Abstract
Purpose.
To investigate effects of aging on endothelial nitric oxide synthase (eNOS) expression and signaling in angular aqueous plexus (AAP) (functional equivalent to human Schlemm's canal) cells subjected to shear stress.
Methods.
The AAP cells were isolated differentially from porcine outflow tissues using puromycin selection. Cell aging was induced by culturing cells in hyperoxia condition (40% oxygen and 5% carbon dioxide) for 14 days. The AAP cells grown in chamber slides were exposed to a shear stress of 8 dynes/cm2 for 24 hours. Expression of eNOS, eNOS-phospho Thr495, eNOS-phospho Ser1177, and Akt-phospho was tested by Western blot analysis and immunofluorescence staining. Nitric oxide (NO) levels were measured using the Griess assay.
Results.
Compared with control, eNOS levels in aged cells were significantly reduced by 60% (P < 0.05; n = 6). Phosphorylation of eNOS at Ser1177 and Akt at Ser473 was 63% and 80% lower in aged cells, respectively, whereas phosphorylation of the eNOS inhibition site (Thr495) increased by 6.1-fold (P < 0.05; n = 6). Shear stress (8 dynes/cm2 for 24 hours) increased eNOS abundance (total protein and at cell borders) and phosphorylation at Ser1177 by 1.7-fold and 1.8-fold, respectively (P < 0.05; n = 6), whereas aged cells were unresponsive. In control cells exposed to shear stress, the NO concentration was 1.8-fold higher than in the static group (P < 0.05; n = 4); however, aged cells were unresponsive to shear stress (mean ± SD, 4.3 ± 1.3 vs. 4.1 ± 1.4 μM).
Conclusions.
Aged AAP cells appear compromised in their mechanotransduction machinery involving eNOS, the protein product of the gene, NOS3, polymorphisms of which impart a risk for the development of glaucoma.
Keywords: aging, Schlemm's canal, eNOS
Aged AAP cells appear compromised in their mechanotransduction machinery involving eNOS, the protein product of the NOS3 gene, polymorphisms of which impart a risk for the development of glaucoma. Aging compromises endothelial nitric oxide synthase–related mechanotransduction in porcine angular aqueous plexus cells.
Introduction
Glaucoma comprises a group of complex and heterogeneous blinding diseases, affecting almost 60 million people worldwide.1 Clinically, glaucoma is characterized by cupping of the optic nerve head and visual field loss. Elevated IOP is the primary risk factor for glaucomatous optic nerve damage, and reducing pressure remains the only treatable end point that slows or stops disease progression. The risk of developing POAG also clearly increases with age.2–8 Primarily, IOP is determined by changes in aqueous humor outflow resistance, which is thought to reside mainly in the juxtacanalicular region, where the trabecular meshwork (TM) and the inner wall of Schlemm's canal (SC) cells interact.9,10 The endothelial cells of the inner wall of SC are important in regulating outflow resistance and thus IOP.11,12
Nitric oxide (NO) regulation appears to have a role in POAG. Family association studies13–15 show a relationship between NOS3 gene polymorphisms, which encode for endothelial NO synthase (eNOS), and POAG. These recent findings are consistent with previous work showing decreased nicotinamide adenine dinucleotide phosphate diaphorase staining, localizing NOS activity in outflow tissues from POAG patients compared with age-matched controls.16–18 Nitric oxide synthases are enzymes that catalyze the conversion of l-arginine to l-citrulline and NO production. Analysis of the aqueous humor from POAG patients showed that the production of NO was also reduced compared with that from control patients.19 The reduced NO in the plasma of POAG patients suggested that it might also alter ocular perfusion pressure, impacting retinal cells, including ganglion cells.20
Nitric oxide appears to control outflow resistance. In human eyes, NOS reactivity was enriched in major sites of outflow resistance, including the TM and SC, as well as in collecting channels.16 The NO-donating compounds effectively decrease conventional outflow resistance, leading to a decrease in IOP and outflow rate in different animal models, including rabbits,21–24 pigs,25 dogs,22 and monkeys,22,26 and in perfused postmortem human eyes.27,28 Our previous study29 further showed that eNOS/NO is a key regulator of outflow facility and IOP in mice. Notably, staining of the mouse eye in cross-section revealed that eNOS expression appeared limited to SC (Stamer WD, unpublished data, 2014). This result is interesting because NO regulates endothelial permeability by assembling and disassembling intercellular junctions,30,31 suggesting a role for NO at the level of the inner wall of SC.
Despite the relative slow flow rate of aqueous humor in the eye, the shear stress in SC was calculated to be in the range of 2 to 20 dynes/cm2 at elevated IOP, which overlaps that of shear stress in large arteries (2–25 dynes/cm2).32 The high shear that SC cells may experience suggests that there could be some underlying mechanisms that regulate SC vessel tone to maintain IOP homeostasis. Consistent with this idea, eNOS expression in vascular endothelia and SC was shown to be shear sensitive.33,34 However, the impact of aging on SC cell responses to shear stress has not been studied to date.
