Abstract
After a complete spinal cord injury, sea lampreys at first are paralyzed below the level of transection. However, they recover locomotion after several weeks, and this is accompanied by short distance regeneration (a few mm) of propriospinal axons and spinal-projecting axons from the brainstem. Among the 36 large identifiable spinal-projecting neurons, some are good regenerators and others are bad regenerators. These neurons can most easily be identified in wholemount CNS preparations. In order to understand the neuron-intrinsic mechanisms that favor or inhibit axon regeneration after injury in the vertebrates CNS, we determine differences in gene expression between the good and bad regenerators, and how expression is influenced by spinal cord transection. This paper illustrates the techniques for housing larval and recently transformed adult sea lampreys in fresh water tanks, producing complete spinal cord transections under microscopic vision, and preparing brain and spinal cord wholemounts for in situ hybridization. Briefly, animals are kept at 16 °C and anesthetized in 1% Benzocaine in lamprey Ringer. The spinal cord is transected with iridectomy scissors via a dorsal approach and the animal is allowed to recover in fresh water tanks at 23 °C. For in situ hybridization, animals are reanesthetized and the brain and cord removed via a dorsal approach.
Keywords: Neuroscience, Issue 92, spinal cord injury, axonal guidance molecules, neurofilaments, regeneration
Introduction
In mammals spinal cord injury (SCI) is a devastating condition that leads to permanent loss of function below the site of injury because injured axons do not regenerate through the trauma zone and reconnect to their appropriate targets. In contrast to mammals, lampreys recover locomotion after a complete spinal cord injury.1 Interestingly, lampreys have a set of 36 spinal cord projecting neurons that are individually identifiable in whole-mount brain preparations because of their big size2,3 (Figure 1). All of these spinal-projecting neurons are axotomized by a high-level complete spinal cord transection. Previous studies of our group and others have shown that even in the presence of functional recovery after SCI some of these neurons show a very low regenerative capacity (they are considered “bad regenerators”), while others usually regenerate their axon through the site of injury (they are considered “good regenerators”).2,3 This characteristic makes lampreys an interesting vertebrate model to study the differences in gene expression between good and bad regenerator spinal-projecting neurons that in turn will lead to the differences in the intrinsic regenerative ability of neurons that attempt to regenerate their axons in the same extrinsic environment.1
Using this model we have previously shown that spinal-projecting neurons with low regenerative ability show expression of axonal guidance molecule receptors like UNC54,5 and neogenin,6 which mediate the inhibitory action of netrin and RGM respectively. In addition, by using this method our group has also shown that only the good regenerators show a recovery of the expression of neurofilaments after the injury and during the regeneration process. Recently, Busch and Morgan7 have shown by immunofluorescence that the bad regenerators show an increased expression of synuclein after injury, which has been related by the authors to the fact that the “bad regenerator” spinal-projecting neurons slowly die after a complete spinal cord transection5,7,8. So, the lamprey model of a complete spinal cord injury has emerged as a very useful model to understand what makes a spinal cord projecting neuron a “bad regenerator” after axotomy.
To conduct our studies we are performing a complete spinal cord transection surgery protocol and a posterior brain dissection at the desired time points after injury to perform wholemount in situ hybridization. In the present methodological article we present a detailed protocol for the proper performance of a complete spinal cord injury surgery in larval lampreys, the subsequent maintenance of the animals and the final brain dissection and preparation of the brain for a wholemount in situ hybridization. A detailed protocol to perform the wholemount in situ hybridization in the brain of larval lampreys has been previously reported.9 In addition, this protocol for spinal cord injury and brain dissection can be also used to then process the brains for immunohistochemistry or other histological methods.
Protocol
See Table 1 for all materials used in this protocol.
Experiments were approved by the Institutional Animal Care and Use Committee at Temple University.
1. Animals
Obtain wildtype larval sea lampreys (Petromyzon marinus L.), 10 – 14 cm in length (4 – 7 years old) from streams feeding Lake Michigan, from tributaries to the Delaware River (Pennsylvania) or streams in Maine.
In the laboratory, maintain larvae in groups of 50 - 100 animals in 50 gallon freshwater tanks at 16 °C. Line the tanks with an inch of gravel for the larvae to burrow in, and the water is charcoal filtered and conditioned with air stones for at least 48 hr to remove chlorine and other impurities before placing the lampreys in them. There is no need to feed the animals, which recycle their own detritus, up to the day of use.
