Abstract
Objective
To determine if medical marijuana provides pain relief for patients with chronic noncancer pain (CNCP) and to determine the therapeutic dose, adverse effects, and specific indications.
Data sources
In April 2014, MEDLINE and EMBASE searches were conducted using the terms chronic noncancer pain, smoked marijuana or cannabinoids, placebo and pain relief, or side effects or adverse events.
Study selection
An article was selected for inclusion if it evaluated the effect of smoked or vaporized cannabinoids (nonsynthetic) for CNCP; it was designed as a controlled study involving a comparison group, either concurrently or historically; and it was published in English in a peer-review journal. Outcome data on pain, function, dose, and adverse effects were collected, if available. All articles that were only available in abstract form were excluded.
Synthesis
A total of 6 randomized controlled trials (N = 226 patients) were included in this review; 5 of them assessed the use of medical marijuana in neuropathic pain as an adjunct to other concomitant analgesics including opioids and anticonvulsants. The 5 trials were considered to be of high quality; however, all of them had challenges with masking. Data could not be pooled owing to heterogeneity in delta-9-tetrahydrocannabinol potency by dried weight, differing frequency and duration of treatment, and variability in assessing outcomes. All experimental sessions in the studies were of short duration (maximum of 5 days) and reported statistically significant pain relief with nonserious side effects.
Conclusion
There is evidence for the use of low-dose medical marijuana in refractory neuropathic pain in conjunction with traditional analgesics. However, trials were limited by short duration, variability in dosing and strength of delta-9-tetrahydrocannabinol, and lack of functional outcomes. Although well tolerated in the short term, the long-term effects of psychoactive and neurocognitive effects of medical marijuana remain unknown. Generalizing the use of medical marijuana to all CNCP conditions does not appear to be supported by existing evidence. Clinicians should exercise caution when prescribing medical marijuana for patients, especially in those with nonneuropathic CNCP.
Résumé
Objectif
Vérifier si la marijuana médicale procure un soulagement de la douleur chez des patients souffrant de douleur chronique non cancéreuse (DCNC), et déterminer sa dose thérapeutique, ses effets indésirables et ses indications spécifiques.
Sources des données
On a consulté MEDLINE et EMBASE en avril 2004 à l’aide des rubriques chronic noncancer pain, smoked marijuana ou cannabinoides, placebo and pain relief, ou side effects, ou adverse events.
Choix des études
Ont été retenus pour cette revue, les articles qui évaluaient l’effet des canabinoïdes (non synthétiques) fumés ou en inhalation pour traiter la DCNC; les études contrôlées comprenant un groupe témoin, que ce soit simultanément ou antérieurement; et les études publiées en anglais dans une revue avec révision par des pairs. Les résultats concernant la douleur, la fonction, la dose et les effets indésirables ont été recueillis lorsque disponibles. Les articles uniquement sous forme de résumé ont tous été exclus.
Synthèse
Un total de 6 essais randomisés contrôlés portant sur 226 patients ont été retenus pour cette revue; 5 d’entre eux évaluaient l’effet de la marijuana médicale associée à des analgésiques, incluant des opiacés et des anticonvulsivants, sur la douleur neuropathique. Ces 5 essais étaient jugés de grande qualité; toutefois, ils avaient tous des problèmes avec l’insu. Les données n’ont pu être combinées en raison de l’hétérogénéité de la puissance par unité de poids sec du delta-9-tétrahydrocannabinol, de traitements de fréquences et de durées différentes et d’une évaluation variable des issues. Toutes les sessions expérimentales dans ces études étaient de courte durée (au plus 5 jours) et rapportaient un soulagement statistiquement significatif de la douleur avec des effets secondaires plutôt légers.
Conclusion
Il existe des données en faveur de l’utilisation de faibles doses de marijuana médicale en association avec des analgésiques traditionnels en cas de douleur neuropathique réfractaire. Toutefois, les essais étaient limités en raison de leurs courtes durées, de variations dans les doses et dans la puissance du delta-9-tétrahydrocannabinol, et de l’absence d’issues fonctionnelles. Même s’ils sont bien tolérés à court terme, les effets psychoactifs et neurocognitifs à long terme de la marijuana médicale demeurent inconnus. L’utilisation généralisée de la marijuana médicale pour toutes les conditions de DCNC ne semble pas soutenue par les données existantes. Les cliniciens devraient user de prudence lorsqu’ils prescrivent la marijuana médicale à des patients, spécialement à ceux qui souffrent de douleur neuropathique chronique non cancéreuse.