Age is a strong risk factor for glaucoma. The risk of developing glaucoma positively correlates with age,6,35–37 and it is a disease of early cell senescence.38 The pressure-dependent outflow resistance increases with age in a linear fashion and begins at a fairly young age.39 At the cellular level, we previously showed that aged porcine angular aqueous plexus (AAP) cells (functional equivalent to human SC endothelial cells) had decreased cell monolayer permeability and were less responsive to elevated pressure gradients.40 We used an established model of chronic oxidative stress to model aging. This is based on one of the most accepted theories of aging known as stress-induced premature senescence.41 It is well known that during cell metabolism oxidizing species are continuously being produced. A buildup of these oxidizing species may consequently cause molecular damage in fibroblast and neuroblastoma cells,41–44 as well as in trabecular/SC endothelial cells, which led to elevation in outflow resistance.45–47 This is a preferred model because it exposed cells to a constant increase in reactive oxygen species without adding chemical or cellular treatments.42–44,48
Endothelial NOS is highly expressed in endothelia, including SC cells, and these cells are sensitive to changes in shear stress. Therefore, we examined whether aging would impact eNOS-related cell mechanotransduction responses to shear stress.
Methods
Cell Culture
The AAP endothelial cells were isolated, characterized, and cultured according to an established method reported by our group.49 For each strain of cells, the conventional outflow tissue from six porcine eyes (4- to 6-month-old pigs) was pooled to generate primary mixed cultures. After collagenase I (1 mg/mL; Sigma-Aldrich Corp., Shanghai, China) digestion, cells were allowed to multiply for 8 days and then were treated with puromycin (4 μg/mL; InvivoGen, San Diego, CA, USA) for 2 days. The cultures were washed free of dead cells and permitted to recover, with the puromycin-resistant cells left to populate the flask. For the experiments, control and aged cells were either cultured in static conditions or exposed to shear stress.
Aging was induced by subjecting cells to normobaric hyperoxia condition (40% oxygen and 5% carbon dioxide) for 14 days in a triple-gas incubator (Smart Cell, Shanghai, China) following an established method.47,50 Control cultures were grown under physiological oxygen conditions (5% oxygen and 5% carbon dioxide) for 14 days in parallel with the experimental group.
Shear Stress
The AAP cells were seeded at confluence into flow chamber slides (μ-Slide I 0.4, cell culture treated; ibidi GmbH, Martinsried, Germany) and cultured until monolayers were stable (typically 3 days). Shear stress was induced by perfusing cells in chamber slides using a pump system (ibidi; ibidi GmbH). Control cells were cultured on the same slides but in static conditions. For the protein expression experiments, shear was set at 8 dynes/cm2 for 24 hours; for the cell alignment experiments, a shear stress of 5 dynes/cm2 was applied continuously for 2 days.
Indirect NO Measurements
Soluble NO (S-NO) concentration was measured indirectly by the Griess method using a nitrate/nitrite assay kit (Sigma; Sigma-Aldrich Corp.) according to the manufacturer's instructions. Briefly, cell culture medium was collected and centrifuged (1000g for 15 minutes), and the supernatant was collected for S-NO analysis. Eighty microliters of sample solution was added to a single well in a 96-well plate, followed by the addition of 20 μL buffer solution and 50 μL Griess reagent A in the kit. After 5 minutes, 50 μL Griess reagent B was added. The mixture was incubated in the dark for 15 minutes before the nitrite content was measured photometrically at 540 nm using a multifunction plate reader (Synergy; BioTek, Beijing, China). The absorbance of the blank solution was subtracted from the absorbance of each well. The sample S-NO concentration was calculated from a standard calibration curve. Six slides were used per cell strain, and four cell strains were used.
Western Blot
Cell lysates were prepared using ristocetin-induced platelet agglutination solution; 50 μg protein was loaded into each lane of gel, and proteins were separated by SDS-PAGE (10% or 12.5% acrylamide). The resolved proteins were transferred by electrophoresis to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 for 2 hours. Membranes were then probed with antibodies that specifically recognize eNOS (1:400; Abcam, Shanghai, China), eNOS-phospho Thr495 (eNOS-P Thr495, 1:1000; Cell Signaling, Shanghai, China), eNOS-phospho Ser1177 (eNOS-P Ser1177, 1:1000; Cell Signaling), or Akt-phospho Ser1177 (Akt-P Ser 1177, 1:2000; Cell Signaling), followed by incubation with peroxidase-linked secondary antibodies. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Signals in the linear range of the x-ray film were captured digitally, and densitometry was performed using molecular imaging software (Carestream, Kodak, Shinkawa, Japan).