2. Complete Spinal Cord Transection
Before the spinal cord surgery prepare a lamprey Ringer solution with the following composition: 110 mM NaCl, 2.1 mM KCl, 2.6 mM CaCl2, 1.8 mM MgCl2 and 10 mM Tris buffer; pH 7.4.
To perform the spinal cord transection, place the larvae in a small container to be anesthetized with 1% tricaine methanesulfonate in ice cold Ringer solution.
Once anesthetized place the animals in a petri dish half filled with Sylgard (silicone) and use 4 insect pins (0.15 mm diameter) to hold the animals in position for the surgery (one is placed at the snout of the animal, one at the tail and two at the level of the 5th gill). Fill the petri dish almost up to the top with Ringer solution. Put the dish with the larva on ice while performing the surgery under the stereomicroscope.
To perform the spinal cord transection a dorsal, midline, make a longitudinal incision with a scalpel (#11) at the level of the 5th gill to visualize the spinal cord. Initially the skin is open by inserting the tip of the scalpel blade facing up and then the muscle tissue is cut very gently until the spinal cord can be seen from above. Fashion two hooks from the insect pins and use them to hold the body walls open while performing the spinal cord transection.
Completely transect the spinal cord at the level of the 5th gill by using a pair of scissors (Castroviejo #8), insert the scissors perpendicularly to the spinal cord. The spinal cord has to be completely transected with one clean cut. Visualize the spinal cord cut ends under the stereomicroscope to confirm that the spinal cord has been completely transected. Then, realign the cut ends of the spinal cord after the transection by using a pair of forceps (#5).
After performing the spinal cord transection close the wound in the dorsal body wall with a pair of fine forceps and put the larvae on ice for 1 hr to allow the wound to dry. Put a piece of filter paper between the ice and the larvae and soak it with lamprey Ringer solution. The low temperature keeps the animals alive at the same time that it allows the wound to air dry and therefore helping to prevent infections.
Then transfer the larvae to small tanks (1 larva per tank) with ice cold water for 24 hr.
Examine the lesioned larvae 1 day after the spinal transection to behaviorally confirm that there is no movement caudal to the site of injury. A spinal cord transection is considered complete if the larva can move only its head rostral to the site of injury (at the level of the 5th gill). If this is the case the larvae can now be transferred to tanks (1 larva per tank) with freshwater at room temperature (23°C) to allow them to recover for the chosen time points.
During the recovery period change the water every 2 to 3 days.
3. Brain Dissection and Preparation of the Brain for in situ Hybridization
At the desired time points after the injury re-anesthetize the larval sea lampreys and place and pin them in the petri dish with Ringer solution to perform the brain dissection as above.
To dissect the brain out, first make a transverse incision with the scalpel between the nostril and the pineal organ. From here begin to cut the tissue surrounding the brain by using a pair of Castroviejo scissors while holding it with a pair of forceps.
Once the brain is exposed, remove the choroid plexus by using a pair of forceps and use the hooks to spread the head of the animal and expose the cranial nerves. Then, cut the spinal cord transversally with the Castroviejo scissors. Hold the spinal cord with a pair of forceps while using another pair of forceps to cut the cranial nerves to dissect the brain out.
After dissection, put the brain on a small piece of silicone and hold it in position by inserting 2 insect pins in the olfactory bulbs and 1 in the spinal cord. Then, cut the posterior and cerebrotectal commissures of the brain along the dorsal midline with the scissors and deflect the alar plates laterally and pin them flat to the silicone with 4 insect pins (Figure 2).
Transfer the piece of silicone with the brain to a tube containing 4% paraformaldehyde in phosphate buffered saline (pH 7.4) to be fixed at room temparature for 3 hr on a rotator.
After fixation use the brain for wholemount in situ hybridization as previously described (Swain et al., 1994). Brains from control or sham operated animals should be run always in parallel with the brains from injured animals. The wholemounted larval brain can be studied by in situ hybridization because of its flat and thin shape and also due to the absence of myelin10, which makes the brain fairly translucent.
