tables.
Practice recommendations
The GDG reviewed the 6 seed guidelines, their companion documents, and the evidence tables during 13 half-day meetings: 1 face-to-face meeting and 12 Web conferences using WebEx (Cisco Systems Inc), which allowed all GDG members to view documents simultaneously and to register their preferences using an online voting system. The 2 GDG cochairs (W.J.B. and P.T.) led all sessions and conducted roundtable discussions for every recommendation to ensure that each GDG member had a voice in the process.
In some cases, the GDG requested additional evidence to resolve uncertainties or disagreements regarding interpretation of the evidence from the seed guidelines or when new interventions were considered that had not been included in the seed guidelines. These “parking lot” requests triggered examination of individual research studies cited by the seed guidelines, as well as additional systematic reviews on headache disorders identified by a supplementary search for literature published between January 2000 and October 2010.11 The parking lot items were referred for further analysis to ad hoc GDG subcommittees that included one or both cochairs, one IHE researcher, and at least one volunteer from the GDG with expertise in the relevant area. Consensus-based decisions made by the subcommittees were then presented to the GDG for final approval. Occasionally new recommendations were generated from parking lot item discussions. A special GDG subcommittee, which included a neuroradiologist, was created for the diagnostic imaging recommendations. The 23-month guideline development process resulted in 91 draft recommendations.
Each recommendation in the Alberta guideline came from 1 or more seed guidelines, was based on evidence from systematic reviews or quasi-systematic reviews, or was created by the GDG members, based on their collective professional opinion and an analysis of relevant evidence. The original wording of the recommendations was retained whenever possible, and designations were used (eg, SR for systematic review, CS for case series) to maintain a link to the evidence cited by the seed guidelines. The principles outlined in the GuideLine Implementability Appraisal tool, which is designed for appraising the implementability of CPGs, were used as a guide when crafting the recommendations.21,22 Standardized definitions for the types of recommendations made in the Alberta CPG were constructed from the evidence-rating scales used by the seed guidelines. The recommendations were categorized as do when the evidence supported the intervention, do not do when the evidence suggested the intervention was ineffective or harmful, or do not know when the evidence was equivocal, conflicting, or insufficient.
A series of companion documents were created, adapted, or adopted to support the implementation of the guideline. These included a quick reference algorithm, a summary document, patient education sheets, and practice tools (a medication table, a headache history form, a patient diary, and a video demonstrating physical examination of the neck).10
The draft guideline was reviewed by the Advisory Committee, a focus group of primary care physicians, and attendees at 2 Alberta physician conferences. The patient information sheets were reviewed by focus groups of patients and laypeople. The feedback was incorporated into the final documents, which were approved by the GDG in February 2012.
Main message
The seed guidelines are listed in Table 1.23–31 The Alberta guideline’s 91 recommendations are organized into 6 sections. The full guideline and accompanying documents are available from the TOP website.10 The quick reference algorithm* information is provided in Figure 1 and Tables 2 to 4.10 Some general practice points are summarized in Box 1.
Table 1.
Seed guidelines used to create the Guideline for Primary Care Management of Headache in Adults
| AUTHOR GROUP | DETAILS |
|---|---|
| US Headache Consortium,23–26 2000 | US: Neuroimaging in patients with nonacute headache23; pharmacologic management of acute migraine attacks24; pharmacologic prevention of migraine25; behavioural and physical treatment of migraine26 |
| European Federation of Neurological Societies,27 2009 | Europe: Pharmacologic treatment of migraine |
| French Society for the Study of Migraine Headache,28 2004 | France: Diagnosis and management of migraine in adults and children |
| Scottish Intercollegiate Guidelines Network,29 2008 | UK: Diagnosis and management of headache in adults |
| European Federation of Neurological Societies,30 2006 | Europe: Treatment of cluster headache and other trigeminal autonomic cephalalgias |
| European Federation of Neurological Societies,31 2010 | Europe: Treatment of tension-type headache |
UK—United Kingdom, US—United States.