Few therapeutic options for chronic noncancer pain (CNCP) provide consistently successful outcomes; many fail to provide clinically meaningful reduction in pain, defined as a decrease in pain scores by at least 30%.1 Even with the widespread use of opioids, improvements in outcomes such as function and mood remain limited.2
Cannabis has had a long history of use for spiritual and religious purposes, as well as for various medical conditions.3 In 1999, an Institute of Medicine report4 supported the use of marijuana in medicine; however, the debate about the usefulness and safety of marijuana remains unresolved.
In Canada, the federal government brought forward the Marihuana for Medical Purposes Regulation in March 2014, replacing the previous Marihuana Medical Access Regulations (MMAR).5 In response to physicians’ concerns, most of the regulatory medical colleges in Canada have published recommendations for prescribing medical marijuana. Most colleges acknowledge the fact that proper studies have not yet been conducted, and one college in the province of Quebec restricts the use of medical marijuana to the context of a research framework.6
The primary objective of this systematic review was to determine whether smoked or vaporized cannabis provides pain relief in the CNCP population. Secondary objectives included determining its effect on function, identifying therapeutic doses, and documenting commonly associated adverse effects.
DATA SOURCES
Literature search
In April 2014, we identified eligible studies through an electronic search of MEDLINE, EMBASE, and the International Pharmaceutical Abstracts. The search strategy encompassed a theme that included the following terms: chronic noncancer pain, smoked marijuana or cannabinoids, placebo and pain relief, or side effects or adverse events.
Study selection
We selected an article for inclusion if it evaluated the effect of smoked or vaporized cannabinoids (nonsynthetic) for CNCP; it was designed as a controlled study involving a comparison group, either concurrently or historically; and it was published in English in a peer-reviewed journal. We excluded all articles that were only available in abstract form.
SYNTHESIS
Data extraction
Two independent reviewers (S.F.L., N.Z.) screened potentially eligible articles, assessed the methodologic quality of each study, and extracted data from included trials. Disagreements were resolved by consensus. For outcomes, pain scores were extracted using the visual analogue scale (VAS) or an alternative numerical pain rating tool. If pain scores were not reported, surrogate measures of effectiveness were included (sleep, function, and quality of life). Frequency of serious and most commonly reported adverse effects was collected. A serious adverse event was based on the definition supplied by Health Canada and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidance documents.7
Quality assessment
To assess quality, we used the Jadad scale, a 5-item tool scored between 0 and 5.8 We categorized the trials as high or low quality with scores greater than 2 or 2 or lower, respectively.
Literature search results
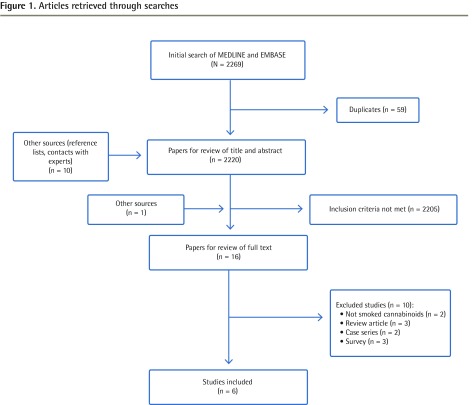
We found 2269 potentially eligible articles from the search strategy and 10 other potential articles through review of references. Sixteen relevant studies were subjected to full-text review (Figure 1) with one study9 identified later in the references of the College of Family Physicians of Canada guidance document on medical marijuana.10 Altogether, this review identified 6 randomized controlled trials,9,11–15 with 5 of them having crossover designs9,11–14; 1 study was performed primarily for spasticity in multiple sclerosis (MS) with pain evaluated as a secondary outcome.11 We did not identify any historically controlled comparative studies.
Figure 1.
Articles retrieved through searches
Study characteristics
Five studies were rated as high quality, scoring 3 out of 5.9,12–15 Allocation concealment was reported in 4 studies.9,13–15 Summaries of the final 6 articles in our review are presented in Tables 1 and 2.8,9,11–15
Table 1.
Characteristics of the 6 studies in this review that examined the use of medical marijuana for CNCP
| STUDY | COUNTRY | FUNDING SOURCE | STUDY DESIGN | QUALITY ASSESSMENT* |
|---|---|---|---|---|
| Abrams et al,15 2007 | US | Center for Medicinal Cannabis Research |
Randomized, double-blind trial | 3 (High) |
| Corey-Bloom et al,11 2012 | US | Center for Medicinal Cannabis Research |
Randomized, double-blind, placebo-controlled, crossover trial | 2 (Low) |
| Ellis et al,13 2009 | US | Center for Medicinal Cannabis Research |
Randomized, double-blind, placebo-controlled, crossover trial | 3 (High) |
| Ware et al,12 2010 | Canada | Canadian Institutes of Health Research | Randomized, double-blind, placebo-controlled, crossover trial | 3 (High) |
| Wilsey et al,9 2013 | US | National Institutes of Health | Randomized, double-blind, placebo-controlled, crossover trial | 3 (High) |
| Wilsey et al,14 2008 | US | Center for Medicinal Cannabis Research |
Randomized, double-blind, placebo-controlled, crossover trial | 3 (High) |
CNCP—chronic noncancer pain, US—United States.