Representative Results
As an example of the results that can be obtained when using this method, representative images of wholemounted brains showing the expression of the neogenin transcripts in identifiable spinal cord-projecting neurons of control and 2 weeks post lesion larval sea lampreys are shown in Figure 2. The readers are referred to a previous study6 reporting the relationship between the expression of neogenin after a complete spinal cord transection and the regenerative ability of the identifiable spinal cord projecting neurons of the sea lamprey for full details. Briefly, results have shown that after the injury there are changes in the expression of the neogenin receptor in identifiable spinal-projecting neurons. Two weeks after a complete spinal cord transection the neogenin receptor is preferentially expressed in bad regenerators, like the M1, I1, I2, I4, or Mth neurons (see Figure 2B) These results demonstrate the outcome of the protocol and suggest that the neogenin receptor could be involved in the failure of the regeneration of these cells.
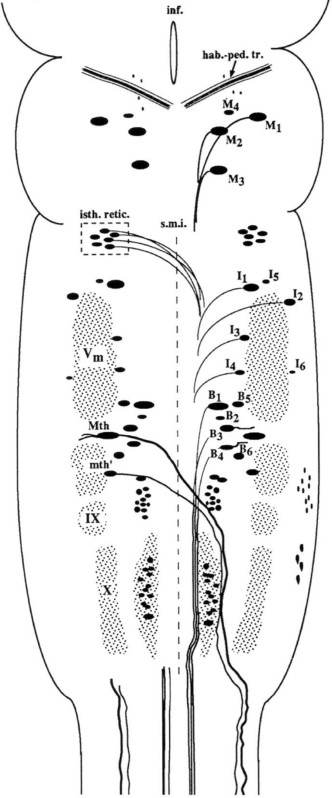 Figure 1: Schematic drawing of a dorsal view of the sea lamprey brain showing the location of identifiable reticulospinal neurons. Reproduced from Barreiro-Iglesias and Shifman.5 For abbreviations, see list below.
Figure 1: Schematic drawing of a dorsal view of the sea lamprey brain showing the location of identifiable reticulospinal neurons. Reproduced from Barreiro-Iglesias and Shifman.5 For abbreviations, see list below.
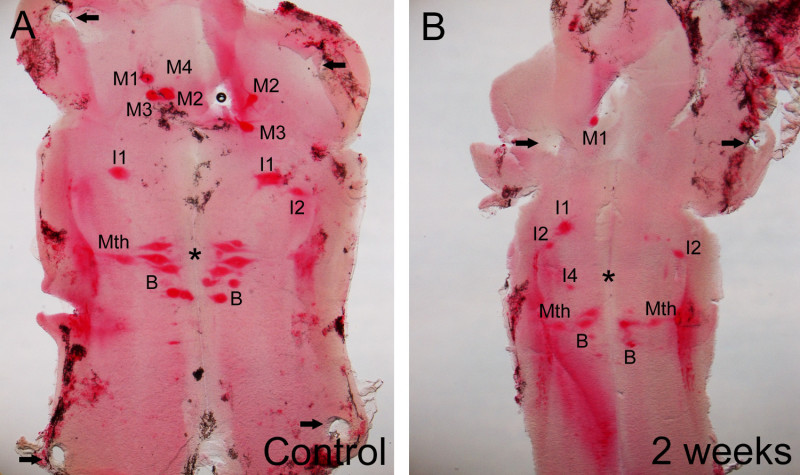 Figure 2. Photomicrographs of dorsal views of the mature larval sea lamprey brain showing the expression of the neogenin transcript in control (A) and 2 weeks post-injury (B) animals. Note in B that after the injury the neogenin transcript is preferentially expressed in neurons known to be bad regenerators (e.g., M1, I1, I2, I4 and Mth neurons). Also, note the presence of the holes left by the pins used to hold the brain (arrows). The readers are referred to the previous study by Shifman and coworkers.6 For abbreviations, see list below.
Figure 2. Photomicrographs of dorsal views of the mature larval sea lamprey brain showing the expression of the neogenin transcript in control (A) and 2 weeks post-injury (B) animals. Note in B that after the injury the neogenin transcript is preferentially expressed in neurons known to be bad regenerators (e.g., M1, I1, I2, I4 and Mth neurons). Also, note the presence of the holes left by the pins used to hold the brain (arrows). The readers are referred to the previous study by Shifman and coworkers.6 For abbreviations, see list below.