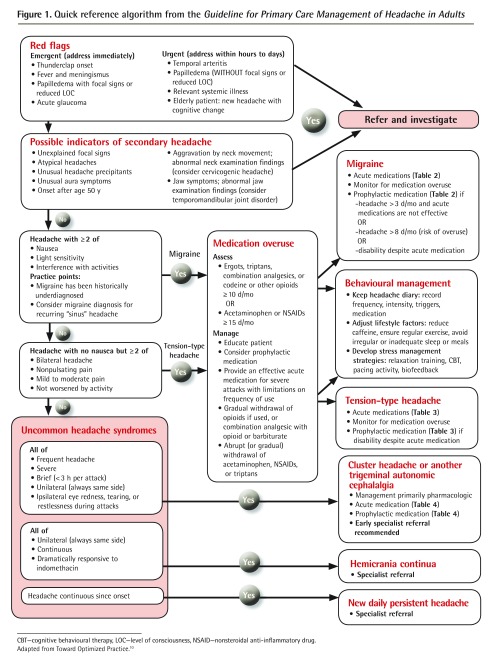
Figure 1.
Quick reference algorithm from the Guideline for Primary Care Management of Headache in Adults
CBT—cognitive behavioural therapy, LOC—level of consciousness, NSAID—nonsteroidal anti-inflammatory drug.
Adapted from Toward Optimized Practice.10
Table 2.
Migraine medications: A) Acute migraine medications. B) Prophylactic migraine medications.
| A) | ||
|---|---|---|
| TYPE | ACUTE MEDICATIONS | |
| First line | Ibuprofen 400 mg, ASA 1000 mg, naproxen sodium 500–550 mg, acetaminophen 1000 mg | |
| Second line | Triptans: oral sumatriptan 100 mg, rizatriptan 10 mg, almotriptan 12.5 mg, zolmitriptan 2.5 mg, eletriptan 40 mg, frovatriptan 2.5 mg, naratriptan 2.5 mg
Antiemetics: domperidone 10 mg or metoclopramide 10 mg for nausea |
|
| Third line | Naproxen sodium 500–550 mg in combination with a triptan | |
| Fourth line | Fixed-dose combination analgesics (with codeine if necessary; not recommended for routine use) |
| B) | ||||
|---|---|---|---|---|
|
| ||||
| PROPHYLACTIC MEDICATIONS | STARTING DOSE | TITRATION,* DAILY DOSE INCREASE | TARGET DOSE OR THERAPEUTIC RANGE† | NOTES |
| First line | ||||
| • propranolol | 20 mg twice daily | 40 mg/wk | 40–120 mg twice daily | Avoid in asthma |
| • metoprolol | 50 mg twice daily | 50 mg/wk | 50–100 mg twice daily | Avoid in asthma |
| • nadolol | 40 mg/d | 20 mg/wk | 80–160 mg/d | Avoid in asthma |
| • amitriptyline | 10 mg at bedtime | 10 mg/wk | 10–100 mg at bedtime | Consider if patient has depression, anxiety, insomnia, or tension-type headache |
| • nortriptyline | 10 mg at bedtime | 10 mg/wk | 10–100 mg at bedtime | Consider if patient has depression, anxiety, insomnia, or tension-type headache |
| Second line | ||||
| • topiramate | 25 mg/d | 25 mg/wk | 50 mg twice daily | Consider as a first-line option if the patient is overweight |
| • candesartan | 8 mg/d | 8 mg/wk | 16 mg/d | Few side effects; limited experience in prophylaxis |
| • gabapentin | 300 mg/d | 300 mg every 3–7 d | 1200–1800 mg/d divided into 3 doses | Few drug interactions |
| Other | ||||
| • divalproex | 250 mg/d | 250 mg/wk | 750–1500 mg/d divided into 2 doses | Avoid in pregnancy or when pregnancy is possible |
| • pizotifen | 0.5 mg/d | 0.5 mg/wk | 1–2 mg twice daily | Monitor for somnolence and weight gain |
| • onabotulinumtoxinA | 155–195 units | No titration needed | 155–195 units every 3 mo | For chronic migraine only (headache on ≥ 15 d/mo) |
| • flunarizine | 5–10 mg at bedtime | No titration needed | 10 mg at bedtime | Avoid in patients with depression |
| • venlafaxine | 37.5 mg/d | 37.5 mg/wk | 150 mg/d | Consider for migraine in patients with depression |
| Over the counter | ||||
| • magnesium citrate | 300 mg twice daily | No titration needed | 300 mg twice daily | Effectiveness might be limited; few side effects |
| • riboflavin | 400 mg/d | No titration needed | 400 mg/d | Effectiveness might be limited; few side effects |
| • butterbur | 75 mg twice daily | No titration needed | 75 mg twice daily | Effectiveness might be limited; few side effects |
| • coenzyme Q10 | 100 mg 3 times daily | No titration needed | 100 mg 3 times daily | Effectiveness might be limited; few side effects |
ASA—acetylsalicylic acid.
