Identification of transcriptional signatures of anti-inflammatory macrophages and their role in lethal endotoxemia.
Keywords: regulatory, RNA-seq, endotoxemia, alternatively activated, transcriptomics
Abstract
Macrophages readily change their phenotype in response to exogenous stimuli. In this work, macrophages were stimulated under a variety of experimental conditions, and phenotypic alterations were correlated with changes in gene expression. We identified 3 transcriptionally related populations of macrophages with immunoregulatory activity. They were generated by stimulating cells with TLR ligands in the presence of 3 different "reprogramming" signals: high-density ICs, PGE2, or Ado. All 3 of these cell populations produced high levels of transcripts for IL-10 and growth and angiogenic factors. They also secreted reduced levels of inflammatory cytokines IL-1β, IL-6, and IL-12. All 3 macrophage phenotypes could partially rescue mice from lethal endotoxemia, and therefore, we consider each to have anti-inflammatory activity. This ability to regulate innate-immune responses occurred equally well in macrophages from STAT6-deficient mice. The lack of STAT6 did not affect the ability of macrophages to change cytokine production reciprocally or to rescue mice from lethal endotoxemia. Furthermore, treatment of macrophages with IL-4 failed to induce similar phenotypic or transcriptional alterations. This work demonstrates that there are multiple ways to generate macrophages with immunoregulatory activity. These anti-inflammatory macrophages are transcriptionally and functionally related to each other and are quite distinct from macrophages treated with IL-4.
Introduction
The plasticity of macrophages allows these cells to undergo dramatic alterations in their phenotype in response to diverse environmental stimuli [1–6]. This phenotypic heterogeneity of macrophages has led to a substantial degree of confusion in the field about how best to name these cells. This is not simply a semantic problem. A better understanding of the phenotypic alterations that macrophages undergo is necessary if we eventually hope to manipulate immune responses at the level of macrophages. Studies to improve understanding of heterogeneity can put us in a better position to generate macrophages with predictable phenotypes, to deplete one set of macrophages while preserving others, or to target drugs to individual subpopulations of macrophages.
The pioneering work of Gordon and colleagues in the 1990s helped to define 2 paradigmatic populations of macrophages, generally referred to as “classical” versus “alternative” but later termed M1 versus M2 or M(IFN-γ) versus M(IL-4) [5, 7–9]. Exposing macrophages to IFN-γ and TLR ligands results in an up-regulation of inflammatory cytokines, an increased MHC-II and costimulatory molecule expression, and the production of antimicrobial products [7, 10–13]. Cells exposed to IL-4, in contrast, fail to up-regulate costimulatory molecules and MHC-II, are poor APCs, and produce negligible amounts of NO. These cells express higher levels of chitinases and lectin-like receptors and are termed AA-Mϕ [7, 14, 15]. In the literature, the various categories of macrophages sometimes get confused, and all TLR-stimulated macrophages are grouped into the M1 category by virtue of the inflammatory cytokines that they produce. In contrast, all noninflammatory macrophages are collectively referred to as M2. This has led to some confusion as to what actually constitutes an M2 macrophage. The linear M1/M2 classification system remained the standard for nomenclature until investigators attempted to include macrophages that were treated with glucocorticoids, anti-inflammatory cytokines, apoptotic cells, ICs, or Ado derivatives, to name a few. These macrophages did not fit into a simple M1/M2 classification scheme.
A color-wheel scheme was proposed to highlight the plasticity of macrophages [4, 16]. This model placed an emphasis on the dynamic nature of macrophage activation and proposed that macrophages can readily transition from one activation state to another. In the present study, we examine 5 macrophage populations from different segments of the color wheel and demonstrate that macrophages treated with IL-4 are transcriptionally and phenotypically distinct from 3 macrophage populations with anti-inflammatory phenotypes. We also show that although the 3 macrophage populations can be distinguished from each other at the global transcriptome level, they share a number of characteristics that endow them with immunoregulatory activity, including the reduced production of inflammatory cytokines and the secretion of growth and angiogenic factors. Therefore, we loosely group them together as R-Mϕ. We describe chemokine and cytokine signatures of R-Mϕ and demonstrate their functionality during inflammatory conditions.
MATERIALS AND METHODS
Mice
Five-week-old BALB/c or C57BL/6 female mice were purchased from Charles River Laboratories (Frederick, MD, USA). Mice were housed at the University of Maryland (College Park, MD, USA) animal facility. All procedures were approved by the University of Maryland Institutional Animal Care and Use Committee.
Murine macrophages
BMDMs were obtained by flushing the femurs and tibiae of BALB/c mice and were cultured in DMEM/F12 medium with 10% FBS, 1% glutamine, 1% penicillin/streptomycin, and 15% L929 cell conditioned media, as described previously [17]. Peritoneal macrophages were obtained by peritoneal flush with PBS from female mice. Cells obtained from 10 to 12 mice were pooled and stimulated with LPS, L+I, L+A, or IL-4 for 4 h.
Human macrophage culture
Peripheral blood was collected from healthy volunteers, and mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation. Monocytes were isolated by attachment and were cultured for 1 wk in RPMI-1640 medium with 10% human AB serum (Life Technologies, Grand Island, NY, USA) and 50 ng/ml M-CSF (PeproTech, Rocky Hill, NJ, USA). All studies on human monocyte-derived macrophages were approved by the University of Maryland Institutional Review Board.
Cell culture and stimulation
M1-Mϕ and AA-Mϕ macrophages were generated by adding 10 ng/ml Ultrapure LPS (InvivoGen, San Diego, CA, USA) and 20 ng/ml mouse IL-4 (R&D Systems, Minneapolis, MN, USA), respectively. R-Mϕ were obtained by adding 10 ng/ml Ultrapure LPS in combination with ICs, generated as described previously [18], 200 nM PGE2 (Cayman Chemical, Ann Arbor, MI, USA), or 200 µM Ado (Sigma-Aldrich, St. Louis, MO, USA). For RNA isolation, 2 × 106 BMDMs were stimulated for 2–6 h, and for cytokine analyses, 2.5 × 105 BMDMs were stimulated for 12–16 h. For the membrane protein array, 2 × 106 BMDMs were stimulated for 12 h. For the bioplex analyses, supernatants were obtained from 5 × 105 human macrophages after 24 h stimulation.
In vivo regulatory induction
Age-matched female BALB/c or C57BL/6 mice were injected with 500 μg LPS alone or a combination of LPS and 400 µl Ova-IC (L+I), 50 μg PGE2 (L+P), 50 μg Ado (L+A), or 50 μg IL-4 intraperitoneally. After 6 h, peritoneal cells were isolated by peritoneal lavage and processed for flow cytometry or real-time PCR.
Lethal endotoxin challenge
BALB/c BMDMs stimulated in vitro under various conditions were injected intraperitoneally into BALB/c mice. Three hours after cell transplantation, mice were challenged with a 10 mg/kg dose of endotoxin (L2630; Sigma-Aldrich). Survival of the mice was recorded for 1 wk. Statistical significance was determined by Mantel-Cox log rank test.
ELISA
IL-12/23p40 and IL-10 levels were measured by the sandwich ELISA method using antibodies purchased from BD PharMingen (San Diego, CA, USA). Mouse IL-1β and IL-6 and human IL-12/IL-23p40 levels (see Fig. 7F) were measured by use of Duoset ELISA kits (R&D Systems). Human IL-10 (see Fig. 7A) and human IL-12p40 (see Fig. 7A) were detected by use of ELISA kits purchased from eBioscience (San Diego, CA, USA).
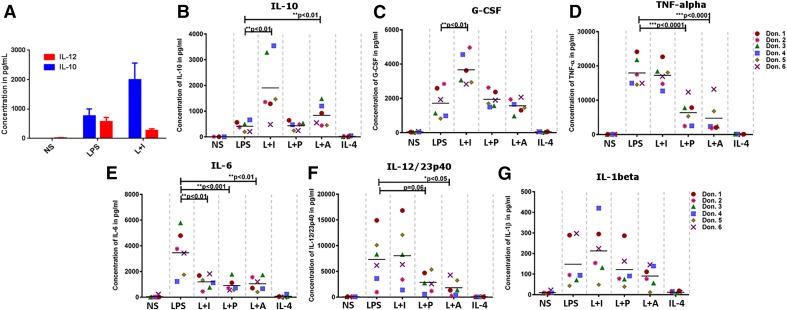
Figure 7. Cytokine profile of human macrophages under regulatory stimulation conditions.