Immunofluorescence Microscopy
Cells cultured in μ-Slides (ibidi GmbH) were fixed with 4% formaldehyde overnight and then washed three times with PBS. Nonspecific binding sites on cells were blocked with goat serum for 1 hour at room temperature and permeabilized with 0.2% Triton X-100 for 5 minutes. Cells were then incubated with polyclonal antibodies against eNOS generated in rabbits (1:400; Abcam) overnight at 4°C. After three washes with PBS, cells were incubated in anti-rabbit secondary antibodies (AlexaFluor 488, goat anti-rabbit; Invitrogen, Shanghai, China) and Hoechst 33258 solution (0.5 μg/mL; Sigma-Aldrich Corp.).
Statistical Analysis
Data distribution normality was first tested, and the protein density ratio (Western blot) and NO concentration data were analyzed by the Mann-Whitney U test (using SPSS 17 for Windows; IBM-SPSS, Chicago, IL, USA). In all cases, differences were considered significant at P < 0.05.
Results
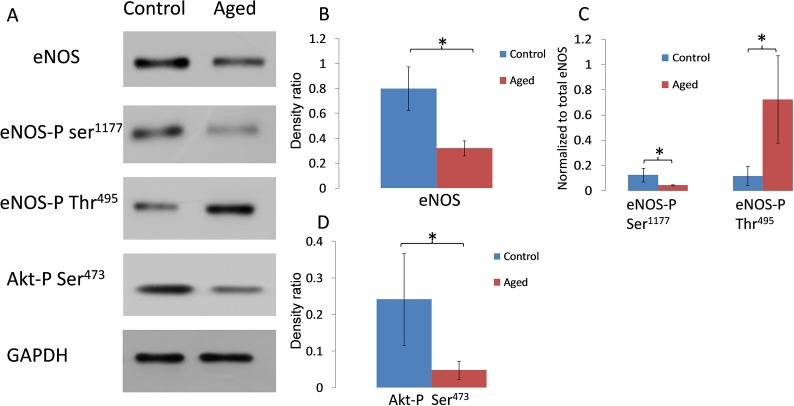
Using AAP cells exposed to hyperoxia as a model for aging, we first investigated how eNOS and its signaling partner Akt are affected (Fig. 1). Western blot analysis showed that eNOS protein levels were reduced in aged cells by 60% compared with normal cells (P < 0.05; n = 6). Since this protein could be regulated by phosphorylation at multiple sites, the most important sites were analyzed. Results demonstrated that eNOS phosphorylation at its activation site Ser1177 was downregulated by 63% (P < 0.05; n = 6), whereas the inhibition site Thr495 was upregulated by 6.1-fold (P < 0.05; n = 6). We also assessed phosphorylation status of the protein kinase Akt, which is known to activate eNOS through Ser1177 phosphorylation. Results showed that Akt-phospho Ser473 was significantly diminished by 80% (P < 0.05; n = 6).
Figure 1.
Western blot analysis of eNOS, eNOS-phospho Ser1177, eNOS-phospho Thr495, and Akt-phospho levels in cultured AAP cells. (A) Representative Western blots showing a comparison of protein/phosphorylation levels in AAP cell monolayers cultured under normal or hyperoxic conditions for 2 weeks (aged). (B–D) Densitometric analyses of Western blots for eNOS-phospho Ser1177 (normalized to total eNOS), eNOS-phospho Thr495 (normalized to total eNOS), or Akt-phospho (normalized to glyceraldehyde 3-phosphate dehydrogenase) from control and aged cells. *P < 0.05; n = 6. Error bar denotes SD.
Next, we explored if aging impacts the mechanotransduction machinery in AAP cell monolayers. We exposed AAP cells to 8 dynes/cm2 for 24 hours and monitored effects on the protein expression of eNOS (Fig. 2) and phosphorylation at Ser1177 (Fig. 3). In control cells exposed to shear stress, we noted significantly increased expression of eNOS by 1.7-fold (P < 0.05; n = 6). In contrast, the level of eNOS in aged cells was similar in static and shear stress conditions. Likewise, there was increased phosphorylation at the eNOS activation site (Ser1177) in cells exposed to shear stress compared with static cultures by 1.8-fold (P < 0.05; n = 6), a result not reproduced in aged cells (Fig. 3).
Figure 2.