Based on the Jadad scale.8
Table 2.
Summaries of the 6 studies in this review that examined the use of medical marijuana for CNCP
| STUDY | CLINICAL CONDITION | STUDY DURATION AND PROTOCOL | PRIMARY OUTCOME MEASURE | STUDY SAMPLE | CONTROL | INTERVENTION | OUTCOME | COMMENTS |
|---|---|---|---|---|---|---|---|---|
| Abrams et al,15 2007 | HIV peripheral neuropathy | Study duration was 3 wk Adults with documented HIV and associated sensory neuropathy in 21-d trial with randomization to control group or intervention group for 5 d |
Daily diary of pain ratings on a VAS (0–100 mm) | There were 55 participants enrolled: 28 randomized to control group, with 25 completing the study; 27 randomized to intervention group, with 25 completing the study | Cigarettes containing 0% delta-9-THC that appeared identical to the cannabis cigarettes | Cigarettes containing 3.56% delta-9-THC and weighing an average of 0.9 g; smoked 3 times per d | A total of 13 of 25 participants in intervention group had > 30% reduction in pain from baseline to end of treatment compared with 6 of 25 participants in control group; median reduction of NP was 34% in the intervention group compared with 17% in the control group | Smoking the first cigarette reduced chronic pain ratings (AUC) by 72% (intervention) versus 15% (control) compared with chronic pain ratings after smoking the last cigarette of 51% (intervention) versus 5% (control) |
| Corey-Bloom et al,11 2012 | MS | Study duration was 17 d Adults with MS and spasticity smoked 1 cigarette per d for 3 d |
Pain intensity measured by VAS (secondary outcome) | There were 37 participants randomized, with 30 completing the study | Cigarettes containing 0% delta-9-THC that appeared identical to the cannabis cigarettes | Cigarettes containing 4% delta-9-THC and weighing an average of 0.8 g; smoked once daily | Smoking cannabis reduced pain scores on the VAS by 5.28 points (95% CI 2.48 to 10.01) more than the control group | There were 17 participants who correctly guessed treatment phase for all 6 visits, with 83% having previous exposure to cannabis. Participants had very low levels of pain to start |
| Ellis et al,13 2009 | HIV peripheral neuropathy | Study duration was 7 wk HIV-infected adults with NP refractory to 2 other analgesics in 5-phase study: 1-wk wash-in phase; randomization to 5-d smoking phase; 2-wk washout phase; 5-d crossover phase; and final 2-wk washout phase |
Pain intensity measured by DDS and VAS, a 10-cm line (secondary outcome) | There were 34 participants randomized, with 28 completing the study | Cigarettes that had all cannabinoids removed and that were identical in appearance to active cigarettes | Cigarettes with 1%–8% delta-9-THC potency titrated to tolerance on d 1, followed by 4 d of smoking target dose, with each d composed of 4 sessions separated by 90–120 min | Median difference in pain reduction was 3.3 DDS points (effect size = 0.6; P = .016); proportion with ≥ 30% pain reduction was greater in the active cannabis wk than the placebo cannabis wk (0.46 [95% CI 0.28 to 0.65] vs 0.18 [95% CI 0.03 to 0.32]. The median (range) change in VAS pain scores were −17 (−58 to 52) for cannabis compared with −4 (−56 to 28) for placebo | Patients correctly guessed when they consumed delta-9-THC; however, subanalysis revealed no difference in final outcome. No breakdown of AEs experienced. There were 2 patients who exited the trial owing to psychosis and intractable cough from cannabis. The UKU and DAIDS side effect frequency was greater in the intervention group and there was a trend toward moderate to severe AEs. Greater increase in heart rate among cannabis group (13 of 28 patients) than placebo group (1 of 28 patients) |
| Ware et al,12 2010 | Posttraumatic neuropathy | Study duration was 8 wk Adults ≥ 18 y with posttraumatic or postsurgical pain for at least 3 mo randomized to a sequence of 4 treatment periods, each 14-d period beginning with 5 d on the study drug followed by a 9-d washout period |
11-Point numeric rating scale (secondary measures included sleep, mood, and quality of life) | There were 23 participants randomized, with 21 completing the study | Cannabis containing 0% delta-9-THC prepared by ethanolic extraction | Over 3, 14-d periods, 25-mg doses of various delta-9-THC potencies (2.5%, 6.0%, 9.4%) were delivered through a pipe 3 times per d for the first 5 d of each cycle, followed by a 9-d washout period | Mean (SD) daily pain intensity was lower among intervention group (5.4 [1.6]) than control group (6.1 [1.7]); difference of 0.7 (95% CI 0.02 to 1.4) | Overall, use of cannabis associated with improvements in pain, sleep, and anxiety. Frequency of AEs increased with potency and was greatest for psychiatric disorders (12 events vs 1). Fixed dose and limited quantity (25 mg) might have limited potential AEs |