Human monocyte-derived macrophages were cultured at the concentration of 5 × 105 cells/500 μL medium and were left unstimulated or stimulated with 30 ng/ml (A) or 10 ng/ml LPS alone or a combination of LPS and 25 µl Ova/anti-Ova ICs (L+I), 200 nM PGE2 (L+P), 200 μM Ado (L+A), or 20 ng/ml IL-4 for 8 (A) or 24 h (B–G). Cytokines in A were measured by use of a sandwich ELISA kit. The levels of all other indicated cytokines were measured by use of the bioplex assay. The horizontal bar represents the mean value, and the asterisks represent the significance of the observed values compared with LPS-treated cells. ***P < 0.001, **P < 0.01, and *P < 0.05.
Membrane protein array
Mouse cytokine antibody array membranes [Proteome Profiler Antibody Array (R&D Systems] were used to assess the relative differences of 40 different cytokines and chemokines in cell culture supernatants. Chemiluminescence signal density was quantified by use of LAS-3000 Imaging Systems from Fujifilm (Tokyo, Japan).
Bioplex assay
Human macrophage cytokines/chemokines were measured by use of a 14-plex magnetic Luminex screening assay (R&D Systems). The samples were acquired in Magpix, and data were analyzed with xPONENT software (Luminex, Austin, TX, USA).
RNA isolation, cDNA synthesis, and PCR
RNA was isolated by use of the TRIzol-chloroform method. Complementary DNA was synthesized with a Thermoscript RT-PCR kit (Invitrogen, Grand Island, NY, USA). Primer pairs used in this study are listed in Supplemental Table 1. Relative quantification of RNA was done by use of SYBR-Green-based real-time PCR. CT value for gapdh was used to calculate relative differences, and fold induction was calculated by use of 2^(−ΔΔCT) [19].
RNA-seq data generation and processing
RNA-seq analyses were performed on 3 different sample sets, obtained on different days from female C57BL/6 mice. Poly(A)-enriched cDNA libraries were generated by use of the TruSeq Sample Preparation Kit (Illumina, San Diego, CA, USA). Paired end reads (100 bp) were obtained from the HiSeq 1500 platform (Illumina). Trimmomatic [20] was used to remove any remaining adapter sequences from reads and to trim off bases with quality scores below 20. Sequence quality metrics were assessed by use of FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were aligned to the Mus musculus genome (v. mm10/GRCm38) obtained from University of California Los Angeles (CA, USA; http://genome.ucsc.edu) by use of TopHat (v 2.0.10) [21]. Reads were allowed to map only to a single locus. The abundance of reads mapping to each gene was determined by use of HTSeq (http://www-huber.embl.de/users/anders/HTSeq/).
Data quality assessment and differential expression analysis
Multiple approaches were used to evaluate replicates, including PCA and Euclidean distances-based hierarchical clustering. All components of the statistical pipeline, named cbcbSEQ, can be accessed on GitHub (https://github.com/kokrah/cbcbSEQ/). Nonexpressed and weakly expressed genes were removed before differential expression analysis, and a quartile normalization scheme was applied to all samples [22]. Limma (a Bioconductor package; http://www.bioconductor.org/packages/release/bioc/html/limma.html) [23] was used to conduct differential expression analyses following log2 data transformation and application of the voom [24] method. Experimental batch effects were adjusted for by including batch/experimental date as a covariate in the statistical model [25]. Differentially expressed genes were defined as genes with a Benjamini-Hochberg multiple-testing adjusted P value of <0.05. The RNA-seq datasets described in this article are available at the National Center for Biotechnology Information Sequence Read Archive under Accession Numbers SRR1918864, SRR1918994, SRR1918999-SRR1919012, and SRR1919014-SRR1919018 (see Supplemental Table 2).
IPAs
IPA software [26] was used to predict "Diseases and Functions" and "Canonical Pathways" associations. Genes that exhibited a <2-fold difference were excluded from the comparisons.
Macrophage metabolism
Glucose levels and L-lactate production were assessed by use of the Glucose Assay Kit (Sigma-Aldrich) and L-Lactate Kit I (Eton Biosciences, San Diego, CA, USA), respectively.
Flow cytometry
Mouse CCR1 surface expression was detected by PE-conjugated antibody (R&D Systems). Data acquisition was carried out in FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA), and analyses were done by use of FlowJo version 10.
Statistics
Nonparametric t-tests were performed to calculate the significance of the observed differences. P < 0.05 was considered to be significant for all analyses.
RESULTS
Macrophages with immunoregulatory activity can offer protection from lethal endotoxin challenge
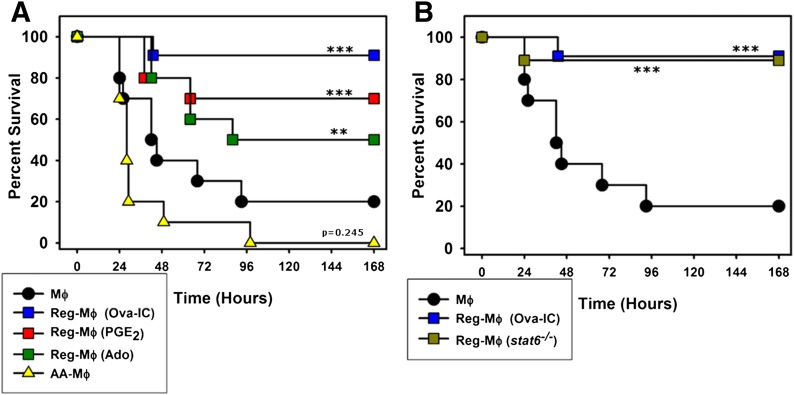
Macrophages were stimulated in a variety of different ways in vitro and then transferred into the peritoneum of mice before an injection of bacterial endotoxin (LPS). The macrophages studied included resting macrophages, macrophages treated with IL-4 (AA-Mϕ), and macrophages that were stimulated with LPS in the presence of 3 different reprogramming stimuli: ICs (RMϕ-IC), PGE2 (RMϕ-PGE2), or Ado (RMϕ-Ado). The goal of this work was to determine whether any of these macrophages could influence the progression of lethal endotoxemia. The administration of 1 × 106 resting macrophages into mice did not influence the progression of lethal endotoxemia, and 80% of the mice succumbed over the first 4 days (Fig. 1A). All 3 of the macrophage populations that were “reprogrammed” provided some level of protection to mice receiving endotoxin. Mice that received RMϕ-IC showed a 90% survival, whereas mice that received RMϕ-PGE2 or RMϕ-Ado showed survival rates of 70% and 50%, respectively. Mice receiving macrophages that were treated with IL-4 (AA-Mϕ) were not protected from lethal endotoxemia and in fact, did slightly worse than mice receiving resting macrophages (Fig. 1A). The mice that received RMϕ-IC had a 90% survival rate, regardless of whether the macrophages that were transferred were from WT or stat6 knockout mice (Fig. 1B). Thus, all 3 of the macrophages that were reprogrammed before stimulation provided some level of protection from lethal endotoxemia, and the ability to provide protection from lethal endotoxin highlights a major functional difference between the R-Mϕ described herein and IL-4-treated AA-Mϕ, both of which have been considered previously by some to be M2 macrophages.
Figure 1. Regulatory activation provides protection from lethal endotoxemia.
(A) BALB/c mice received 1 × 106 resting, nonstimulated macrophages (Mϕ, filled circles) intraperitoneally or macrophages stimulated in vitro with LPS in combination with Ova-IC (blue squares), PGE2 (red squares), or Ado (green squares) or macrophages treated with IL-4 (yellow triangles), 3 h before challenge with a lethal dose of endotoxin (10 mg/kg). The survival of the mice was recorded every 8 h over the next week. (B) A similar survival experiment was carried out in WT mice that received 1 × 106 L+I macrophages from WT (blue squares) or stat6−/− (olive squares) mice before endotoxin challenge. Each graph represents data of 2 independent experiments with 10 mice/experiment for each condition. The data from the control group and WT RMϕ-IC were shared between the 2 graphs. ***P < 0.0001, and **P = 0.0006, Kaplan-Meier estimates obtained for mice treated with resting macrophages versus other macrophage treatments.