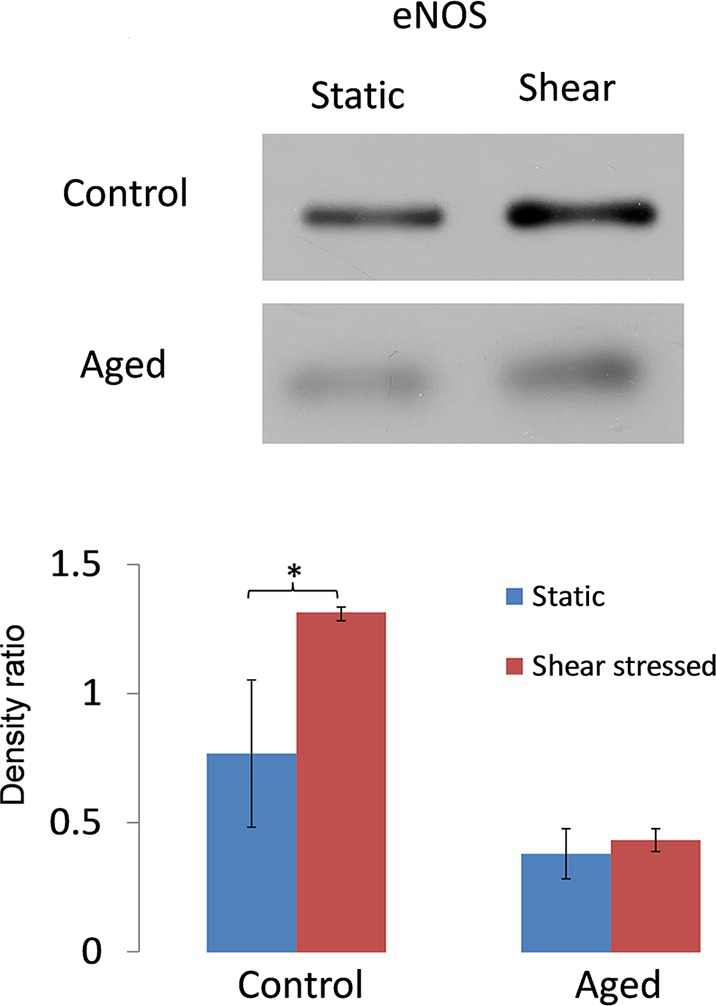
Effect of shear stress on eNOS expression by AAP cells. (A) Representative Western blots for eNOS expression by AAP cells (control versus aged) exposed to a shear stress of 8 dynes/cm2 for 24 hours or remaining in static culture. (B) Densitometric analysis of combined data from Western blots comparing AAP cells exposed to shear stress and static culture. *P < 0.05; n = 6. Data are expressed as the mean ± SD.
Figure 3.
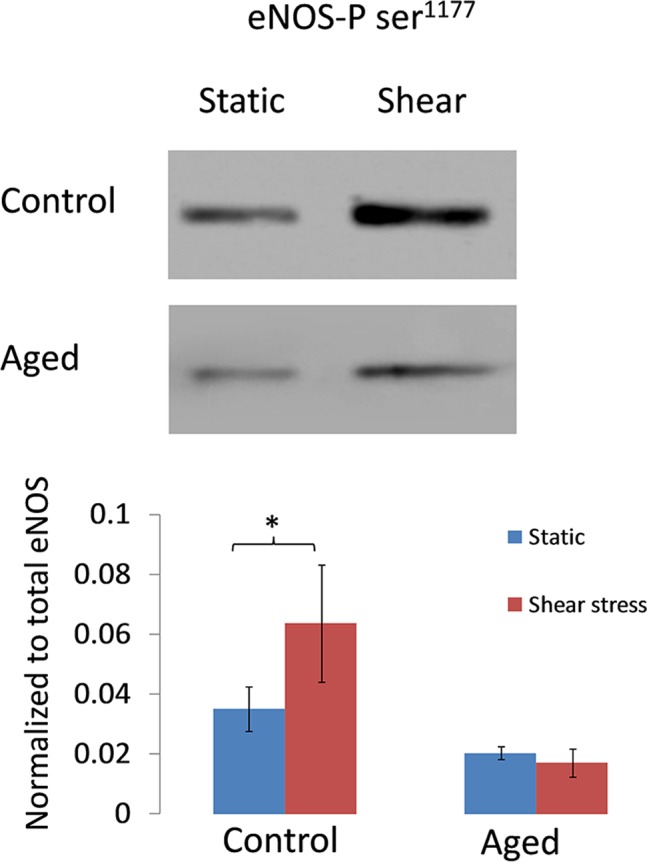
Effect of shear stress on phosphorylation status of eNOS in control and aged AAP cells. (A) Representative Western blot showing phosphorylation levels of eNOS-phospho Ser1177 in AAP cells (normal and aged) subjected to shear stress (8 dynes/cm2 for 24 hours) or static condition. (B) Blots were analyzed by densitometry, phosphorylation levels were normalized to total eNOS protein, and comparisons are shown in the bar graph. *P < 0.05; n = 6. Data are expressed as the mean ± SD.
When eNOS expression by AAP cells exposed to shear stress was examined by immunostaining, results were consistent with Western blots showing more abundant protein in control cells exposed to shear stress (Figs. 4A, 4B) compared with static cultures (Figs. 4C, 4D). Upon closer inspection, we noted stronger staining at the cell periphery of control cells exposed to shear stress. However, we did not quantify the magnitude or percentage of cells that showed this stronger staining. In aging cells, the amount of protein and the distribution of the protein did not differ markedly between cells exposed to shear stress and static cultures of cells. When cells were inspected by phase-contrast microscopy (Leica DMi1; Leica, Shanghai, China), we observed that cell alignment was evident after a continuous shear stress exposure of 5 dynes/cm2 for 2 days in both normal cells and aged cells (Figs. 4E, 4F). We used a lower shear stress here for the cell alignment study because cells were more prone to detachment with the higher shear stress of 8 dynes/cm2 for 48 hours. We found that 5 dynes/cm2 was still able to induce cell alignment in vitro after 2 days of perfusion.