| Wilsey et al,9 2013 | NP | Study duration was 3, 6-h experimental sessions; there were 3- to 14-d intervals between sessions Adults with type 1 CRPS, spinal cord injury, peripheral neuropathy, or nerve injury |
Measured with VAS (0–100 mm) and the NP scale | There were 39 participants randomized and who completed at least 1 session (no dropouts from AEs or experimental intervention) | Placebo cannabis made from whole plant with cannabinoid extraction | Participants were randomized to 1 of 3, 6-h sessions. Cued puff (vaporized) procedure of 0%, 1.29% (low dose), or 3.53% (medium dose) delta-9-THC, with cumulative 8-12 puffs per session | A 30% reduction in pain intensity: 10 of 38 (26%) placebo patients; 21 of 37 (57%) low-dose patients; 22 of 36 (61%) medium-dose patients. For placebo vs low dose, NNT was 3.2 (P = .0069); for placebo vs medium dose, NNT was 2.9 (P = .0023) | Both 1.29% and 3.53% delta-9-THC potencies produced equal antinociception with minimal effect on cognitive testing. Greatest dose effects were noted in learning and memory, with effect sizes in small or medium range |
| Wilsey et al,14 2008 | NP | Study duration was 3, 6-h experimental sessions; there were 3- to 14-d intervals between sessions Adults with type 1 CRPS, spinal cord injury, peripheral neuropathy, or nerve injury | Measured with VAS (0–100 mm) and the NP scale | There were 38 participants randomized, with 32 completing all sessions | Cigarettes made from whole cannabis with cannabinoid extraction | Participants were randomized to 1 of 3, 6-h sessions. Cued puff procedure of 0%, 3.5%, or 7% delta-9-THC | A 0.0035 reduction in VAS pain intensity per min was noted from both 3.5% and 7% cannabis, with cumulative 9 puffs per session | Ceiling effect noted with cumulative dosing, as 3.5% and 7% potencies produced equal antinociception; secondary outcomes improved, including pain unpleasantness (mean difference = −0.21 [95% CI −0.33 to −0.09]; P < .01) and global impression of change (mean difference = 0.12 [95% CI 0.064 to 0.18]; P < .01) |
AE—adverse event, AUC—area under curve, CRPS—complex regional pain syndrome, DAIDS—Division of AIDS, DDS—descriptor differential scale, delta-9-THC—delta-9-tetrahydrocannabinol, MS—multiple sclerosis, NNT—number needed to treat, NP—neuropathic pain, UKU—Udvalg for Kliniske Undersøgelser, VAS—visual analogue scale.
In total, 226 adults (mean age of 45 to 50 years across trials) with chronic neuropathic pain were randomized, with 189 adults specifically identified as having chronic neuropathic pain.9,12–15 Two studies focused on HIV-associated neuropathy,13,15 1 on posttraumatic neuropathy,12 and 2 on mixed neuropathic conditions.9,14 The study involving patients with MS did not discriminate between spasticity pain and neuropathic pain.11 Three studies limited enrolment to patients with previous cannabis exposure,9,14,15 while 2 had no limitations.11,12 All trials excluded individuals with a history of psychotic disorders and previous history of cannabis abuse or dependence. All trials, except 1,15 reported the use of urine toxicology or other screening tools before starting the trial. Pain duration (6 to 9 years) was specifically mentioned in 3 trials,9,14,15 with 4 trials identifying baseline pain in the moderate range.9,12,14,15 Four9,12–14 of the 5 trials9,12–15 that allowed participants to continue to use opioids, anticonvulsants, and antidepressants reported that more than 50% of participants used concomitant opioids. Studies did not report the baseline dose of concurrent analgesics.
Trial duration varied from 17 days11 to 8 weeks,12 with the actual intervention (smoking cannabinoids) varying from a minimum of 3 experimental session days each lasting 6 hours9,14 to a maximum of 5 days.12,13,15 One study had an intervention period of 3 days.11
Only 1 trial administered delta-9-tetrahydrocannabinol (delta-9-THC) through the use of a vaporizer.9 The strength of delta-9-THC employed in the trials for smoked cannabinoids ranged from a low of about 1%9,12 to a high of 9.4%12 as measured by the percentage of dry weight. The total daily delta-9-THC consumption was reported only in 1 trial.14 In 3 studies the total daily delta-9-THC consumption was calculated based on the reported percentage of dry weight delta-9-THC and the cigarette weight.11,12,15 The total daily delta-9-THC exposure could not be determined in 1 study because of missing information13 and in another study owing to flexible dosing.9 The total daily delta-9-THC consumed during the trials ranged between a low of 1.875 mg per day12 and a high of 34 mg per day14 (Table 3).9,11–15
Table 3.