Macrophages with this immunoregulatory phenotype have distinct cytokine and chemokine profiles
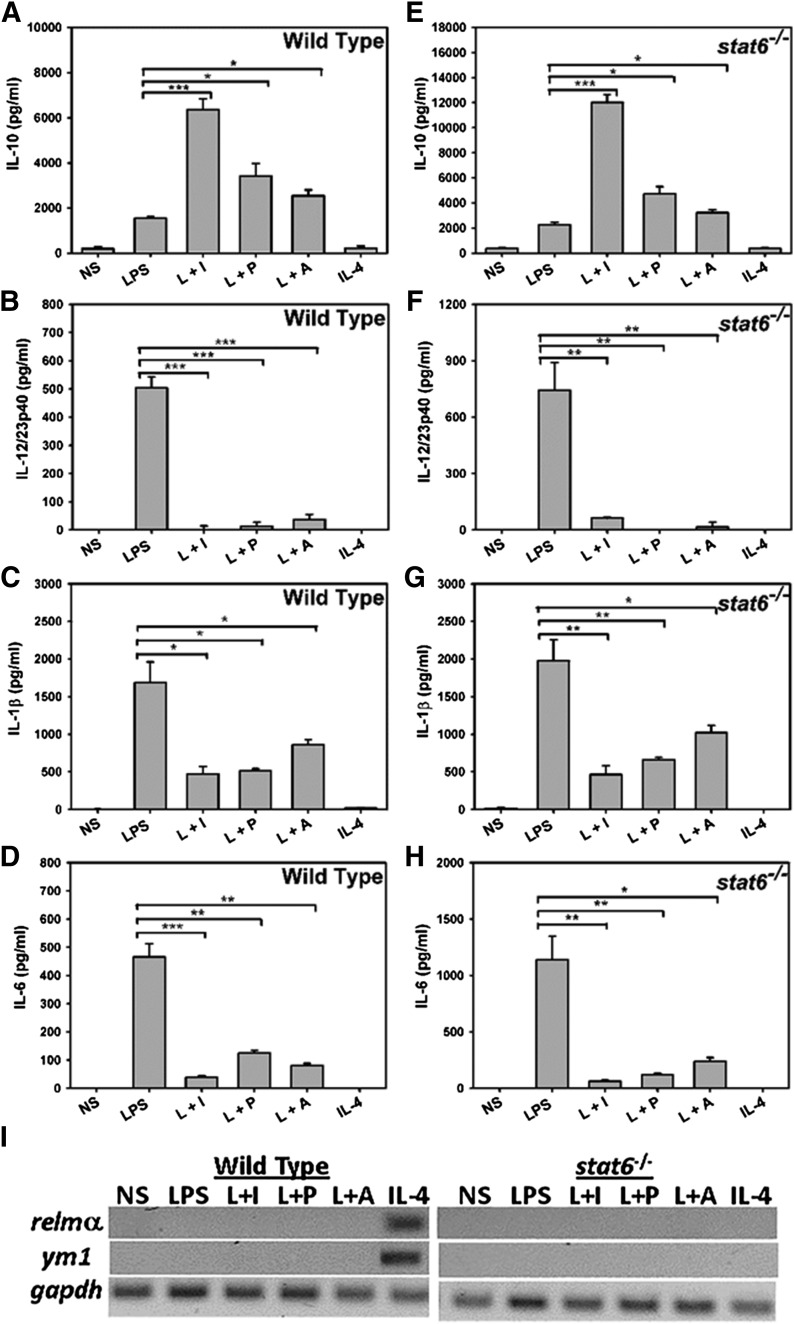
Cytokine production from the various macrophage populations was measured. The addition of the various reprogramming signals to macrophages resulted in dramatic changes in their cytokine and chemokine expression. As expected, M1-Mϕ stimulated with LPS exhibited an inflammatory phenotype, secreting high IL-12/23p40, IL-1β, and IL-6 but low levels of IL-10 (Fig. 2A–D). Macrophages stimulated with LPS and reprogrammed with IC, PGE2, or Ado were substantially less inflammatory. They secreted higher levels of IL-10 and suppressed the production of IL-12/23p40, IL-1β, and IL-6 (Fig. 2A–D). To test if regulatory functions were dependent on STAT6, macrophages from stat6 knockout mice were stimulated in the presence of IC, PGE2, or Ado. These cells produced higher levels of IL-10 and reduced levels of inflammatory cytokines IL-12/23p40, IL-1β, and IL-6, indicating that STAT6 signaling is dispensable for generating macrophages with this immunoregulatory phenotype (Fig. 2E–H). In contrast to R-Mϕ, AA-Mϕ produced little or no detectable levels of the tested cytokines (Figure 2A–D). RT-PCR analyses of AA-Mϕ from WT macrophages revealed high transcription of relmα and ym1 [14], confirming that our IL-4 treatment had indeed generated AA-Mϕ (Fig. 2I). Macrophages from mice genetically deficient in stat6 failed to transcribe relmα and ym1 in response to IL-4, as expected (Fig. 2I).
Figure 2. R-Mϕ induction is independent of STAT6 signaling pathway.
BALB/c WT and stat6−/− BMDMs were treated with 10 ng/ml LPS alone or a combination of LPS and 25 µl Ova/anti-Ova ICs (L+I), 200 nM PGE2 (L+P), 200 μM Ado (L+A), or 20 ng/ml IL-4 for 16 h. The levels of IL-10 (A and E), IL-12/23p40 (B and F), IL-1β (C and G), and IL-6 (D and H) were measured in their supernatants by ELISA. Error bars indicate means ± sem of 3 independent experiments. ***P < 0.001, **P < 0.01, and *P < 0.05. Representative RT-PCR analysis of alternate activation markers relmα and ym1 from WT or stat6−/− BMDMs stimulated for 4 h with their respective stimuli before RNA isolation. The gapdh is used as the internal control (I).
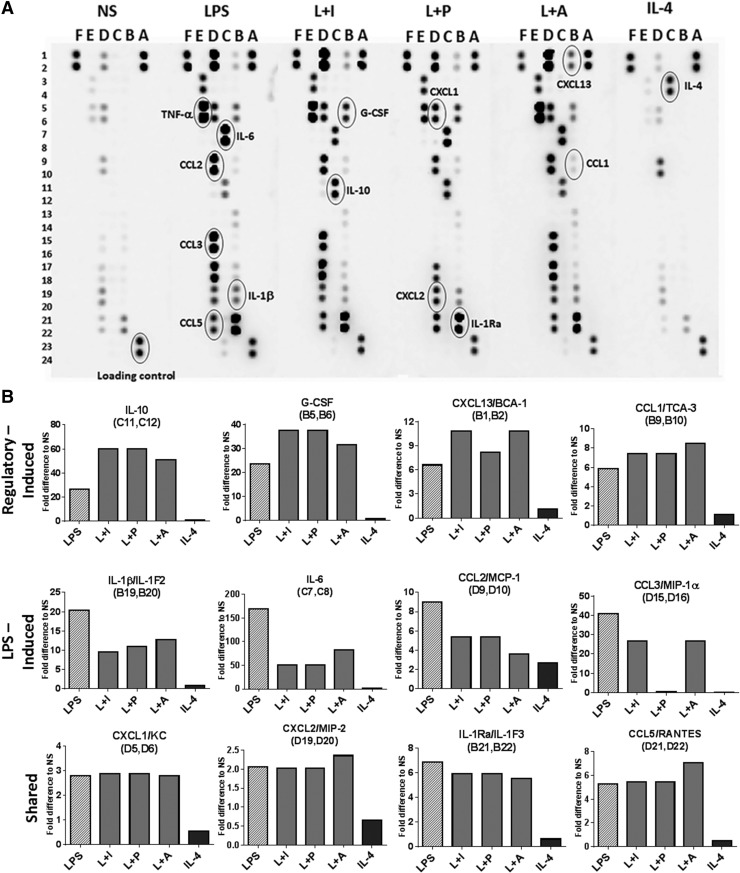
Further analyses of cytokines and chemokines by membrane arrays revealed an increased expression of G-CSF, CXCL13, and CCL1, as well as IL-10 in the R-Mϕ, relative to LPS or IL-4-treated macrophages (Fig. 3A and B). The chemokines CCL2 and CCL3 were down-regulated by at least 2-fold in intensity in R-Mϕ (Fig. 3A and B). Most of the tested chemokines and cytokines showed little or no expression in AA-Mϕ, except CCL2 (Fig. 3A and B). Together, the data suggest that there are multiple ways to generate macrophages with immunoregulatory activity and that these R-Mϕ exhibit unique expression patterns of cytokines and chemokines that are quite distinct from macrophages treated with IL-4.