Dosage can be increased every 2 wk to avoid side effects. For most drugs, slowly increase to the target dose; a therapeutic trial requires several months. The expected outcome is reduction not elimination of attacks.
If the target dose is not tolerated, try a lower dose. If the medication is effective and tolerated, continue it for at least 6 mo. If several preventive drugs fail, consider a specialist referral.
Adapted from Toward Optimized Practice.10
Table 3.
Medications for tension-type headache
| MEDICATION | DOSE |
|---|---|
| Acute | |
| Ibuprofen | 400 mg |
| ASA | 1000 mg |
| Naproxen sodium | 500–550 mg |
| Acetaminophen | 1000 mg |
| Prophylactic | |
| First line | |
| • amitriptyline | 10–100 mg/d |
| • nortriptyline | 10–100 mg/d |
| Second line | |
| • mirtazapine | 30 mg/d |
| • venlafaxine | 150 mg/d |
ASA—acetylsalicylic acid.
Adapted from Toward Optimized Practice.10
Table 4.
Medications for cluster headache: Consider early specialist referral.
| MEDICATION | DOSE |
|---|---|
| Acute | |
| Subcutaneous sumatriptan | 6 mg |
| Intranasal zolmitriptan | 5 mg |
| 100% oxygen | 12 L/min for 15 min through non-rebreathing mask |
| Prophylactic* | |
| First line | |
| • verapamil | 240–480 mg/d (higher doses might be required) |
| Second line | |
| • lithium | 900–1200 mg/d |
| Other | |
| • topiramate | 100–200 mg/d |
| • melatonin | Up to 10 mg/d |
If the patient has more than 2 attacks daily, consider transitional therapy while verapamil is built up (eg, 60 mg of prednisone for 5 d, then reduced by 10 mg every 2 d until discontinued).
Adapted from Toward Optimized Practice.10
Box 1. General practice points for managing primary headache in adults.
The following are general practice points for the management of primary headache in adults:
|
NSAID—nonsteroidal anti-inflammatory drug.
Section 1: headache diagnosis and investigation
Box 2 presents important elements of the history for patients presenting with first-time headache or a change in headache pattern. Box 3 presents an approach to the physical examination specifically for primary care providers.29 Box 4 presents red flags and other potential indicators of secondary headache.29 Table 5 presents a simplified strategy for diagnosing primary headache disorders.32,33
Box 2. Important elements of the headache history in patients presenting with headache for the first time or those with a change in headache pattern.
Explore the following important elements of the headache history:
|
Based on expert opinion of the Guideline Development Group.
Box 3. Approach to the physical examination of a patient presenting with headache for the first time or with a change in headache pattern.
The physical examination should incorporate the following elements:
|
Based on the Scottish Intercollegiate Guidelines Network guideline29 and expert opinion of the Guideline Development Group.
Box 4. Red flags and other potential indicators of secondary headache: Appropriate referral or investigation should be considered.
Red flags: emergent (address immediately)
Red flags: urgent (address within hours to days)
Other possible indicators of secondary headache (less urgent)
|
Based on the Scottish Intercollegiate Guidelines Network guideline29 and expert opinion of the Guideline Development Group.