Incidence of AEs reported in the 6 studies in this review
| STUDY | DELTA-9-THC POTENCY, % | DOSING FREQUENCY | DELTA-9-THC POTENCY DOSE, mg/D | AEs | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| MINIMUM | MAXIMUM | MINIMUM | MAXIMUM | NEUROCOGNITIVE | NONCOGNITIVE | ||
| Abrams et al,15 2007 | 3.56 | 3.56 | 3 times daily | 32 | 32 | Mean side effect scores (95% CI) were as follows for cannabis group and placebo group, respectively:
|
NR |
| Corey-Bloom et al,11 2012* | 4 | 4 | 1 time daily | 32 | 32 | Dizziness, feeling “too high,” and headaches were all greater in treatment group | Fatigue and nausea were higher in treatment group |
| Ellis et al,13 2009 | 1 | 8 | 4 times daily | NR | NR | Combined UKU and DAIDS side effect (concentration difficulties, fatigue, sleepiness or sedation, increased duration of sleep) frequency was greater in cannabis group than in placebo group. There was a trend for moderate or severe AEs to be more frequent during active cannabis than placebo administration | Increases in heart rate by ≥ 30 points were more frequent in cannabis group than placebo group |
| Ware et al,12 2010† | 2.5 | 9.4 | 3 times daily | 1.875 | 7 | Of the total number of neurocognitive events, 15 of 91, 23 of 91, 23 of 91, and 30 of 91 reported AEs for 0%, 2.5%, 6.0%, and 9.4% delta-9-THC, respectively | Of the total number of noncognitive events, 12 of 52, 13 of 52, 14 of 52, 13 of 52 reported AEs at site of administration for 0%, 2.5%, 6.0%, and 9.4% delta-9-THC, respectively; 5 of 28, 5 of 28, 7 of 28, and 7 of 28 for respiratory AEs, respectively; and 9 of 39, 9 of 39, 12 of 39, 9 of 39 for systemic and nonspecific AEs, respectively |
| Wilsey et al,9 2013‡ | 1.29 | 3.53 | 8–12 puffs per session | Unknown | 19.25§ | Feeling “high” or feeling “stoned” was greater in treatment groups and was dose dependent, but effect was relatively small. Feeling “anxiety” or feeling “down” was not prominent. Neuropsychological tests found psychomotor slowing in dominant hand and impaired learning or memory that was dose dependent, while delayed memory was not affected by delta-9-THC use. Effect sizes were generally small across groups | NR |
| Wilsey et al,14 2008 | 3.5 | 7 | 9 puffs per session | 19.25‖ | 34‖ | Feeling “high” scored greatest for the high-dose group (P < .001) and both dose groups differed from placebo group (P < .05). Sedation occurred more in both dose groups compared with placebo group (P < .01). Cannabis produced significantly more confusion than placebo (P = .03). The 7% cannabis demonstrated evidence of neurocognitive impairment in attention, learning and memory, and psychomotor speed, whereas the 3.5% cannabis resulted in a decline in learning and memory only. When looking across at all measures, participants using 7% cannabis had greater impairment than those using 3.5% cannabis, who in turn had greater impairment than placebo participants | NR |
AE—adverse events, DAIDS—Division of AIDS, delta-9-THC—delta-9-tetrahydrocannabinol, NR—not reported, UKU—Udvalg for Kliniske Undersøgelser.
There were 5 participants who withdrew from treatment owing to AEs including uncomfortable “high” (n = 2), dizziness (n = 2), and fatigue (n = 1).
Overall, 248 mild and 6 moderate AEs. Total number of AEs and number of participants reporting at least 1 AE increased with delta-9-THC potency.
While there were neurocognitive symptoms, there were generally small-medium effect sizes and the authors believed that they were not likely to affect daily functioning.
Study authors were unable to comment on dose owing to flexible dosing (maximum assumes participant inhaled all medication in vaporizer).
Based on average cigarette weight.
The 2 trials open to cannabis-naïve participants reported dropouts or withdrawals owing to potential adverse effects of smoked cannabis11,12 such as psychosis (n= 1), persistent cough (n = 1), feeling “high” (n = 2), dizziness (n = 2), and fatigue (n = 1). Causes for the remaining dropouts in the 5 studies were unrelated to delta-9-THC consumption (eg, personal reasons, withdrawal of consent, medical causes unrelated to cannabis).