Figure 3. Regulatory activation results in alterations in cytokine/chemokine profiles.
(A) Chemokine and cytokine secretion by BMDMs was measured by a proteome profiler membrane antibody array. Supernatants from nonstimulated macrophages were compared with macrophages treated with 10 ng/ml LPS or a combination of LPS and 100 µl Ova-IC (L+I), 200 nM PGE2 (L+P), 200 μM Ado (L+A), or 20 ng/ml IL-4 for 12 h. The proteins that are of interest to this study are indicated in circles, and the letters and numbers are provided to identify the position of the analyte in the membrane. The profiling was done on pooled supernatants collected from 3 separate experiments from 3 different mice. (B). Mean fold differences in intensity of the duplicate samples for relevant analytes are compared with supernatants from nonstimulated. The alphanumeric values within parentheses indicate their position in the membrane array. BCA-1, B Cell-attracting chemokine-1; TCA-3, T cell activation-3; KC, keratinocyte-derived chemokine.
RNA-seq analysis of murine macrophages
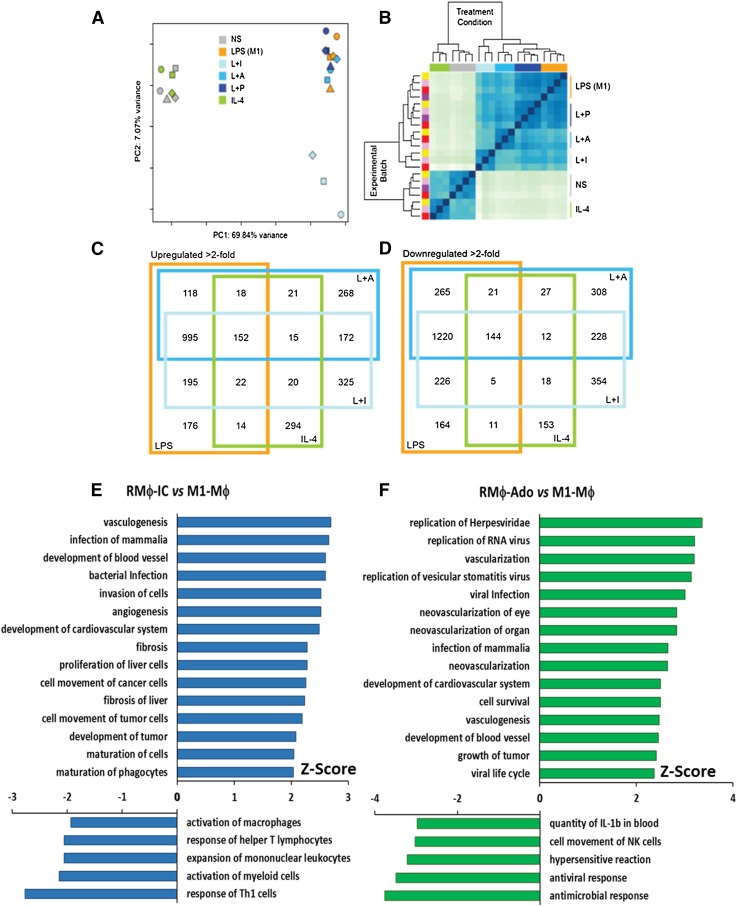
To dissect further differences between the activation states of primary macrophages, we used high-throughput RNA-seq technology to assess the transcriptomes of differentially stimulated peritoneal macrophages. PCA results revealed that AA-Mϕ cluster with nonstimulated macrophages, whereas M1-Mϕ, RMϕ-IC, RMϕ-PGE2, and RMϕ-Ado align together along principal component 1 (the x-axis), which accounts for ∼70% of the variability observed between samples (Fig. 4A). Likewise, when Euclidean distance heat-map analysis was used to visualize the relationships between the samples, IL-4-treated AA-Mϕ grouped closely with nonstimulated cells, and the 3 R-Mϕ subtypes clustered with LPS-treated cells (Fig. 4B). Thus, macrophages with immunoregulatory activity were transcriptionally distinct from IL-4-treated AA-Mϕ.
Figure 4. Global RNA expression profiles of macrophage samples.
RNA-seq was carried out on an Illumina platform, comparing nonstimulated (NS) murine peritoneal macrophages and macrophages exposed to LPS, L+I, L+P, L+A, or IL-4. A Principal Component Analysis (PCA) plot (A) and heat map of a hierarchical clustering analysis that uses the Euclidean distance metric (B) are shown. (A) In the PCA plot, each symbol represents an experimental sample with symbol color indicating macrophage treatment condition (NS, gray; LPS, orange; L+I, light blue; L+A, medium blue; L+P, navy blue; and IL-4, green), and symbol shape indicates batch. (B) Colors along the top of the heat map indicate the treatment condition (same color codes as in A), and colors along the left side of the heat map indicate the batch/experimental date. (C and D) Overlap of differentially expressed genes up-regulated (C) or down-regulated (D) by >2-fold relative to nonstimulated macrophages are displayed in Venn diagrams. Each large-colored square represents the treatment condition (same color codes as in A). The changes associated with Diseases and Functions in R-Mϕ were predicted by the IPA program. Genes that showed a changed in L+I or L+A of at least 2-fold when compared with LPS were selected to identify pathways associated with regulatory functions (E and F). Fold changes were uploaded to IPA, and the Diseases and Functions predicted to be altered based on a significant Z-score were selected for these graphs. A Z-score above 1.65 (activated) or below −1.65 (inhibited) is considered statistically significant.
Differential expression analysis was used to generate lists of genes that were >2-fold different between each macrophage population and nonstimulated macrophages (P < 0.05). RMϕ-IC and RMϕ-Ado shared a total of 172 genes that were not up-regulated in other macrophage populations, whereas only 15 genes were up-regulated in R-Mϕ and AA-Mϕ but not M1-Mϕ (Fig. 4C). Likewise, 228 genes were down-regulated in R-Mϕ-IC and RMϕ-Ado, but only 12 genes were down-regulated in R-Mϕ and AA-Mϕ (Fig. 4D). The gene products that were uniquely up-regulated in R-Mϕ have the potential to be used as biomarkers for defining these macrophages and providing further insights into their immunoregulatory functions. It should be noted that the 2 populations of R-Mϕ showed substantial transcriptional diversity: 325 genes were uniquely up-regulated, and 354 were uniquely down-regulated in RMϕ-IC; 268 genes were uniquely up-regulated, and 308 genes were down-regulated in RMϕ-Ado (Fig. 4C and D). The fold induction of regulatory genes following reprogramming by PGE2 was insufficient to yield a reliable transcriptional signature.
Differentially expressed genes
R-Mϕ and AA-Mϕ are often grouped together as M2 macrophages [8, 27]. However, our functional profiles, PCA, and heat map analyses revealed that these macrophages are quite distinct. To identify the genes that define each population, we analyzed the differentially expressed genes in each of the subsets. The top 20 genes that were induced in AA-Mϕ relative to unstimulated macrophages are listed in Table 1. All of these genes are induced 32-fold or greater in response to IL-4 treatment. Only 2/60 of the most highly up-regulated genes (Itgb3 and Chi3l3) were significantly induced in any of the 3 R-Mϕ populations, and the highest induction was <3-fold. Therefore, the transcripts that define the major functions of AA-Mϕ are largely lacking in R-Mϕ. Previously defined markers for murine AA-Mϕ, including ym1 & relmα, were confirmed by our RNA-seq analyses [14]. Genes with at least a 2-fold up-regulation and with an adjusted P value of <0.01 were considered to be immunoregulatory genes. Table 2 lists 20 genes significantly up-regulated in all 3 R-Mϕ populations relative to unstimulated macrophages. As expected, IL-10 was high on the list of genes associated with regulatory activation (Table 2). In addition to this list of genes with known function, there were another 26 genes that had no known function that were substantially up-regulated in R-Mϕ (Supplemental Table 3). Studies to address the relevance of these induced genes are ongoing.