Table 5.
Diagnosing primary headache syndromes
| DESCRIPTION | HEADACHE SYNDROME |
|---|---|
| Patients with recurrent headache attacks and normal neurologic examination findings (in some patients other clinical symptoms might also need to be considered)* |
|
| Patients with headache on ≥ 15 d/mo for > 3 mo and with normal neurologic examination findings‡ |
|
| Patients with continuous daily headache for > 3 mo with normal neurologic examination findings§ |
|
NSAID—nonsteroidal anti-inflammatory drug.
Modified from the International Classification of Headache Disorders32; data from Lipton et al33; and based on expert opinion of the Guideline Development Group.
If patients do not meet migraine diagnostic criteria.
Modified from the International Classification of Headache Disorders32 and based on expert opinion of the Guideline Development Group.
Modified from the International Classification of Headache Disorders32 and based on expert opinion of the Guideline Development Group.
This less common headache syndrome should be considered in patients with continuous headache.
Section 2: migraine
A comprehensive approach to migraine management is summarized in Box 5. Section 2 of the guideline contains recommendations for lifestyle management, acute treatment, prophylaxis, menstrual migraine, and migraine treatment during pregnancy. The full guideline provides a detailed medication table for migraine that includes available formulations, usual doses, relative and absolute contraindications, and adverse events. Boxes 6 and 7 show the indications and considerations for prescribing prophylactic drugs for migraine.28,29 Recommended medications are outlined in Table 2.10
Box 5. Comprehensive migraine management.
Consider the following when managing patients with migraine:
|
Based on expert opinion of the Guideline Development Group.
Box 6. Pharmacologic prophylaxis for migraine.
Prophylactic medication is indicated in the following circumstances:
|
NSAID—nonsteroidal anti-inflammatory drug.
Based on expert opinion of the Guideline Development Group.
Box 7. Pharmacologic prophylaxis for migraine.
Consider the following when prescribing prophylactic medication:
|
Section 3: tension-type headache
This section contains recommendations on lifestyle, acute and prophylactic drug therapy, and management of tension-type headache during pregnancy. Recommended medications are outlined in Table 3.10
Section 4: medication-overuse headache
Migraine sufferers are particularly prone to developing medication-overuse headache. Recommendations for diagnosis and management of medication-overuse headache are shown in Boxes 8 and 9.29
Box 8. Diagnosis of medication-overuse headache.
Consider the following in the diagnosis of medication-overuse headache:
|
NSAID—nonsteroidal anti-inflammatory drug.
Based on the Scottish Intercollegiate Guidelines Network guideline29 and expert opinion of the Guideline Development Group.
Box 9. Management of medication-overuse headache.
Treatment plans for patients with medication-overuse headache should include the following:
|
NSAID—nonsteroidal anti-inflammatory drug.
Based on the Scottish Intercollegiate Guidelines Network guideline29 and expert opinion of the Guideline Development Group.
Section 5: cluster headache
Cluster headache is managed with a number of pharmacologic therapies. These can be initiated and monitored in primary care, but early specialist referral is recommended because this headache type is uncommon, disabling, and challenging to manage. Recommended medications are outlined in Table 4.10
Section 6: other headache disorders
This section of the guideline focuses on hemicrania continua, cervicogenic headache, and headache secondary to temporomandibular joint disorders. Treatment of these conditions will likely involve referral to an appropriately trained therapist or specialist.
Implementation and update plans
The guideline has been disseminated through presentations and workshops at provincial, regional, and national conferences. It is also listed in the CMA Infobase,34 where it was among the 10 most downloaded guidelines for nearly 6 months. It also appears on the Michael G. DeGroote National Pain Centre website35 and is listed by the US National Guideline Clearing House.36 A pilot project is under way at the University of Calgary in Alberta to present the headache guideline using interactive webinars.
The evidence base for the Alberta CPG will be assessed annually and will be updated when new evidence is found that changes the recommendations.