Figure 4.
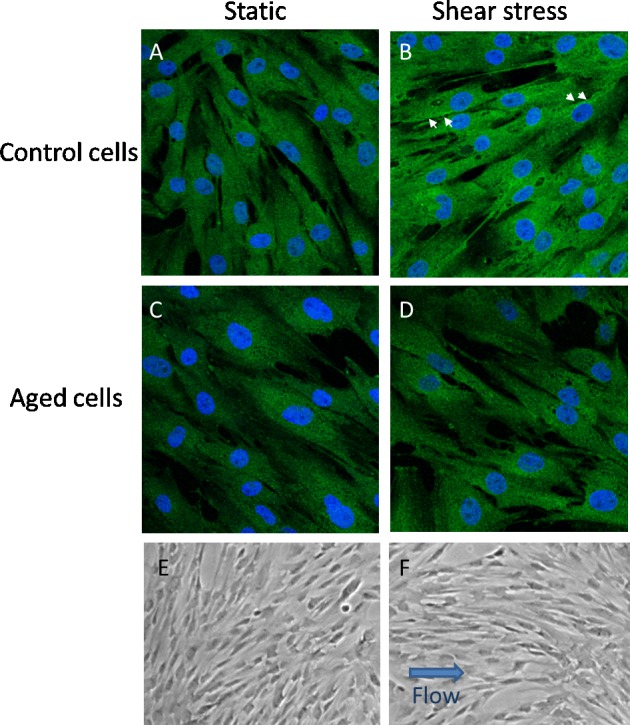
Effects of shear stress on AAP cell alignment plus eNOS expression and localization. (A–D) Immunofluorescence images taken of cells (control [A, B] versus aged [C, D]) subjected to static culture or shear stress (8 dynes/cm2 for 24 hours) and then labeled with antibodies specific for eNOS. Arrows point to labeling at cell boundaries in control cells; however, this is not apparent in aged cells. Phase-contrast images of AAP cells (control versus aged) were exposed to 2 days of 5 dynes/cm2 shear stress or remained in static culture (E, F). An arrow indicates the direction of flow in cells subjected to shear stress. Shown are representative results from one experiment using one cell line of four in total.
To examine the amount of NO produced by cells exposed to shear versus cells in static culture, the concentration of NO released was indirectly measured using the Griess assay (Sigma; Sigma-Aldrich Corp.) (Fig. 5). In control cells, the mean ± SD NO concentrations were 4.8 ± 0.8 and 8.7 ± 1.9 μM in the static and shear stress groups, respectively. In contrast, the mean ± SD NO concentrations in aged cells were 4.0 ± 0.9 and 4.6 ± 1.3 μM in the static and shear stress groups, respectively. Control cells had a significantly 1.8-fold higher NO concentration after shear stress than in the static group (P < 0.05; n = 4), while the NO concentration after shear stress was no more statistically significant than static cultures in aged cells.
Figure 5.
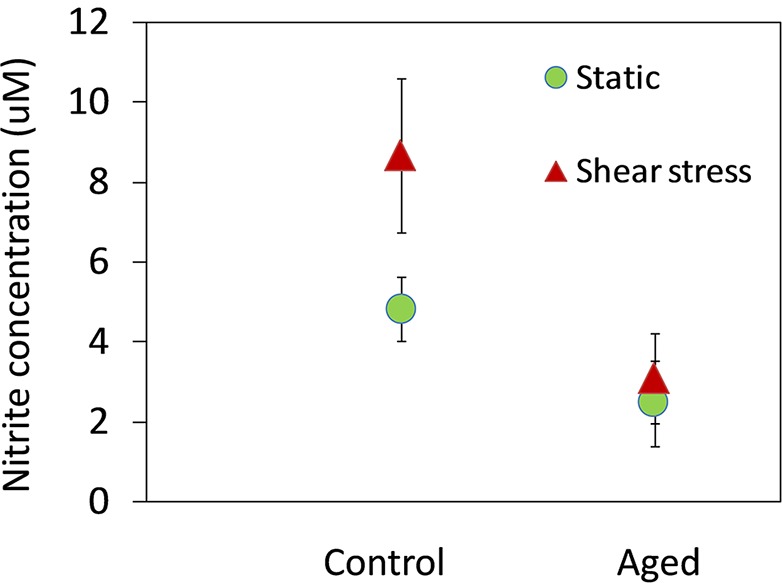
Effect of aging on nitrite concentration in AAP cells exposed to shear stress. The NO concentration was measurements by the Griess assay (Sigma; Sigma-Aldrich Corp.). Shown are combined data from four experiments (mean ± SD) showing the nitrite content in AAP cells (control versus aged) exposed to shear stress (10 dynes/cm2 for 24 hours) compared with cells in static culture. *P < 0.05.