TABLE 1.
Top 20 genes induced following IL-4 stimulation
| Symbol | Name | IL-4 (log2) | R-IC | R-Ado | R-PGE2 | |
|---|---|---|---|---|---|---|
| 1 | chi3l3 | Chitinase 3-like 3 | 8.85 | ns | 0.94 | ns |
| 2 | cd209e | CD209e antigen | 6.83 | ns | ns | ns |
| 3 | itgb3 | Integrin β 3 | 6.80 | 1.46 | ns | ns |
| 4 | serpina3g | Serine peptidase inhibitor clade A-3G | 6.71 | ns | −2.19 | ns |
| 5 | flt1 | FMS-like tyrosine kinase 1 | 6.21 | −1.47 | ns | ns |
| 6 | pdcd1lg2 | Programmed cell death 1 lig2 | 5.78 | ns | ns | ns |
| 7 | slc7a2 | Solute carrier family 7 | 5.69 | ns | ns | ns |
| 8 | rnase2a | Ribonuclease RNase A family 2a | 5.62 | ns | ns | ns |
| 9 | cish | Cytokine-inducible Src homology 2-containing protein | 5.55 | ns | ns | ns |
| 10 | tslp | Thymic stromal lymphopoietin | 5.49 | ns | ns | ns |
| 11 | chil4 | Chitinase-like 4 | 5.41 | ns | ns | ns |
| 12 | tmem26 | Transmembrane protein 26 | 5.30 | ns | ns | ns |
| 13 | il4i1 | IL-4-induced 1 | 5.26 | −0.73 | ns | ns |
| 14 | cdh1 | Cadherin 1 | 5.25 | ns | ns | ns |
| 15 | apol7c | Apolipoprotein L 7c | 5.20 | ns | ns | ns |
| 16 | socs1 | Suppressor cytokine signaling 1 | 5.04 | −0.94 | ns | ns |
| 17 | mrc1 | Mannose receptor C type 1 | 5.02 | ns | ns | ns |
| 18 | ddx4 | DEAD box polypeptide 4 | 5.02 | ns | ns | ns |
| 19 | il31ra | IL-31R A | 5.00 | ns | ns | ns |
| 20 | insrr | Insulin receptor-related receptor | 5.00 | ns | ns | ns |
Values represent the fold induction (log 2) over nonstimulated macrophages. R-IC, RMϕ-IC; R-Ado, RMϕ-Ado; R-PGE2, RMϕ-PGE2; DEAD (Asp-Glu-Ala-Asp); ns, not significant.
TABLE 2.
Genes induced during regulatory activation
| Symbol | Name | L+I (log2) | L+A (log2) | L+P (log2) | |
|---|---|---|---|---|---|
| 1 | lif | Leukemia inhibitory factor | 9.65 | 9.64 | 8.21 |
| 2 | il10 | IL-10 | 8.73 | 6.84 | 5.99 |
| 3 | ildr1 | Ig-like domain receptor 1 | 6.59 | 4.46 | 2.93 |
| 4 | flrt3 | Fibronectin leucine-rich transmembrane protein 3 | 6.27 | 6.40 | 5.28 |
| 5 | xcr1 | Chemokine (c-motif) receptor-1 | 6.30 | 5.07 | 2.52 |
| 6 | il33 | IL-33 | 5.75 | 9.06 | 5.21 |
| 7 | ckap2l | Cytoskeleton-associated protein 2-like | 5.51 | 6.06 | 3.92 |
| 8 | ndrg1 | N-myc downstream-regulated gene 1 | 5.38 | 5.09 | 2.97 |
| 9 | itga2 | Integrin α 2 | 5.24 | 4.83 | 3.91 |
| 10 | gem | GTP-binding protein | 5.72 | 4.72 | 3.10 |
| 11 | mid1 | Midline 1 | 4.81 | 3.38 | 1.50 |
| 12 | odc1 | Ornithine decarboxylase structural 1 | 5.16 | 5.72 | 3.98 |
| 13 | hephl1 | Hephaestin-like 1 | 4.83 | 4.87 | 4.41 |
| 14 | gdnf | Glial cell line-derived neurotrophic factor | 5.42 | 6.17 | 5.18 |
| 15 | klk9 | Kallikrein-related peptidase 9 | 4.45 | 6.67 | 4.78 |
| 16 | dusp14 | Dual-specificity phosphatase 14 | 5.25 | 8.00 | 4.07 |
| 17 | gprc5a | G protein-coupled receptor family C member A | 4.73 | 4.04 | 2.80 |
| 18 | tmem88 | Transmembrane protein 88 | 5.08 | 5.77 | 3.30 |
| 19 | hrc | Histidine-rich calcium-binding protein | 4.56 | 2.99 | 1.94 |
| 20 | nptx2 | Neuronal pentraxin 2 | 4.49 | 7.62 | 3.14 |
Values represent induction over the nonstimulated and are expressed as a log2.
Activated Diseases and Functions identified by IPA analysis
The IPA platform was used to identify differences in Diseases and Functions associations between R-Mϕ and M1-Mϕ. The attributes most closely associated with 2 populations of R-Mϕ were the development of new blood vessels, the proliferation of cells, and the development of tumors (Fig. 4E and F). As expected, R-Mϕ down-regulated myeloid cell activation, the induction of TH1-associated functions, and antimicrobial and antiviral functions (Fig. 4E and F). The induction of regulatory-associated transcripts in PGE2-programmed cells was not robust enough to allow a similar analysis of these cells. The previously reported functions associated with LPS stimulation [28–31] were confirmed by our RNA-seq and IPA analyses (Supplemental Fig. 1A). AA-Mϕ appeared to be more closely associated with the maintenance of connective tissue and tissue development in general (Supplemental Fig. 1B), thus agreeing with previously described phenotypes for these cells [27, 32]. The IPA analysis of these data clearly demonstrate that R-Mϕ have functions that are distinct from AA-Mϕ.
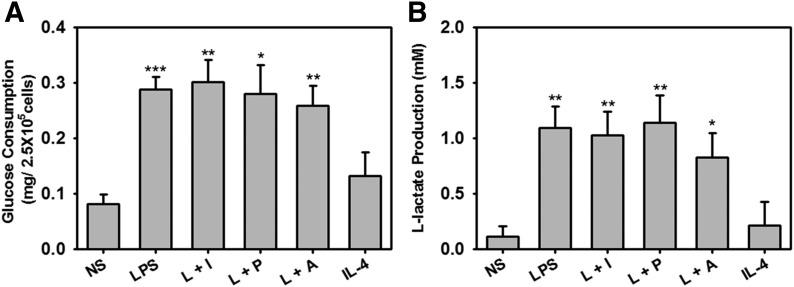
Metabolism in regulatory activation is similar to that in LPS-treated cells
Several studies have identified metabolic alterations among the various macrophage activation states [33–35]. IPA analysis of the RNA-seq data identified several metabolic changes that occurred in M1-Mϕ and R-Mϕ but not in AA-Mϕ (Table 3). Glucose consumption by R-Mϕ was comparable with M1-Mϕ, and much higher than AA-Mϕ and nonstimulated cells (Fig. 5A). Likewise, the secretion of L-lactate, a metabolic product of the fermentation pathway, was higher in M1-Mϕ and R-Mϕ than AA-Mϕ (Fig. 5B). These results suggest that macrophages with an immunoregulatory phenotype share metabolic similarities with M1-Mϕ and are distinct from AA-Mϕ. Thus, the reprogramming signals that so substantially change the phenotype of these cells (Fig. 2) do not alter the metabolic alterations associated with inflammatory M1 macrophages.
TABLE 3.