ABBREVIATIONS
| B (B1-B6) | Müller cells of the bulbar region (middle rhombencephalic reticular nucleus) |
| hab.-ped. tr. | habenulopeduncular tract |
| I1-I6 | Müller cells of the isthmic region |
| IX | glossopharyngeal motor nucleus |
| inf. | infundibulum |
| isth. retic. | isthmic reticular formation |
| M1-M4 | Müller cells 1 to 4 |
| Mth | Mauthner cell |
| mth’ | auxiliary Mauthner cell |
| s.m.i. | sulcus medianus inferior |
| Vm | trigeminal motor nucleus |
| X | vagal motor nucleus |
Discussion
Here we present a detailed protocol to perform a complete spinal cord transection and posterior brain dissection in larval sea lampreys. This procedure allows analyzing differences in gene expression between identifiable spinal cord projecting neurons after spinal cord injury by means of a whole-mount brain in situ hybridization. The critical step in the procedure is the correct performance of a complete spinal cord transection, which can be controlled by observing the cut ends of the spinal cord under the stereomicrocope and confirmed 24 hr later by gently touching the snout of the larva with a pair of forceps to see if there is no movement caudal to the site of injury. It is also important for the recovery of the animal that the scissors used for the surgery are not blunt and that a single and clean cut is done to completely transect the spinal cord.
The survival of the animals after the injury is highly improved by keeping the animals on ice during the surgery and during the hour used to allow the wound to air dry and then in ice cold water for the first 24 hr. After this period the wound is closed and the animals can already be transferred to tanks with freshwater at room temperature.
Larval lampreys are allowed to recover at room temperature and not at their usual cold temperature because a greater majority of the animals recover full locomotor behavioral function after the injury at higher temperatures.11 The recovery of a normal pattern of locomotion is observed 8 to 10 weeks after the complete spinal cord transection.
After performing the complete spinal cord transection and the brain dissection at the chosen time points the larval brains can be processed not only to perform a whole-mount in situ hybridization as shown here (Figure 2), but also for other histological methods like immunohistochemistry (e.g. 7), the detection of neuronal tracers that can be applied at the time of transection at the spinal cord injury site (e.g. 8) or the detection of activated caspases by using fluorochrome labeled caspase inhibitors.5
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
Supported by NIH Grants NS14837, R01 NS38537, R24 HD050838 to Dr. Michael E. Selzer; Shriners Research Grant SHC-85220 to Dr. Michael E Selzer; and Shriners Research Grant SHC-85310 to Dr. Michael I. Shifman. Dr. Antón Barreiro-Iglesias was supported by the Fundación Barrié (Spain) and the Xunta de Galicia (Galicia, Spain).
References
- Rodicio MC, Barreiro-Iglesias A. Lampreys as an animal model in regeneration studies after spinal cord injury. Rev Neurol. 2012;55:157–166. [PubMed] [Google Scholar]
- Davis GR, Jr, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinalcord-transected lamprey. J Comp Neurol. 1994;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci. 1997;17:5206–5220. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Expression of the netrin receptor UNC-5 in lamprey brain modulation by spinal cord transection. Neurorehabil Neural Repair. 2000;14:49–58. doi: 10.1177/154596830001400106. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Laramore C, Shifman MI. The sea lamprey UNC5 receptors cDNA cloning, phylogenetic analysis and expression in reticulospinal neurons at larval and adult stages of development. J Comp Neurol. 2012;520:4141–4156. doi: 10.1002/cne.23143. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Yumu lRE, Laramore C, Selzer ME. Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey. Exp Neurol. 2009;217:242–251. doi: 10.1016/j.expneurol.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DJ, Morgan JR. Synuclein accumulation is associated with cell-specific neuronal death after spinal cord injury. J Comp Neurol. 2012;520:1751–1771. doi: 10.1002/cne.23011. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J Comp Neurol. 2008;510:269–282. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- Swain GP, Jacobs AJ, Frei E, Selzer ME. A method for in situ hybridization in wholemounted lamprey brain neurofilament expression in larvae and adults. Exp. Neurol. 1994;126:256–269. doi: 10.1006/exnr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Kiemel T, Pate V, Blinder J, Guan L. Temperature can alter the function outcome of spinal cord regeneration in larval lampreys. Neuroscience. 1999;90:957–965. doi: 10.1016/s0306-4522(98)00502-8. [DOI] [PubMed] [Google Scholar]