Discussion
In this study, we explored the impact of AAP cell aging on eNOS and responses to a shear stress of 8 dynes/cm2, which is within the physiological range. As in human SC cells, we confirm that AAP cells are mechanosensitive to shear stress. For the first time to date, we report that eNOS expression and phosphorylation status at Ser1177 (activation site) and Thr495 (inhibition site) were altered in aged cells. Consistent with these findings, phosphorylated Akt was also lower in aged cells. When exposed to shear stress, aged AAP cells did not upregulate eNOS, eNOS-phospho Ser1177, or NO production as in control cells but did align with the direction of shear stress. Together, these results suggest that mechanotransduction via eNOS signaling is compromised in aging.
Shear stress is one of the most important physiological stimuli for vascular endothelial cells. It is the frictional force that a fluid exerts as it flows over a surface per unit area.51 Shear stress is parallel to the surface, is proportional to the viscosity and velocity of the fluid, and is inversely proportional to the radius of the vessel. In normal AAP cells, shear stress induced eNOS expression, eNOS phosphorylation (Fig. 2), and NO release (Fig. 5). This is consistent with findings in human eyes (Stamer WD, unpublished data, 2014). The SC cells from normal and glaucomatous human donor eyes were subjected to continuous shear stress (0.1 or 10 dynes/cm2) for 6, 24, or 168 hours, and it was found that found shear stress increased NO levels in normal SC cells at as early as 6 hours.
In contrast, aging significantly impacted mechanotransduction through eNOS in AAP cells. Previous investigations using other endothelial cells showed similar findings that shear stress upregulated eNOS mRNA and the posttranslational activation of eNOS.52 However, aged cells did not respond to mechanical stimulus as young cells do. In our aging model, cells stained positive for the aging marker β-galactosidase and the DNA damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) as shown previously.53 After cells were subcultured once, they still showed signs of aging (e.g., increased cell size, growth rate reduction, and cell cycle arrest) after 3 days in normal cell culture condition. In future studies, longer recovery periods need to be evaluated. Comparing the sensitivity of human umbilical vein endothelial cells to apoptosis in young and aged cells, Hoffman et al.54 showed that shear stress could not upregulate eNOS in aged human umbilical vein endothelial cells (HUVEC) cells (14th passage) as it did in controls, nor could it reverse the decreased S-NO content in aged cells. Together, these data suggest that aging reduces cellular mechanosensitivity to shear stress. Although it is difficult to elucidate the exact relationship between aging and glaucoma, these data may at least suggest that aging itself could lead to reduction in eNOS and NO levels as seen in glaucoma.
Our present findings in aged AAP cells fit well with recent clinical discoveries. Several groups have shown that vitrectomy and cataract surgery significantly increase IOP and the risk of developing POAG,55,56 which is due to exposure of the TM and SC to a higher level of oxygen causing oxidative damage to these tissues.57,58 We demonstrated in vitro in an aging model of hyperoxia that it altered eNOS and its phosphorylation status and resulted in reduced NO production. Together with our previous data39,49 showing that hyperoxia can cause increased resistance at the level of SC, these findings suggest that vitrectomy and phacoemulsification and the related oxygen concentration elevation could cause increased IOP by damaging SC.
In most individuals, IOP is maintained in an acceptable range throughout their lifetime, and a homeostatic mechanism must exist to keep it this way. Our present results and previous data40 suggest that SC can sense shear stress and respond to pressure fluctuation by adjusting the aqueous humor outflow resistance, restoring IOP to physiological levels. Other groups have shown that perfused anterior segments and perfused eyes can sense pressure elevation and respond by adjusting outflow resistance.59,60 In POAG, IOP homeostasis appears to be defective, resulting in ocular hypertension. Elevated IOP exerts preferential stress on the optic nerve head, placing retinal ganglion cell axons in danger. Defective IOP control could be due to loss of mechanosensitivity,40 inadequate outflow adjustment,61 or a disrupted feedback mechanism of sensing and responding to shear stress at the level of SC.
Previously, we reported that aging of AAP cells led to increased cell monolayer resistance, which could result in IOP elevation.53 We hypothesize that on pressure elevation SC begins to collapse, and shear stress in SC theoretically increases.12,62 Increased shear in turn leads to increased NO production, which acts in an autocrine fashion to increase the permeability of the inner wall of SC and in a retrograde paracrine fashion to relax juxtacanalicular/TM cells. Such a scenario would lead to decreased outflow resistance and quickly normalize IOP, preventing full collapse of SC and maintaining IOP homeostasis. However, aging rendered cells less able to respond to pressure elevation.40,53 Based on this series of data, we suggest that with aging the mechanism for maintaining IOP homeostasis may be compromised and may contribute to the development of ocular hypertension.