Limitations
The guideline adaptation process precluded an in-depth analysis of the validity or a formal assessment of the strength and quality of the underlying empirical evidence, which made categorizing the strength and type of recommendations problematic. To counter this problem, standardized definitions were constructed for the types of recommendations made in the Alberta CPG (eg, what constituted a do or do not do recommendation) from the overlapping evidence-rating scales used by the seed guidelines, and designations were used (eg, SR for systematic review) to maintain a link to the evidence type referenced by the seed guidelines in support of their recommendations.10,11
The lack of high-quality scientific evidence for headache investigations, diagnosis, red flags, and specialist referral meant that many recommendations in these areas relied on the opinions of the GDG or the experts who developed the seed guidelines. However, these issues were overcome by using credible seed guidelines, scrupulously listing the evidence type and source for all recommendations, and clearly documenting the subjective contextualization process.
Adaptation processes are limited by the time lag between the publication of primary studies and their incorporation into guidelines, which means that recently published evidence was not necessarily incorporated into the Alberta CPG and that not all of the treatment options available were covered by the seed guidelines. To help offset this, the research team updated searches regularly throughout the Alberta guideline adaptation process.
There was debate among the GDG members about incorporating newly emerging headache treatments that were not identified in the seed guidelines. A conservative approach was adopted whereby a recommendation for an emerging intervention was created only if it had been assessed in a systematic review.
None of the seed guidelines included formal economic evaluations or cost analyses, nor did they discuss the economic implications of their recommendations. Owing to time and resource constraints, a formal cost analysis or economic evaluation of the effect of the Alberta CPG was not conducted. However, any statements on economic aspects made by the seed guidelines were noted in the accompanying background document.11
Conclusion
The format and brevity of the Guideline for Primary Care Management of Headache in Adults reflects its intent—to provide Canadian primary care providers across multiple disciplines with a comprehensive suite of resources for assessing and managing headaches in adults. A guideline summary and algorithm, as well as practice tools and patient information sheets, are provided to support comprehensive headache management that emphasizes patient engagement and self-management, as well as evidence-informed interventions.
Acknowledgments
We thank the members of the Ambassador Program Guideline Development Group, the Advisory Committee, and the Steering Committee for their commitment and hard work. We also thank all of the organizations that provided support during the guideline development process, particularly Toward Optimized Practice for guidance in the final stages of the guideline process and for hosting the completed guideline online. Special thanks to Dr James N. Scott (radiology) and Dr Robert Ashforth (radiology) for their invaluable contributions to the diagnostic imaging components of the guideline. We also thank Ms Wendy McIndoo for administrative assistance and Ms Liz Dennett and Ms Dagmara Chojecki for information services support. Funding for this initiative was provided by Alberta Health. The views expressed herein do not necessarily represent the official policy of Alberta Health.
EDITOR’S KEY POINTS
Headache is a common reason why patients seek help from family physicians, and treatment is often suboptimal. This article outlines the development and key recommendations of the clinical practice guideline created by a multidisciplinary guideline development group to assist Canadian primary care practitioners with providing evidence-informed care for patients with headache.
Migraine, which is historically underdiagnosed, is by far the most common headache type in patients seeking help for headache. Neuroimaging, sinus or cervical spine x-ray scans, and electroencephalograms are not recommended for the routine assessment of patients with headache: history and physical and neurologic examination findings are usually sufficient to make a diagnosis. Comprehensive migraine therapy includes management of lifestyle factors and triggers, acute and prophylactic medications, and migraine self-management strategies. Treatment of tension-type, cluster, and medication-overuse headache is also outlined.
Footnotes
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro d’août 2015 à la page e353.
The original quick reference algorithm is available in an easy-to-print format at www.cfp.ca. Go to the full text of the article online and click on CFPlus in the menu at the top right-hand side of the page.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
Dr Becker served on medical advisory boards for AGA Medical, Allergan, Merck, and Pfizer; received speaker’s honoraria from Allergan, Merck, Pfizer, Serono, and Teva; and received research support as part of multicenter clinical trials (served as local principal investigator) from AGA Medical, Allergan, Medtronic, and Merck. However, these interests had no influence on the design, data analysis, formulation, or content of the guideline. None of the other authors has any conflict of interest to declare.