Efficacy
A meta-analysis of the efficacy of using delta-9-THC could not be completed owing to the heterogeneity of interventions and outcome variables.
All studies reported a statistically significant benefit in terms of pain relief. Ware et al reported a difference of 0.7 in average daily VAS between the placebo group (score of 6.1) and the 9.4% delta-9-THC intervention group (score of 5.4).12 The cigarettes with the lower delta-9-THC potency (2.5% and 6.0%) were associated with more modest reductions in average daily pain scores of 5.9 and 6.0, respectively.12 Wilsey et al reported statistically significant improvement in the cannabis group for pain reduction over time (0.0035 reduction in VAS per minute),14 noting a ceiling effect with equal antinociception between the high (7%) and low (3.5%) delta-9-THC concentrations. A 2013 study also by Wilsey et al reported similar findings, in which vaporized cannabis provided substantial analgesia compared with placebo, while noting that the 1.29% and 3.53% delta-9-THC doses were equianalgesic to one another.9 While there was a statistically significant mean difference in VAS reduction between the delta-9-THC group and the placebo group in the study involving MS patients, the baseline pain level of participants was low, 14.51 (95% CI 9.16 to 21.75) and 16.61 (95% CI 10.79 to 24.93) in the placebo and intervention groups, respectively.11 Clinically meaningful pain reduction was reported in 3 studies,9,13,15 with 46%, 52%, and 61% of cannabis users reporting benefit versus 18%, 24%, and 26% of the placebo group (Ellis et al,13 Abrams et al,15 and Wilsey et al,9 respectively). The effect of medical marijuana on the dose of other analgesic drugs, including opioids, was reported in 1 study, which noted that opioid doses did not differ statistically significantly from baseline.13 Functional outcomes were absent in all studies; however, 2 studies assessed quality of life and both reported no statistically significant improvement.12,13
Adverse events
While there were no serious adverse events reported in any of the trials, smoking cannabis was associated with a greater incidence of adverse events compared with placebo in each of the studies (Table 3).9,11–15
While all trials captured neurocognitive side effects, only 1 trial reported detailed incidence of adverse effects across multiple organ systems (eg, visual symptoms, gastrointestinal, musculoskeletal).12 Adverse neurologic or psychiatric events (eg, headaches, sedation, dysphoria, and poor concentration) increased with cannabis use versus placebo and with higher delta-9-THC concentrations.12 Another study noted statistically significantly (P < .001) increased incidence of sedation, disorientation, confusion, and dizziness in the cannabis group.15 Wilsey et al reported that feeling “high,” “stoned,” and “impaired” scored statistically greater in the cannabis group compared with the placebo group and appeared to be dose dependent.14 On specific neuropsychological tests, the 7% delta-9-THC concentration was associated with impaired attention, learning, memory, and psychomotor speed, while the 3% delta-9-THC concentration resulted in learning and memory decline.14 For patients using lower doses (1.29% and 3.53%) and a vaporizer, similar effects were noted in a dose-dependent manner for feeling “high,” “stoned,” “drunk,” and “sedated”; however, the effect sizes for all psychoactive outcomes were small.9 In the same study, outcomes of neuropsychological testing noted a general cognitive decline (small effect size) with the greatest effect on learning and memory (small to medium effect size). In the study involving patients with MS, 6% of the delta-9-THC group reported feeling “too high” posttreatment as compared with 0% of the placebo group.11 For noncognitive effects, fatigue, throat irritation, and anxiety were noted in a number of studies.11,13
DISCUSSION
This systematic review found that the use of medical marijuana in the management of CNCP of primarily neuropathic origin was associated with a reduction in pain and a number of short-term neurocognitive adverse effects. While most of the trials were of high quality, the psychoactive effect of delta-9-THC versus inactive placebo resulted in unmasking in many trials. Only 2 studies reported maintaining a positive but smaller effect size when correcting for this factor,9,13 consistent with the finding that inappropriate blinding has been shown to cause larger treatment effects.16
While statistical reduction in pain was reported in all studies, a more fundamental outcome is clinically meaningful pain reduction (a decrease of 2 points on a 0-to-10 numerical pain rating or a 30% improvement in pain intensity), which has been associated with an improvement in a patient’s global impression of change.17,18 Only 3 of the 6 trials evaluated and reported positive findings in this respect. Functional assessment has also been designated as a core outcome domain in CNCP trials,17 but its measurement was absent in all included studies. With quality of life unchanged in 2 trials, the question of whether patients experience functional improvement with medical marijuana remains unanswered. Finally, there was a notable absence of effectiveness trials comparing outcomes with other known treatments in CNCP. Most studies, in fact, employed medical marijuana as an adjunct to participants’ existing opioids and adjuvant medications suggesting it might only have a role in refractory pain in conjunction with other analgesics.