Recent advances in SC research showed that SC is derived from sprouting limbal veins in a unique manner.63–65 Lymphangiogenic growth factor VEGF-C and its receptor VEGFR-3 are essential for SC development.66 The SC endothelial cells were shown to express vascular marker vascular endothelial cadherin (VE-cadherin), eNOS, and VEGFR-1 and VEGFR-2 in vitro67 and lymphatic marker Prospero homeobox protein 1 (Prox-1) ex vivo (but did not express lymphatic vessel endothelial hyaluronan receptor [LYVE-1]).63–65 We confirmed that AAP cells expressed VE-cadherin, eNOS, vascular cell adhesion molecule 2 (VCAM2), and von Willebrand factor in vitro.40,49,53 However, we were unable to test expression of Prox-1 because the porcine antibody is not yet commercially available to date.
In summary, our study confirms in a second SC model (after human SC cells) that shear stress upregulates eNOS expression and for the first time to date reports that eNOS expression and phosphorylation status were altered in aged cells. We further show that aged cells failed to respond to shear stress, implicating a mechanism by which aging may contribute to aberrant IOP autoregulation and homeostasis.
Acknowledgments
We thank Huang Yalin from the Stem Cell Research Centre of Fudan University for her technical support in confocal imaging.
Supported by Grants 81100662 and 81371015 from the National Science Foundation China, Grant XYQ2013083 from the Shanghai Municipal Health Bureau Young Outstanding Scientist Program, Grant EHF158351211 from the Project of Fudan University, The Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry), and Grant EY022359 from Research to Prevent Blindness, New York, New York, United States (WDS). XHS was supported by grants from Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (81020108017), National Health and Family Planning Commission, China (201302015), National Major Scientific Equipment program, the Ministry of Science and Technology, China (2012YQ12008003), State Key Program of National Natural Science Foundation of China (81430007), New Technology Research Project, and Shanghai Municipal Commission of Health and Family Planning (2013SY058).
Disclosure: Y. Lei, Allergan (F); W.D. Stamer, None; J. Wu, None; X. Sun, None
References
- 1. Quigley HA. Glaucoma. Lancet. 2011; 377: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 2. Friedman DS. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004; 122: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989; 96: 26–32. [DOI] [PubMed] [Google Scholar]
- 4. Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001; 131: 699–708. [DOI] [PubMed] [Google Scholar]
- 5. Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies, part I: prevalence findings. Ophthalmology. 1989; 96: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 6. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994; 112: 821–829. [DOI] [PubMed] [Google Scholar]
- 7. Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998; 105: 733–739. [DOI] [PubMed] [Google Scholar]
- 8. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991; 266: 369–374. [PubMed] [Google Scholar]
- 9. Stamer WD, Roberts BC, Epstein DL. Hydraulic pressure stimulates adenosine 3′,5′-cyclic monophosphate accumulation in endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1999; 40: 1983–1988. [PubMed] [Google Scholar]
- 10. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989; 8: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 11. Ethier CR. The inner wall of Schlemm's canal. Exp Eye Res. 2002; 74: 161–172. [DOI] [PubMed] [Google Scholar]
- 12. Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J. 2004; 87: 2828–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henry E, Newby DE, Webb DJ, O'Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 1710–1714. [PubMed] [Google Scholar]
- 14. Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol. 2011; 129: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010; 51: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide–producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36: 1765–1773. [PubMed] [Google Scholar]
- 17. Selbach JM, Gottanka J, Wittmann M, Lutjen-Drecoll E. Efferent and afferent innervation of primate trabecular meshwork and scleral spur. Invest Ophthalmol Vis Sci. 2000; 41: 2184–2191. [PubMed] [Google Scholar]
- 18. Hamanaka T, Bill A. Effects of alpha-chymotrypsin on the outflow routes for aqueous humor. Exp Eye Res. 1988; 46: 323–341. [DOI] [PubMed] [Google Scholar]
- 19. Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002; 12: 44–48. [DOI] [PubMed] [Google Scholar]
- 20. Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004; 88: 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotikoski H, Vapaatalo H, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003; 26: 119–123. [DOI] [PubMed] [Google Scholar]
- 22. Borghi V, Bastia E, Guzzetta M, et al. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010; 26: 125–132. [DOI] [PubMed] [Google Scholar]
- 23. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37: 1711–1715. [PubMed] [Google Scholar]
- 24. Carreiro S, Anderson S, Gukasyan HJ, Krauss A, Prasanna G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J Ocul Pharmacol Ther. 2009; 25: 105–112. [DOI] [PubMed] [Google Scholar]
- 25. Shahidullah M, Yap M, To CH., Cyclic GMP. sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005; 145: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heyne GW, Kiland JA, Kaufman PL, Gabelt BT. Effect of nitric oxide on anterior segment physiology in monkeys. Invest Ophthalmol Vis Sci. 2013; 54: 5103–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schneemann A, Dijkstra BG, van den Berg TJ, Kamphuis W, Hoyng PF. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 936–941. [DOI] [PubMed] [Google Scholar]