References
- 1.Bigal ME, Lipton RB, Stewart WF. The epidemiology and impact of migraine. Neurol Neurosci Rep. 2004;4(2):98–104. doi: 10.1007/s11910-004-0022-8. [DOI] [PubMed] [Google Scholar]
- 2.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30(2):107–19. doi: 10.1055/s-0030-1249220. Epub 2010 Mar 29. [DOI] [PubMed] [Google Scholar]
- 3.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooke LJ, Becker WJ. Migraine prevalence, treatment and impact: the Canadian Women and Migraine Study. Can J Neurol Sci. 2010;37(5):580–7. doi: 10.1017/s0317167100010738. [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 6.Latinovic R, Gulliford M, Ridsdale L. Headache and migraine in primary care: consultation, prescription, and referral rates in a large population. J Neurol Neurosurg Psychiatry. 2006;77(3):385–7. doi: 10.1136/jnnp.2005.073221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigal M, Krymchantowski AV, Lipton RB. Barriers to satisfactory migraine outcomes. What have we learned, where do we stand? Headache. 2009;49(7):1028–41. doi: 10.1111/j.1526-4610.2009.01410.x. Epub 2009 Apr 6. [DOI] [PubMed] [Google Scholar]
- 8.Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value Health. 2009;12(1):55–64. doi: 10.1111/j.1524-4733.2008.00404.x. Epub 2008 Jul 30. [DOI] [PubMed] [Google Scholar]
- 9.Becker WJ, Gladstone JP, Aube M. Migraine prevalence, diagnosis, and disability. Can J Neurol Sci. 2007;34(Suppl 4):S3–9. [PubMed] [Google Scholar]
- 10.Guideline for primary care management of headache in adults. Edmonton, AB: Toward Optimized Practice; 2012. Toward Optimized Practice. Available from: www.topalbertadoctors.org/cpgs/10065. Accessed 2013 Aug 26. [Google Scholar]
- 11.Institute of Health Economics . Ambassador program guideline for management of primary headache in adults: background document. Edmonton, AB: Institute of Health Economics; 2013. Available from: www.ihe.ca/research-programs/hta/aagap/headache. Accessed 2013 Aug 26. [Google Scholar]
- 12.Harstall C, Taenzer P, Angus DK, Moga C, Schuller T, Scott NA. Creating a multidisciplinary low back pain guideline: anatomy of a guideline adaptation process. J Eval Clin Pract. 2011;17(4):693–704. doi: 10.1111/j.1365-2753.2010.01420.x. Epub 2010 Sep 16. [DOI] [PubMed] [Google Scholar]
- 13.Fervers B, Burgers JS, Haugh MC, Latreille J, Mlika-Cabanne N, Paquet L, et al. Adaptation of clinical guidelines: literature review and proposition for a framework and procedure. Int J Qual Health Care. 2006;18(3):167–76. doi: 10.1093/intqhc/mzi108. [DOI] [PubMed] [Google Scholar]
- 14.Groot P, Hommersom A, Lucas P. Adaptation of clinical practice guidelines. Stud Health Technol Inform. 2008;139:121–39. [PubMed] [Google Scholar]
- 15.Muth C, Gensichen J, Beyer M, Hutchinson A, Gerlach FM. The systematic guideline review: method, rationale, and test on chronic heart failure. BMC Health Serv Res. 2009;9:74. doi: 10.1186/1472-6963-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The AGREE Collaboration . AGREE instrument. London, UK: The AGREE Collaboration; 2001. Available from: www.agreetrust.org/resource-centre/the-original-agree-instrument/the-original-agree-instrument-translations. Accessed 2013 Aug 26. [Google Scholar]
- 17.The AGREE Collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott NA, Moga C, Harstall C. Making the AGREE tool more user friendly: the feasibility of a user guide based on Boolean operators. J Eval Clin Pract. 2009;15(6):1061–73. doi: 10.1111/j.1365-2753.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 19.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. CMAJ. 2010;182(18):E839–42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The AGREE Next Steps Consortium . Appraisal of Guidelines for Research & Evaluation II. London, UK: The AGREE Next Steps Consortium; 2009. Available from: www.agreetrust.org. Accessed 2013 Aug 26. [Google Scholar]