The trials in our review reported short-term psychoactive and neuropsychological effects without evidence of serious adverse effects, measured over hours or days. Of note, one study specifically commented that the small to medium effect sizes of cognitive effects were unlikely to affect daily functioning.9 These cognitive adverse effects in the short term are similar to those experienced with opioids19 and suggest that the same precautions employed with opioids would be in order with the use of medical marijuana. In particular, its use in elderly patients or those with pre-existing cognitive impairments might not be ideal. These short-term findings contrast with a recent review of observational data collected over years reporting several high-confidence-level adverse effects (eg, addiction, diminished life achievement, and motor vehicle accidents).20Analogous to trials of opioids, medical marijuana trials, including those in our review, have been of short duration and not designed to detect longer-term sequelae.21
Finally, the amount of exposure to delta-9-THC in all studies was extremely low in contrast to that available in the marketplace. According to Health Canada’s website, the average amount of dried marijuana dispensed under the old MMAR was 1.0 to 3.0 g per day containing delta-9-THC concentrations of 12.5%.22 With an average dry weight of only 2.0 g per day, the available delta-9-THC exposure under the old MMAR program was 250 mg, or nearly 8-fold the maximum amount used in clinical trials. Now, under the newer regulations (Marihuana for Medical Purposes Regulation), industry producers can provide even higher delta-9-THC concentrations (up to 20% delta-9-THC by dried weight as shown on industry websites), suggesting a potential gap between evidence and product offerings.
Comparison with previous systematic reviews
Previous systematic reviews have assessed the available evidence for the use of cannabinoids in chronic pain23,24; however, none commented systematically on the level of delta-9-THC consumption. The review by Martín-Sánchez and colleagues assessed the use of cannabinoids in chronic pain of any cause, with a third of the trials focused on cancer pain and interventions restricted to synthetic cannabinoids only.23 The authors commented on a positive, moderate, short-term trend toward pain reduction but noted serious adverse effects.
The Lynch and Campbell review on cannabinoids in CNCP included oral or smoked synthetic and natural cannabinoids.24 The authors included 4 trials contained in our review.12–15 While they opined that larger trials were necessary with additional reporting requirements, they concluded that there was support for the use of cannabinoids in CNCP to provide modestly effective and safe treatment.24
Conclusion
The current evidence suggests that very low-dose medical marijuana (< 34 mg/d) is associated with an improvement in refractory neuropathic pain of moderate severity in adults using concurrent analgesics. There were no studies evaluating other CNCP causes including rheumatologic conditions.25 The generalizability of the results in CNCP is limited by factors such as the quality of studies, small sample sizes, very short duration, and dose and scheduling variability. Neurocognitive adverse effects such as learning, memory, and psychomotor deficits are common even with low-dose, short-term use but they appear well tolerated. However, the longer-term consequences of medical marijuana still remain unknown. These findings are consistent with existing guidance documents.10 Future trials should consider incorporation of standard outcome measures beyond pain, such as function and quality of life, similar to other interventions in CNCP.26 It might also be advantageous to enable prospective observational studies through creation of registries, protocols, and mandatory reporting of adverse events. Without additional evidence and a clear understanding as to the indications for and dosing of cannabis, there remains a risk that clinicians might unwittingly propagate similar issues that we now face with opioids in the management of CNCP.
EDITOR’S KEY POINTS
Medical marijuana has been proposed as a potential treatment for use in pain management. However, there is still uncertainty about the specific indications, ideal doses, and adverse effects that are related to this substance when used for medical purposes.
While statistical reduction in pain was reported in all studies in this review, a more fundamental outcome is clinically meaningful pain reduction (a decrease of 2 points on a 0-to-10 numerical pain rating or a 30% improvement in pain intensity); only 3 of the 6 studies reported positive findings in this respect. Most of the studies employed medical marijuana as an adjunct to participants’ existing opioids and adjuvant medications, suggesting it might only have a role in refractory pain in conjunction with other analgesics.
Neurocognitive adverse effects such as learning, memory, and psychomotor deficits are common even with low-dose, short-term use of medical marijuana but they appear well tolerated. However, the long-term consequences of medical marijuana remain unknown.
POINTS DE REPÈRE DU RÉDACTEUR
On a proposé d’utiliser la marijuana médicale comme traitement potentiel de la douleur. Toutefois, on connaît encore mal les indications spécifiques, le dosage idéal et les effets indésirables de cette substance lorsqu’on l’utilise à des fins médicales.