Metabolic pathways identified by IPA analysis (Z-score)
| Canonical pathways related to energy metabolism | LPS | L+I | L+A | IL-4 |
|---|---|---|---|---|
| Pentose phosphate pathway (oxidative branch) | 2.54 | 2.37 | 2.42 | 0.73 |
| Pentose phosphate pathway | 2.43 | 2.20 | 2.26 | 0.44 |
| Fatty acid β-oxidation I | 1.82 | 2.46 | 3.12 | 0 |
| Glycogen degradation II | 1.89 | 1.70 | 2.53 | 0 |
| Glycogen degradation III | 2.19 | 1.33 | 2.79 | 0 |
| Gluconeogenesis I | 1.66 | 3.73 | 1.06 | 0.21 |
| Oleate biosynthesis II (animals) | 0.85 | 1.77 | 1.82 | 0 |
| Glycolysis I | 1.12 | 3.51 | 0.97 | 0 |
The top energy metabolism-related canonical pathways that show significant up-regulation in at least 1 of the 4 conditions analyzed by RNA-seq. Numbers are expressed as the Z-score, which was generated from a Fisher's exact test when comparing stimulated with nonstimulated macrophages. Numbers that had a Z-score of at least 1.65 (P < 0.05) are considered significant.
Figure 5. Glucose and lactate production in R-Mϕ.
BMDMs were left unstimulated or stimulated with a combination of 10 ng/ml LPS alone or LPS in combination with 25 µl Ova-IC (L+I), 200 nM PGE2 (L+P), 200 μM Ado (L+A), or 20 ng/ml IL-4 for 24 h. (A) Glucose consumption was determined 24 h poststimulation by an enzymatic assay, as described in Materials and Methods. (B) l-Lactate concentrations in the supernatants were obtained 8 h poststimulation by use of the NADH-coupled enzyme reaction that reduces tetrazolium salt to formazan, which is measured at an absorbance of 490 nm. The error bars represent means ± sem values calculated from values of 4 separate experiments. ***P < 0.001, **P < 0.01, and *P < 0.05.
Identification of candidate biomarkers
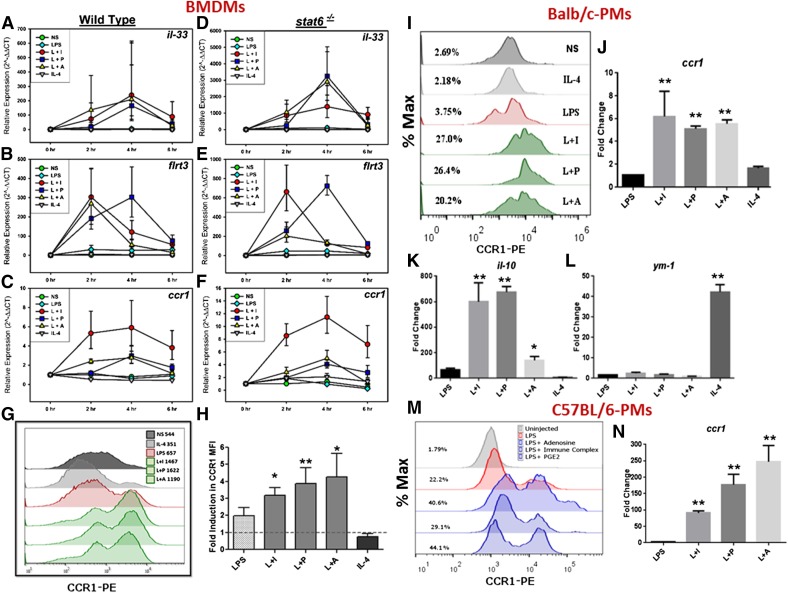
To validate the RNA-seq analyses, qRT-PCR was performed to examine several regulatory and AA-Mϕ-associated genes. Activation states were confirmed through the examination of well-established markers (Supplemental Fig. 2). Among tested genes, the mRNA levels of il-33, flrt3, and ccr1 were induced in all regulatory conditions but not by LPS or IL-4 stimulation, and their induction was STAT6 independent (Fig. 6A–F). Thus, these genes represent potential biomarkers for R-Mϕ. Additionally, some genes were induced in individual regulatory populations but not shared by all 3 R-Mϕ. For example, mRNAs encoding gem, ildr1, and epithelial membrane protein 1 were induced in RMϕ-IC but not in RMϕ-PGE2/RMϕ-Ado (Supplemental Fig. 3). The mRNA expression of il-4i1, earl1, and cd209e was specifically induced in WT AA-Mϕ but not in stat6−/− macrophages (Supplemental Fig. 3), reiterating the importance of STAT6 in alternative macrophage activation.
Figure 6. Regulatory gene induction is independent of STAT6 signaling.
RT-PCR analyses were carried out at indicated time-points on cDNA obtained from BALB/c WT and stat6−/− BMDMs, which were left unstimulated or stimulated with 10 ng/ml LPS alone or a combination of LPS and 100 µl Ova-IC (L+I), 200 nM PGE2 (L+P), 200 μM Ado (L+A), or 20 ng/ml IL-4 (A–F). Each data point represents mean values ± sem from duplicate values of 4 separate experiments. (G) The relative expression of genes up-regulated after treatment of macrophages with L+I is shown. (H) The relative expression of genes up-regulated after treatment of macrophages with IL-4 is shown. Each error bar represents mean values ± sem from duplicate values of 4 separate experiments. Surface expression of CCR1 on cells stimulated under the same conditions was assessed by flow cytometry by use of a PE-conjugated antibody to CCR1, 24 h after stimulation. A representative histogram (I) and the fold induction in CCR1 mean fluorescence intensity (MFI) over nonstimulated cells are represented from 5 separate experiments (J). The mean values ± sem are depicted, and *P < 0.05, and **P < 0.01. Regulatory gene induction was also assessed in the peritoneal macrophages (PMϕ) of Balb/c (I–L) or C57BL/6 mice (M and N) mice injected intraperitoneally with 500 μg LPS alone or in combination with 400 µl Ova-IC (L+I), 50 μg PGE2 (L+P), 50 μg Ado (L+A), or 50 μg IL-4. Macrophages were isolated 6 h later, and surface expression of CCR1 on macrophages was assessed by flow cytometry. Cells were gated on their expression of F4/80 (allophycocyanin) but not for other cell markers (CD3, CD4, CD19, and FITC). (I and M) The expression of regulatory (ccr1 and IL-10)- or alternate (ym1)-activated gene induction was evaluated by real-time PCR (J–L and N). Data were obtained from injections of 3 different mice. The numbers on the left side of the histogram denote the percentage of positive cells. *P < 0.05; **P < 0.01.
Chemokine receptors form an important component of immune responses, and the polarized macrophages subsets exhibit differences in their surface chemokine receptor expression [36]. To correlate transcription with surface protein levels, we performed flow cytometric analysis of surface expression of CCR1 on the different macrophage populations stimulated in vitro in the presence or absence of reprogramming. There was a 3- to 4-fold increase in mean fluorescent intensity observed in all of the R-Mϕ, with only a 2-fold induction in the LPS-treated cells and no induction in IL-4-treated cells (Fig. 6I and J). Thus, CCR1 represents a potential biomarker for R-Mϕ.
A further analysis of CCR1 expression was performed on R-Mϕ from mice injected in vivo with LPS intraperitoneally in the presence or absence of reprogramming signals. Peritoneal macrophages from BALB/c (Fig. 6I–L)and C57BL/6 mice (Fig. 6M and N) were analyzed. Similar to the in vitro results reported above, all 3 reprogrammers induced IL-10 transcripts from PEC (Fig. 6K), but only IL-4 injections resulted in macrophage expression of ym-1 (Fig. 6L). CCR1 was analyzed ex vivo by flow cytometry. PEC from BALB/c (Fig. 6I and J) and C57BL/6 (Fig. 6M and N) mice up-regulated CCR1 in response to all 3 reprogramming signals, indicating that the regulatory phenotype was readily inducible in vivo.