- 21.Shiffman RN, Dixon J, Brandt C, Essaihi A, Hsiao A, Michel G, et al. The GuideLine Implementability Appraisal (GLIA): development of an instrument to identify obstacles to guideline implementation. BMC Med Inform Decis Mak. 2005;5:23. doi: 10.1186/1472-6947-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yale University . GuideLine Implementability Appraisal (GLIA) New Haven, CT: Yale University; 2005. Available from: http://nutmeg.med.yale.edu/glia/login.htm;jsessionid%20=DFE8740FF9FF152296DD79BFBAA723B6. Accessed 2013 Aug 26. [Google Scholar]
- 23.Frishberg BM, Rosenberg JH, Matchar DB, McCrory DC, Pietrzak MP, Rozen TD, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul, MN: US Headache Consortium; 2000. [Google Scholar]
- 24.Matchar DB, Young WB, Rosenberg JH, Pietrzak MP, Silberstein SD, Lipton RB, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management of acute attacks. St Paul, MN: US Headache Consortium; 2000. [Google Scholar]
- 25.Ramadan NM, Silberstein SD, Freitag F, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for prevention of migraine. St Paul, MN: US Headache Consortium; 2000. [Google Scholar]
- 26.Campbell JK, Penzien DB, Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. St Paul, MN: US Headache Consortium; 2000. [Google Scholar]
- 27.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 28.Géraud G, Lantéri-Minet M, Lucas C, Valade D, French Society for the Study of Migraine Headache (SFEMC) French guidelines for the diagnosis and management of migraine in adults and children. Clin Ther. 2004;26(8):1305–18. doi: 10.1016/s0149-2918(04)80161-9. [DOI] [PubMed] [Google Scholar]
- 29.Scottish Intercollegiate Guidelines Network . Diagnosis and management of headache in adults. A national clinical guideline. Publication no. 107. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2008. Available from: www.sign.ac.uk/guidelines/fulltext/107/index.html. Accessed 2013 Aug 26. [Google Scholar]
- 30.May A, Leone M, Afra J, Linde M, Sandor PS, Evers S, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066–77. doi: 10.1111/j.1468-1331.2006.01566.x. Available from: www.guideline.gov/content.aspx?id=34898. Accessed 2015 Jun 10. [DOI] [PubMed] [Google Scholar]
- 31.Bendtsen L, Evers S, Linde M, Mitsikostas DD, Sandrini G, Schoenen J. EFNS guideline on the treatment of tension-type headache—report of an EFNS task force. Eur J Neurol. 2010;17(11):1318–25. doi: 10.1111/j.1468-1331.2010.03070.x. [DOI] [PubMed] [Google Scholar]
- 32.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 33.Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;61(3):375–82. doi: 10.1212/01.wnl.0000078940.53438.83. [DOI] [PubMed] [Google Scholar]
- 34.Canadian Medical Association . CMA Infobase: clinical practice guidelines database. Record ID 13271. Ottawa, ON: Canadian Medical Association; 2013. Available from: https://www.cma.ca/en/Pages/cpg-details.aspx?cpgId=13271&la_id=1. Accessed 2013 Aug 26. [Google Scholar]
- 35.Michael G. Guidelines. Hamilton, ON: McMaster University; 2013. DeGroote National Pain Centre [website] Available from: http://nationalpaincentre.mcmaster.ca/guidelines.html. Accessed 2015 Jun 11. [Google Scholar]
- 36.Guideline summary. Rockville, MD: Agency for Healthcare Research and Quality; 2013. National Guideline Clearinghouse [website] Available from: www.guideline.gov/content.aspx?id=47060&search=guideline+for+primary+care+management+of+headache+in+adults. Accessed 2015 Jun 11. [Google Scholar]