Même si toutes les études de cette revue rapportent une réduction significative de la douleur, une réduction cliniquement significative de la douleur (c.-à-d. une diminution de 2 points sur une échelle de la douleur entre 0 et 10 ou une amélioration de 30 % de l’intensité de la douleur) serait une issue plus fondamentale; seulement 3 des 6 études ont rapporté des résultats positifs de cette nature. Dans la plupart des études, on utilisait la marijuana médicale comme complément aux opiacées existants ou à des médications adjuvantes, ce qui suggère qu’elle pourrait n’être utile qu’en association avec d’autres analgésiques.
Des effets indésirables d’ordre neurocognitif tels que des troubles d’apprentissage, de mémoire ou de psychomotricité sont fréquents, même avec des doses faibles et pour de courtes périodes, mais ces effets semblent bien tolérés. Toutefois, les conséquences à long terme de la marijuana médicale demeurent inconnues.
Footnotes
Cet article a fait l’objet d’une révision par des pairs.
This article has been peer reviewed.
Contributors
All authors contributed to the concept and design of the study and data gathering. Ms Lakha, Dr Zoheiry, and Dr Deshpande contributed to the analysis of the study. Dr Deshpande and Dr Mailis-Gagnon contributed to the interpretation of the study and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377(9784):2226–35. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 2.Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;(8):CD004959. doi: 10.1002/14651858.CD004959.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalant H. Medical use of cannabis: history and current status. Pain Res Manag. 2001;6(2):80–91. doi: 10.1155/2001/469629. [DOI] [PubMed] [Google Scholar]
- 4.Joy JE, Watson SJ Jr, Benson JA Jr, editors. Marijuana and medicine. Assessing the science base. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 5.Health Canada [website]. Medical use of marijuana. Ottawa, ON: Health Canada; 2015. Available from: www.hc-sc.gc.ca/dhp-mps/marihuana/index-eng.php. Accessed 2015 Jul 10. [Google Scholar]
- 6.Collège des médecins du Québec. Guidelines concerning the prescription of dried cannabis for medical purposes. Montreal, QC: Collège des médecins du Québec; 2014. Available from: www.cmq.org/en/MedecinsMembres/DossierMembreFormulaires/~/media/Files/Cannabis/Guidelines-prescription-cannabis.pdf?111402. Accessed 2015 Jul 10. [Google Scholar]
- 7.Health Canada [website]. Adverse reaction information. Ottawa, ON: Health Canada; 2012. Available from: www.hc-sc.gc.ca/dhp-mps/medeff/advers-react-neg/index-eng.php. Accessed 2015 Jul 10. [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 9.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136–48. doi: 10.1016/j.jpain.2012.10.009. Epub 2012 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.College of Family Physicians of Canada. Authorizing dried cannabis for chronic pain or anxiety. Preliminary guidance. Mississauga, ON: College of Family Physicians of Canada; 2014. [Google Scholar]
- 11.Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143–50. doi: 10.1503/cmaj.110837. Epub 2012 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694–701. doi: 10.1503/cmaj.091414. Epub 2010 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):672–80. doi: 10.1038/npp.2008.120. Epub 2008 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506–21. doi: 10.1016/j.jpain.2007.12.010. Epub 2008 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):515–21. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 16.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. Epub 2007 Dec 11. [DOI] [PubMed] [Google Scholar]
- 18.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 19.Højsted J, Kurita GP, Kendall S, Lundorff L, de Mattos Pimenta CA, Sjøgren P. Non-analgesic effects of opioids: the cognitive effects of opioids in chronic pain of malignant and non-malignant origin. An update. Curr Pharm Des. 2012;18(37):6116–22. doi: 10.2174/138161212803582522. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7(5):R1046–51. doi: 10.1186/ar1782. Epub 2005 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health Canada. Daily amount and dosing information sheet. Cannabis (marijuana, marihuana). Ottawa, ON: Health Canada; 2014. Available from: www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/marihuana/med/daily-quotidienne-eng.pdf. Accessed 2015 Jul 10. [Google Scholar]
- 23.Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10(8):1353–68. doi: 10.1111/j.1526-4637.2009.00703.x. Epub 2009 Sep 1. [DOI] [PubMed] [Google Scholar]
- 24.Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–44. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzcharles MA, Clauw DJ, Ste-Marie PA, Shir Y. The dilemma of medical marijuana use by rheumatology patients. Arthritis Care Res (Hoboken) 2014;66(6):797–801. doi: 10.1002/acr.22267. [DOI] [PubMed] [Google Scholar]
- 26.Chapman JR, Norvell DC, Hermsmeyer JT, Bransford RJ, DeVine J, McGirt MJ, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine (Phila Pa 1976) 2011;36(Suppl 21):S54–68. doi: 10.1097/BRS.0b013e31822ef74d. [DOI] [PubMed] [Google Scholar]