- 28. Chuman H, Chuman T, Nao-i N, Sawada A. The effect of l-arginine on intraocular pressure in the human eye. Curr Eye Res. 2000; 20: 511–516. [PubMed] [Google Scholar]
- 29. Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donnini S, Ziche M. Constitutive and inducible nitric oxide synthase: role in angiogenesis. Antioxid Redox Signal. 2002; 4: 817–823. [DOI] [PubMed] [Google Scholar]
- 31. Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci. 2000; 21: 249–252. [DOI] [PubMed] [Google Scholar]
- 32. Ethier CR, Simmons CA. Introductory Biomechanics: From Cells to Organisms (Cambridge Texts in Biomedical Engineering). New York: Cambridge University Press; 2007: 262. [Google Scholar]
- 33. Cheng C, van Haperen R, de Waard M, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005; 106: 3691–3698. [DOI] [PubMed] [Google Scholar]
- 34. Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998; 32: 351–355. [DOI] [PubMed] [Google Scholar]
- 35. Tielsch JM, Katz J, Singh K, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991; 134: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 36. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1992; 99: 1499–1504. [DOI] [PubMed] [Google Scholar]
- 37. Varma R, Wang D, Wu C, et al. Los Angeles Latino Eye Study Group. Four-year incidence of open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2012; 154: 315–325.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caprioli J. Glaucoma: a disease of early cellular senescence. Invest Ophthalmol Vis Sci. 2013; 54: ORSF60–67. [DOI] [PubMed] [Google Scholar]
- 39. Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005; 24: 612–637. [DOI] [PubMed] [Google Scholar]
- 40. Lei Y, Stamer WD, Wu J, Sun X. Cell senescence reduced the mechanotransduction sensitivity of porcine angular aqueous plexus cells to elevation of pressure. Invest Ophthalmol Vis Sci. 2014; 55: 2324–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campisi J. d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007; 8: 729–740. [DOI] [PubMed] [Google Scholar]
- 42. Sitte N, Huber M, Grune T, et al. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000; 14: 1490–1498. [DOI] [PubMed] [Google Scholar]
- 43. Terman A, Brunk UT. Ceroid/lipofuscin formation in cultured human fibroblasts: the role of oxidative stress and lysosomal proteolysis. Mech Ageing Dev. 1998; 104: 277–291. [DOI] [PubMed] [Google Scholar]
- 44. Zheng L, Roberg K, Jerhammar F, Marcusson J, Terman A. Oxidative stress induces intralysosomal accumulation of Alzheimer amyloid beta–protein in cultured neuroblastoma cells. Ann N Y Acad Sci. 2006; 1067: 248–251. [DOI] [PubMed] [Google Scholar]
- 45. Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthalmic Res. 1995; 27 (suppl 1): 143–149. [DOI] [PubMed] [Google Scholar]
- 46. Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001; 7: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci. 2008; 49: 3961–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Landis GN, Abdueva D, Skvortsov D, et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004; 101: 7663–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lei Y, Overby DR, Read AT, Stamer WD, Ethier CR. A new method for selection of angular aqueous plexus cells from porcine eyes: a model for Schlemm's canal endothelium. Invest Ophthalmol Vis Sci. 2010; 51: 5744–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54: 4827–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009; 10: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992; 103 (pt 1): 23–37. [DOI] [PubMed] [Google Scholar]
- 53. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54: 4827–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001; 89: 709–715. [DOI] [PubMed] [Google Scholar]
- 55. Koreen L, Yoshida N, Escariao P, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012; 32: 160–167. [DOI] [PubMed] [Google Scholar]
- 56. Parke DW III, Sisk RA, Houston SK, Murray TG. Ocular hypertension after intravitreal triamcinolone with vitrectomy and phacoemulsification. Clin Ophthalmol. 2012; 6: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beebe DC, Shui YB, Siegfried CJ, Holekamp NM, Bai F. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Jpn J Ophthalmol. 2014; 58: 225–231. [DOI] [PubMed] [Google Scholar]
- 58. Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010; 51: 5731–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003; 44: 5174–5181. [DOI] [PubMed] [Google Scholar]
- 60. Borras T, Rowlette LL, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002; 43: 33–40. [PubMed] [Google Scholar]
- 61. Acott TS, Kelley MJ, Keller KE, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014; 30: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Avtar R, Srivastava R. Aqueous outflow in Schlemm's canal. Appl Math Computation. 2006; 174: 316–328. [Google Scholar]
- 63. Park DY, Lee J, Park I, et al. Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity. J Clin Invest. 2014; 124: 3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Truong TN, Li H, Hong YK, Chen L. Novel characterization and live imaging of Schlemm's canal expressing Prox-1. PLoS One. 2014; 9: e98245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014; 12: e1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aspelund A, Tammela T, Antila S, et al. The Schlemm's canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. J Clin Invest. 2014; 124: 3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perkumas KM, Stamer WD. Protein markers and differentiation in culture for Schlemm's canal endothelial cells. Exp Eye Res. 2012; 96: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]