Cytokine expression from human macrophages
To extend our observations from mouse to human, we stimulated human monocyte-derived macrophages under the same conditions used for mouse BMDMs. The results were generally consistent with the murine data, although there was substantially more variability in this small patient sample. Most of the human macrophages responded to reprogramming with IC by increasing their secretion of IL-10 production and decreasing IL-12/23p40 secretion (Fig. 7A, B, and F). By bioplex assay, the inflammatory cytokines TNF-α (Fig. 7D), IL-6 (Fig. 7E), and IL-12/23p40 (Fig. 7F) were generally down-regulated in human R-Mϕ, but only IL-6 was decreased significantly in all 3 R-Mϕ groups (Fig. 7E). The other 2 inflammatory cytokines were down-regulated in ⅔ of the human R-Mϕ groups. Likewise, IL-10 (Fig. 7A and B) was up-regulated in ⅔ of the human R-Mϕ groups. The biggest difference between the murine and human cells was that the levels of IL-1β were unaffected by any of the regulatory signals in human macrophage (Fig. 7G). These results demonstrate that regulatory signals are inducible in human and murine macrophages, and when induced, they produce unique cytokine signatures that differentiate them from M1-Mϕ and AA-Mϕ.
DISCUSSION
There remains a substantial degree of confusion regarding what constitutes an M2-Mϕ. The original grouping of all macrophages that were not M1-Mϕ into the M2 category was initially instructional, as it fostered the idea that not all stimulated macrophages are the same. However, it has also led to the misconception that all M2-Mϕ are similar, and this does not appear to be the case. In this work, we describe populations of macrophages with potent anti-inflammatory activity and demonstrate that they can be generated via a STAT6-independent mechanism. We also demonstrate that R-Mϕ are transcriptionally and functionally distinct from the originally described AA-Mϕ.
In earlier reports, we demonstrated that macrophages stimulated with TLR ligands in the presence of high-density ICs assumed immunoregulatory functions by dampening inflammatory cytokine production and enhancing IL-10 secretion [18, 37]. The induction of R-Mϕ required 2 concurrent signals: 1 to activate the transcription factors necessary for cytokine production and the 2nd to “reprogram” the cell to secrete immunoregulatory cytokines. In this study, we show that there are many potential reprogramming signals that can change the phenotype of stimulated macrophages, including Ado and PGE2. All of these macrophages exhibit common characteristics, such as the production of higher levels of the immunomodulatory cytokine IL-10 and the secretion of reduced levels of inflammatory cytokines, such as IL-1β, IL-6, and IL-12/23. IPA analysis revealed that R-Mϕ were associated with increases in cell growth and proliferation. Thus, it is likely that these R-Mϕ contribute to the maintenance of homeostasis by dampening immune responses and promoting cellular repair.
To address whether IL-4 or STAT6 signaling is required for R-Mϕ functions, we studied cytokine production from macrophages from stat6−/− mice stimulated under these immunoregulatory conditions. Signaling through STAT6 was previously demonstrated to be important for the anti-inflammatory properties of IL-4 [38, 39] and the generation of AA-Mϕ [39]. In our hands, STAT6 signaling was dispensable for the generation of R-Mϕ. These cells produced similar amounts of regulatory transcripts and provided equal degrees of protection of mice from lethal endotoxemia. Furthermore, the addition of IL-4 to WT macrophages failed to induce transcripts associated with R-Mϕ and failed to rescue mice from lethal endotoxemia. Thus, these 2 macrophage populations are functionally and transcriptionally distinct.
The relationship between AA-Mϕ and R-Mϕ is fairly complex. They share some common activities, in that both appear to be induced in response to tissue injury, and both may contribute to wound healing and tissue regeneration. Furthermore, antigen presentation by R-Mϕ can give rise to TH2 T cells [40], which have the potential to generate AA-Mϕ. However, there are some clear distinctions between these 2 cell types. AA-Mϕ are uniquely involved in thermoregulation [41], and they alone are induced during helminthic infections. The depletion of AA-Mϕ can ameliorate pathologic responses in allergic disease and helminthic infections, suggesting that these cells can also regulate immune responses, but clearly, these cells are phenotypically distinct from the R-Mϕ that we describe here. In the lethal endotoxemia model shown in Fig. 1, the 2 cells have the opposite effect. The increased mortality following the addition of AA-Mϕ may be explained by the previous observations of others that IL-4 treatment of macrophages can augment their production of IL-12 [42, 43]. From these studies, one may conclude that R-Mϕ can exert a strong anti-inflammatory influence on innate-immune responses, whereas AA-Mϕ may influence adaptive immunity. Part of the confusion pertaining to M2-Mϕ stems from the fact that IL-4 and IL-10 can inhibit IFN-γ production and prevent the generation of cell-mediated immunity. The immunomodulatory effects of IL-10 appear to be most pronounced at the level of APCs. Therefore, the high levels of IL-10 production from R-Mϕ suggest that macrophages themselves are the main regulators of macrophage activation. We suggest that this is a primary function of R-Mϕ. The results from our studies clearly demonstrate that R-Mϕ and AA-Mϕ have different functions and should not be considered part of the same (M2) class.
RNA-seq analysis of various human [44] and mouse [45] macrophage subsets has been reported previously, and these studies highlight differences between macrophage subsets. Most of the genes that we report to be up-regulated specifically in AA-Mϕ agree with previously published reports [45], but our analysis emphasizes the comparative differences in transcripts by AA-Mϕ relative to R-Mϕ. By our analysis, AA-Mϕ are predicted to be involved in cell differentiation, whereas R-Mϕ are predicted to promote angiogenesis, cell growth, and repair. In addition to examining differences in the transcripts produced by AA-Mϕ relative to R-Mϕ, our analysis examined similarities among different R-Mϕ populations. This analysis was undertaken to identify a “core transcriptome” that would define macrophages with an immunoregulatory phenotype. We identified some 182 genes that were uniquely up-regulated in R-Mϕ relative to other macrophage populations, including resting, M1, and AA-Mϕ. Studies that use a NanoString platform are under way to determine whether these transcripts can collectively lead to the identification of R-Mϕ in tissue.
The identification of individual, stable, and reliable protein biomarkers for R-Mϕ proved to be much more difficult. The plasticity of macrophages poses a particular challenge to the identification of macrophage biomarkers. We demonstrated recently that M1-Mϕ gradually transition into R-Mϕ following stimulation [46], and this transition makes it difficult to establish a baseline biomarker expression level from which to compare. The chemokine receptor CCR1 was up-regulated at mRNA and protein levels in R-Mϕ, and this up-regulation occurred independent of STAT6 expression. Thus, CCR1 represents a potential biomarker for R-Mϕ. Attempts to identify R-Mϕ in tissue based on CCR1 expression are under way. This chemokine receptor has been implicated in immune regulatory functions in inflammatory and infection models [47, 48]. How this receptor affects the migratory pattern of R-Mϕ is of future interest to us.
Metabolic reprogramming has been implicated in the polarization of macrophages into M1 and M2 phenotypes [49, 50]. Metabolic pathways shift to anaerobic glycolysis in M1-Mϕ and to oxidative glucose metabolism in M2-Mϕ [33]. We demonstrate that R-Mϕ undergo enhanced glycolysis and produce L-lactate similarly to M1-Mϕ. This observation may not be surprising, as M1 and R-Mϕ are stimulated with LPS, but it does reveal that the reprogramming signals that so dramatically alter cytokine production in these cells do extend to alterations in cellular metabolism. R-Mϕ exhibit an increase in anaerobic glycolysis, despite their potent anti-inflammatory activity. Our analyses of metabolic pathways in R-Mϕ further confirm that these macrophages are distinct from AA-Mϕ.
In this study, we demonstrate that R-Mϕ distinguish themselves from M1-Mϕ by a relatively small and unique set of transcripts and immunoregulatory functions. Although there are subtle differences in the gene expression patterns and cytokine/chemokine responses among the differently generated R-Mϕ, it should be appreciated that these macrophages are biochemically and functionally related and distinct from M1-Mϕ and AA-Mϕ. Hence, we recommend the consideration of R-Mϕ as a separate macrophage population and not as a subset of M2-Mϕ.
AUTHORSHIP
Mouse cytokine experiments, RNA profiles, and endotoxin experiments were performed by B.D.F. RNA-seq experiments were performed by L.A.L.D., R.S., and N.M.E.-S. IPA analysis was performed by E.D. and B.D.F. Metabolism experiments were performed by E.D. Membrane array, bioplex, and in vivo injection studies were performed by P.C. Flow cytometry analysis was performed by P.C. and A.S. Cytokine/chemokine membrane array and human cytokine experiments were performed by P.C. and B.D.F. The manuscript was drafted by B.D.F. and D.M.M., with critical revisions made by P.C. and N.M.E.-S. This study was conceived of and designed by D.M.M.
Acknowledgments
This study was funded by grants from the U.S. National Institutes of Health (R01 GM102589-01, U01 AI088650, and R01 AI-094773). The authors thank Andrew Stewart for the critical review of the manuscript and advice pertaining to statistical analysis of qRT-PCR data.
Glossary
- AA-Mϕ
alternatively activated macrophages
- Ado
adenosine
- BMDM
bone marrow-derived macrophages
- Chi3l3
chitinase 3-like protein 3
- CT
comparative threshold cycle
- flrt3
fibronectin leucine-rich transmembrane protein 3
- gem
GTP-binding protein
- IC
immune complex
- ildr1
Ig-like domain receptor 1
- IPA
Ingenuity Pathway Analysis
- Itgb3
integrin β3
- L+A
LPS + adenosine
- L+I
LPS + immune complex
- L+P
LPS + PGE2
- MHC-II
MHC class II
- PCA
Principal Component Analysis
- PEC
peritoneal exudate cell
- qRT-PCR
quantitative RT-PCR
- R-Mϕ
regulatory macrophages
- relmα
resistin-like molecule α
- RNA-seq
RNA-Sequencing
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
D.M.M. has a financial interest in a company (LeukoSight) that seeks to manipulate immune responses at the level of macrophages. All other authors declare no competing financial interests.
REFERENCES
- 1.Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussell T., Bell T. J. (2014) Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 14, 81–93. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185. [DOI] [PubMed] [Google Scholar]
- 4.Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn T. A., Chawla A., Pollard J. W. (2013) Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez F. O., Gordon S. (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173. 10843666 [Google Scholar]
- 10.Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E. II (1986) Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J. Immunol. 136, 963–970. [PubMed] [Google Scholar]
- 11.Foss D. L., Zilliox M. J., Murtaugh M. P. (1999) Differential regulation of macrophage interleukin-1 (IL-1), IL-12, and CD80-CD86 by two bacterial toxins. Infect. Immun. 67, 5275–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay S., Peiser L., Gordon S. (2004) Activation of murine macrophages by Neisseria meningitidis and IFN-gamma in vitro: distinct roles of class A scavenger and Toll-like pattern recognition receptors in selective modulation of surface phenotype. J. Leukoc. Biol. 76, 577–584. [DOI] [PubMed] [Google Scholar]
- 13.Canton J. (2014) Phagosome maturation in polarized macrophages. J. Leukoc. Biol. 96, 729–738. [DOI] [PubMed] [Google Scholar]
- 14.Raes G., De Baetselier P., Noël W., Beschin A., Brombacher F., Hassanzadeh Gh G. (2002) Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71, 597–602. [PubMed] [Google Scholar]
- 15.Murray P. J., Wynn T. A. (2011) Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming B. D., Mosser D. M. (2011) Regulatory macrophages: setting the threshold for therapy. Eur. J. Immunol. 41, 2498–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosser D. M., Zhang X. (2008) Activation of murine macrophages. Curr. Protoc. Immunol. 83, 14.2.1–14.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson C. F., Mosser D. M. (2002) Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J. Immunol. 168, 3697–3701. [DOI] [PubMed] [Google Scholar]
- 19.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 20.Bolger A. M., Lohse M., Usadel B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C., Pachter L., Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193. [DOI] [PubMed] [Google Scholar]
- 23.Smyth G. K. (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, 1–25. [DOI] [PubMed] [Google Scholar]
- 24.Law C. W., Chen Y., Shi W., Smyth G. K. (2014) voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leek J. T., Scharpf R. B., Bravo H. C., Simcha D., Langmead B., Johnson W. E., Geman D., Baggerly K., Irizarry R. A. (2010) Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 11, 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krämer A., Green J., Pollard J. Jr., Tugendreich S. (2014) Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483. [DOI] [PubMed] [Google Scholar]
- 28.Edwards J. P., Zhang X., Frauwirth K. A., Mosser D. M. (2006) Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J. J., Diesl V., Wittmann T., Morrison D. C., Ryan J. L., Vogel S. N., Follettie M. T. (2002) Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J. Leukoc. Biol. 72, 1234–1245. [PubMed] [Google Scholar]
- 30.Gao J. J., Diesl V., Wittmann T., Morrison D. C., Ryan J. L., Vogel S. N., Follettie M. T. (2003) Bacterial LPS and CpG DNA differentially induce gene expression profiles in mouse macrophages. J. Endotoxin Res. 9, 237–243. [DOI] [PubMed] [Google Scholar]
- 31.Palaga T., Buranaruk C., Rengpipat S., Fauq A. H., Golde T. E., Kaufmann S. H., Osborne B. A. (2008) Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur. J. Immunol. 38, 174–183. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Prados J. C., Través P. G., Cuenca J., Rico D., Aragonés J., Martín-Sanz P., Cascante M., Boscá L. (2010) Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614. [DOI] [PubMed] [Google Scholar]
- 34.Odegaard J. I., Chawla A. (2011) Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6, 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colegio O. R., Chu N. Q., Szabo A. L., Chu T., Rhebergen A. M., Jairam V., Cyrus N., Brokowski C. E., Eisenbarth S. C., Phillips G. M., Cline G. W., Phillips A. J., Medzhitov R. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. [DOI] [PubMed] [Google Scholar]
- 37.Gallo P., Gonçalves R., Mosser D. M. (2010) The influence of IgG density and macrophage Fc (gamma) receptor cross-linking on phagocytosis and IL-10 production. Immunol. Lett. 133, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohmori Y., Hamilton T. A. (1998) STAT6 is required for the anti-inflammatory activity of interleukin-4 in mouse peritoneal macrophages. J. Biol. Chem. 273, 29202–29209. [DOI] [PubMed] [Google Scholar]
- 39.Huber S., Hoffmann R., Muskens F., Voehringer D. (2010) Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood 116, 3311–3320. [DOI] [PubMed] [Google Scholar]
- 40.Anderson C. F., Mosser D. M. (2002) A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 72, 101–106. [PubMed] [Google Scholar]
- 41.Nguyen K. D., Qiu Y., Cui X., Goh Y. P., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R. M., Chawla A. (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Y., Li W., Kaplan M. H., Chang C. H. (2005) Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J. Exp. Med. 201, 1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bullens D. M., Kasran A., Thielemans K., Bakkus M., Ceuppens J. L. (2001) CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand. J. Immunol. 53, 455–463. [DOI] [PubMed] [Google Scholar]
- 44.Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., De Nardo D., Gohel T. D., Emde M., Schmidleithner L., Ganesan H., Nino-Castro A., Mallmann M. R., Labzin L., Theis H., Kraut M., Beyer M., Latz E., Freeman T. C., Ulas T., Schultze J. L. (2014) Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gundra U. M., Girgis N. M., Ruckerl D., Jenkins S., Ward L. N., Kurtz Z. D., Wiens K. E., Tang M. S., Basu-Roy U., Mansukhani A., Allen J. E., Loke P. (2014) Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen H. B., Briggs K. T., Marino J. P., Ravid K., Robson S. C., Mosser D. M. (2013) TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 122, 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seki E., De Minicis S., Gwak G. Y., Kluwe J., Inokuchi S., Bursill C. A., Llovet J. M., Brenner D. A., Schwabe R. F. (2009) CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuichi K., Gao J. L., Horuk R., Wada T., Kaneko S., Murphy P. M. (2008) Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. J. Immunol. 181, 8670–8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haschemi A., Kosma P., Gille L., Evans C. R., Burant C. F., Starkl P., Knapp B., Haas R., Schmid J. A., Jandl C., Amir S., Lubec G., Park J., Esterbauer H., Bilban M., Brizuela L., Pospisilik J. A., Otterbein L. E., Wagner O. (2012) The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 15, 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biswas S. K., Mantovani A. (2012) Orchestration of metabolism by macrophages. Cell Metab. 15, 432–437. [DOI] [PubMed] [Google Scholar]